Abstract
Background
Transitions between different stages of age-related macular degeneration (AMD) are not completely captured by traditional survival models with an end point of advanced AMD.
Objectives
This study aimed to explore the transitions from early and intermediate AMD to higher non-advanced and advanced stages and determine the contributions of nutritional factors to these outcomes.
Methods
Eyes with early or intermediate AMD at baseline, classified according to the Age-Related Eye Disease Study severity scale, were included in this prospective longitudinal analysis. Foods and the biologically active nutrients associated with AMD [green leafy vegetables, fish, lutein/zeaxanthin (LZ), and ω-3 (n–3) fatty acids] were determined by a baseline food frequency questionnaire. Progression was defined as eyes transitioning to higher severity groups including non-advanced and advanced stages over 5 years, confirmed at 2 consecutive visits. Cox proportional hazards models for foods and nutrients were analyzed adjusting for demographics, lifestyle, baseline macular status, a family history of AMD, caloric intake, and genetic risk.
Results
Among 2697 eyes, 616 (23%) progressed to higher severity groups. In the food group model, higher intake of green leafy vegetables reduced incidence of transitions {hazard ratio [HR] (≥2.7 servings/wk compared with none): 0.75; 95% confidence interval [CI]: 0.59, 0.96; P = 0.02}. Higher fish intake was also protective [HR (≥ two 4-ounce servings/wk compared with <2): 0.79; 95% CI: 0.65, 0.95; P = 0.01]. In the nutrient model, LZ intake was protective [HR (≥2 mg/d compared with <2): 0.76; 95% CI: 0.60, 0.96; P = 0.02]. Higher intake of ω-3 fatty acids also tended to be beneficial [HR (≥0.7 g/wk compared with <0.7): 0.85; 95% CI: 0.71, 1.01; P = 0.06].
Conclusions
Increased consumption of green leafy vegetables, LZ, and fish nutritionally rich in ω-3 fatty acids during the initial stages of AMD may reduce rates of progression to higher severity of this debilitating disease.
This trial was registered at clinicaltrials.gov as NCT00594672.
Keywords: age-related macular degeneration, fish, green leafy vegetables, increasing severity of AMD, lutein/zeaxanthin, ω-3 fatty acids, severity groups, transitions to higher non-advanced and advanced stages of age-related macular degeneration
Introduction
Age-related macular degeneration (AMD) is a multifactorial disease causing vision loss in an estimated 20 million people living in the United States, of which there are 18 million people affected with early and intermediate non-advanced AMD [1]. Across the globe, prevalence of AMD is expected to surge to 288 million in 2040 [2], and with the recent increase in life expectancies, this chronic neurodegenerative disease heavily impacts vision loss and quality of life in industrialized nations [3,4]. Therefore, prevention is of utmost importance.
AMD is usually initially diagnosed by the presence of drusen abnormalities under the retinal pigment epithelium (RPE) or retina. Even before noticeable visual acuity changes, other visual disturbances such as loss of dark adaptation owing to photoreceptor loss and RPE dysfunction [[5], [6], [7]] are often seen with earlier non-advanced stages of AMD. As the disease advances from early and intermediate non-advanced stages to later advanced stages of geographic atrophy (GA) and neovascularization (NV), individuals experience central vision loss, making it harder to see faces, read, or drive [4]. Because earlier non-advanced stages precede advanced end points of GA with limited therapeutic options and NV with a high burden of intravitreal injections, there is a growing need for prevention to decrease the rate of progression to both non-advanced and advanced stages of macular degeneration.
Previous studies have highlighted the contribution of factors that increase rates of progression of AMD over the life course, for example, age, smoking, BMI, exercise, education, diet, inflammatory biomarkers, baseline macular status, a family history of AMD, and genetic variants are significantly associated with AMD [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]]. We have developed predictive models incorporating various non-genetic and genetic variables to distinguish between eyes likely to progress to advanced age-related macular degeneration (AAMD) and those that do not progress [[31], [32], [33], [34], [35], [36], [37]]. Our latest AMD calculator incorporates a family history and selective genetic variants to predict risk of AAMD, and AUC has reached 0.94 [38]. There is a paucity of information, however, on predictive factors in the earlier stages of disease.
In this study, we focus on transitions beyond the usual paradigm of progression to AAMD. Our primary objective was to investigate progression from early and intermediate AMD to higher non-advanced and advanced stages and analyze whether modifiable nutritional factors that have previously been associated with progression to AAMD [8,10,15,33] are also associated with these transitions, independent of other risk factors including genetic risk. Early disease prevention plays a pivotal role in slowing disease progression, thereby mitigating the transition to both non-advanced and advanced irreversible stages. This proactive approach might result in both improvement in personal quality of life and substantial mitigation of the socioeconomic burden related to AMD [4,39].
Methods
Study population
Deidentified data from the Age-Related Eye Disease Study (AREDS), a multicenter randomized clinical trial, was accessed from NIH Database of Genotype and Phenotypes through accession number phs000001.v3.p1. This trial was registered at clinicaltrials.gov as NCT00594672, enrolling participants from November 1992 to January 1998, with the clinical trial lasting until April 2001 and continued follow-up until December 2005 [40]. Research adhered to the tenets of the Declaration of Helsinki and was performed under approved institutional review board protocols of the participating sites.
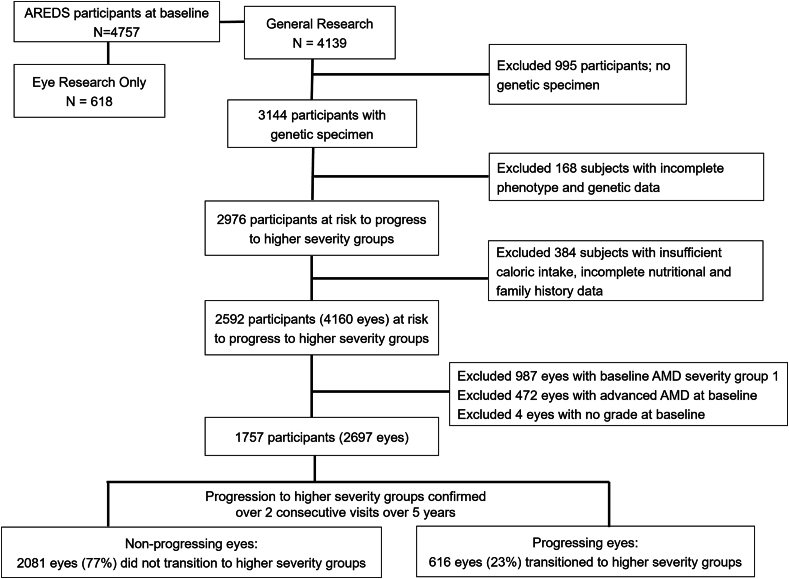
Figure 1 displays the selection of subjects and eyes for the analytic data set. Patients with no genetic specimen, with incomplete phenotypic, genetic, nutritional, and family history data, and with baseline AMD severity group 1 (severity scale 1) were excluded. The analytic data set included eyes with scales 2–8 (non-advanced AMD) at baseline and complete genetic, phenotype, family history data, with ≥1 year follow-up and valid daily caloric intake (females: 600–3200 kcal; males: 600–4200 kcal), which yielded 2697 eyes obtained from 1757 subjects.
FIGURE 1.
Flowchart showing selection of subjects for this study who are at risk of progression to higher AMD severity groups from AREDS cohort (N = 2697 eyes). AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study.
Nondietary covariates at baseline
Data on age (55–64, 65–74 and ≥75 years), sex (female/male), race, education (≤high school, >high school), and smoking status were collected at baseline and included as covariates. Race was reported as ‘White—not of Hispanic origin’, ‘Black—not of Hispanic origin’, ‘Hispanic’, ‘Asian’ or ‘Pacific Islander’, and ‘other’ and grouped for analysis as ‘non-Hispanic White’ or ‘other’. Smoking status was categorized as ‘Never’: those who never smoked cigarettes; ‘Past’: those who ever reported smoking 6 months or longer and were not smoking at baseline; and ‘Current’: those who reported smoking at baseline. BMI was not included because it may be in the causal pathway between dietary intake and AMD risk. AREDS treatment was defined as ‘Active’ for subjects in the antioxidants only, zinc only, or antioxidants–zinc group and ‘Placebo’ as subjects with placebo assignment. Multivitamin intake was defined as ‘No’ for those who never reported taking multivitamins or Centrum and ‘Yes’ for those who had taken a multivitamin supplement/Centrum in the past. Using the AREDS severity scale [41], non-advanced AMD was defined based on drusen area and size, increased pigment or depigmentation, and each eye was graded separately at baseline. AMD severity group 1 (scale 1, eyes with no AMD) was not included in our analytic data set. Scales 2 to 8 were then grouped into 2 categories to increase sample size within each group representing non-advanced levels of AMD risk—1) baseline AMD severity group 2: early AMD (scales 2–4) and 2) baseline AMD severity group 3: intermediate AMD (scales 5–8). Baseline AMD status of the fellow eye was categorized as non-advanced (scales 1–8) or AAMD with GA or NV (scales 9–12). A family history of AMD was self-reported as no family member affected with AMD, 1 family member affected with AMD, or ≥2 family members affected with AMD. A genetic risk score (GRS) was computed from β estimates of 12 loci from 9 genes shown to be most strongly associated with progression to AAMD, adjusted for age, sex, and race—complement factor H (CFH) Y402H (rs1061170); age-related maculopathy susceptibility 2/high-temperature requirement A serine peptidase 1 (ARMS2/HTRA1) A69S (rs10490924); CFH (rs1410996); CFH R1210C (rs121913059); complement component 3 (C3) R102G (rs2230199); C3 K155Q (rs147859257); RAD51 paralog B (RAD51B; rs8017304); transforming growth factor β receptor type 1 (TGFBR1; rs334353); ATP-binding cassette transporter (ABCA1; rs1883025); heat shock protein family H (Hsp110) member 1/β3-glucosyltransferase (HSPH1/B3GALTL; rs9542236); TIMP metallopeptidase inhibitor 3 (TIMP3; rs9621532); and solute carrier family 16 member 8 (SLC16A8; rs8135665) [38]. The follow-up time was restricted to 5 years to reduce misclassification due to changes in diet.
Exposure
Dietary data were obtained at baseline using a validated, self-administered, 90-item semiquantitative AREDS food frequency questionnaire based on the National Cancer Institute Health Habits and History Questionnaire (v2.1). Individuals were asked to report frequency of consumption of each food or beverage item on average, during the past year. The University of Minnesota Nutrition Coordinating Center Food Composition Database (v31) was used to estimate quantity of nutrient and caloric intake, and DietSys software (v3.0: Block Dietary Data Systems) was used to derive individual nutrient values for each food frequency questionnaire item. Nutrient estimates excluded any additional intake from oral supplementation. Weekly food consumption of green leafy vegetables [consisting of weekly ½ cup serving of spinach (raw or cooked), greens (cooked), mustard greens, turnip greens, collards] and fish intake (broiled or baked fish such as tuna, salmon, mackerel, trout) were categorized as sex-specific quintiles. Nutrient estimates of the active biological constituents of the foods: lutein/zeaxanthin (LZ) and ω-3 (n–3) fatty acids (FAs) were calorie adjusted separately for men and women and were then categorized as quintiles.
Outcome
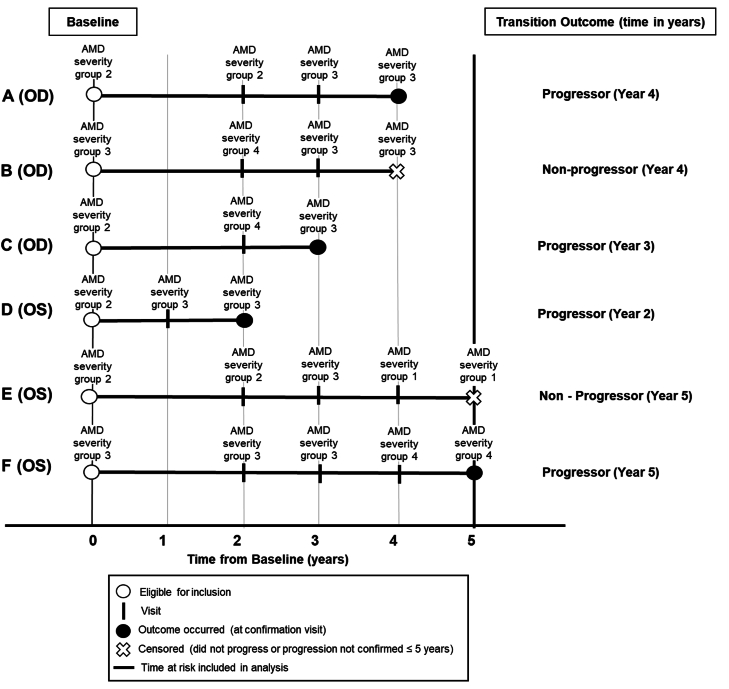
We defined progression as an eye transitioning from baseline AMD severity group 2 (early AMD) to either group 3 (intermediate AMD) or group 4 (AAMD) or from a baseline AMD severity group 3 (intermediate AMD) to group 4 (AAMD). Progression was defined to include either of these transition stages and had to be confirmed at 2 consecutive visits, ≥6 months apart over 5 years (some examples are shown in Figure 2). Time since baseline (quantified by the visit number) was used as the time scale for this analysis. An eye was censored at the earliest time of reaching a positive outcome, at 5 years if an outcome was not reached, loss to follow-up, or death. For example, an eye with AMD severity group 2 at baseline, which transitioned to AMD severity group 3 at visits in both years 3 and 4 was considered to be an outcome at year 4 and follow-up was stopped at that point (Figure 2, patient A).
FIGURE 2.
Illustration of time at risk for progression to outcome and censoring. Patients A–E had AMD severity group 2 or 3 at baseline (year 0). Patient A with baseline severity group 2 and follow-up visits in year 2, 3, and 4 with AMD severity group for their eye under study graded as 2, 3, and 3, respectively, and thus was considered a progressor at year 4 (at the confirmation visit). Patient B (OD) with baseline AMD severity group 3 was considered as a non-progressor in year 4 (at the end of follow-up). Patient C (OD) with AMD severity group 2 at baseline, transitioned to AMD severity group 4 in year 2 and was graded as AMD severity group 3 in year 3. The eye transitioned through both the higher advanced and non-advanced severity stages and was confirmed as an outcome in year 3 at a non-advanced stage. Patient D (OS) with baseline AMD severity group 2, was considered a progressor as there were 2 consecutive visits with AMD severity group 3 in years 1 and 2. Patient E (OS) with baseline AMD severity group 2 was graded as AMD severity group 3 in year 3 with no transition confirmation in any of the consecutive visits and was considered as a non-progressor at year 5. Patient F (OS) with baseline AMD severity group 3 was considered a progressor as there were 2 consecutive visits with AMD severity group 4 in years 4 and 5. AMD, age-related macular degeneration; OD, right eye; OS, left eye.
Statistical analyses
We assessed transition to higher severity groups over 5 years using a Cox proportional hazards model, with the individual eye as the unit of analysis to account for inter-eye correlation (PROC PHREG of SAS with the covariate aggregate option). After initial assessment of individual food groups and nutrients, we further assessed the combined contributions of dietary factors on progression to higher severity groups and incorporated foods (green leafy vegetables, fish) and nutrients (LZ, ω-3 FAs) in 2 separate multivariate models, adjusting for baseline nondietary covariates [38]. In addition, we estimated direct adjusted cumulative incidence curves for 4 levels of adherence to dietary consumption of the food groups: 1) high adherence to both green leafy vegetables and fish; 2) high intake of green leafy vegetables only; 3) high intake of fish only; and 4) low adherence to both green leafy vegetables and fish, assuming mean levels of the other covariates. Statistical tests were performed as 2-tailed at an α level of 0.05. All statistical analyses were performed using SAS software (v9.4).
Results
AMD transitions
Table 1 displays the changes in AREDS severity scale from baseline to 5 years in our analytic data set of 2697 eyes. In eyes with baseline non-advanced AMD severity scales 2–8, while 17% of eyes progressed to AAMD, 37% of eyes transitioned to other higher non-advanced severity scales at 5 years, which would not be counted as outcomes in typical survival analyses using only AAMD as an end point. Nevertheless, an eye usually transitions through these stages before eventually progressing to AAMD. After confirmation of outcome across 2 consecutive visits, 268 eyes (10%) progressed to higher non-advanced stages and 348 eyes (13%) progressed to AAMD. Among the 616 progressing eyes (23%), 300 were in baseline severity group 2 and 316 were in baseline severity group 3 (Table 2). The mean baseline age was 69.4 years (SD: 5.2) and mean follow-up time was 4.7 years (SD: 0.8; range: 1–5 years). A cross-tabulation of individual AREDS severity scale at baseline compared with AREDS severity scale at 5 years helped visualize these transitions through stages of AMD (Supplemental Table 1). Because most of the transitions occurred in subjects with baseline severity scales 2–8, we restricted our analyses to these eyes and excluded eyes with severity scale 1 at baseline. Similar to our analytic data set, the full AREDS data set (Supplemental Tables 2 and 3) also had a higher percentage of eyes transitioning to a higher non-advanced stage than those reaching AAMD at 5 years (29% compared with 10%). The focus of this study was to study associations of risk and protective factors (particularly nutritional factors) with these transitions.
TABLE 1.
Change in AREDS severity scale from baseline to 5 years for the analytic data set (N = 2697 eyes).
| AREDS severity scale at baseline | Decreased (%)1 | Remained the same (%)1 | Outcome not confirmed over 2 consecutive visits over a 5 year period (%)1 |
Confirmed as an outcome at second consecutive visit (%)1n = 616 (23%) |
Total | ||
|---|---|---|---|---|---|---|---|
| Transitioned to higher non-advanced stages | Progressed to AAMD | Transitioned to higher non-advanced stages | Progressed to AAMD | ||||
| 2 | 177 (28) | 236 (37) | 199 (31) | 2 (0) | 24 (4) | 5 (1) | 643 |
| 3 | 74 (28) | 55 (21) | 93 (35) | 2 (1) | 37 (14) | 5 (2) | 266 |
| 4 | 103 (21) | 106 (21) | 56 (11) | 2 (0) | 198 (40) | 31 (6) | 496 |
| 5 | 74 (24) | 59 (19) | 140 (45) | 13 (4) | 0 (0) | 24 (8) | 310 |
| 6 | 60 (15) | 106 (26) | 152 (37) | 35 (9) | 3 (1) | 53 (13) | 409 |
| 7 | 54 (13) | 77 (19) | 88 (21) | 47 (11) | 6 (1) | 142 (34) | 414 |
| 8 | 38 (24) | 23 (14) | 0 (0) | 10 (6) | 0 (0) | 88 (55) | 159 |
| Total | 580 (22) | 662 (25) | 728 (27) | 111 (4) | 268 (10) | 348 (13) | 2697 |
AAMD, advanced age-related macular degeneration; AREDS, Age-Related Eye Disease Study.
All percentages are row percentages.
TABLE 2.
Distribution of subjects and eyes according to baseline AMD severity group in the analytic dataset (N = 2697 eyes), according to progression status.
| Baseline severity group | Subjects1 (n) | Eyes (n) | Non-progressing eyes2, n (%) | Progressing eyes2, n (%) |
|---|---|---|---|---|
| Group 2 | 1052 | 1405 | 1105 (79) | 300 (21) |
| Group 3 | 885 | 1292 | 976 (75) | 316 (25) |
| Total | 17571 | 2697 | 2081 (77) | 616 (23) |
Abbreviation: AMD, age-related macular degeneration.
Only one eye was included for 817 subjects, while both eyes were included for 940 subjects in either baseline severity groups 2 or 3; therefore, since some subjects had one eye in group 2 and one eye in group 3 the number of subjects in the rows does not equal the total.
Progression defined as eye in AMD severity group 2 or group 3 at baseline progressing to higher severity groups at 2 consecutive visits over a 5 year follow-up.
Associations of non-dietary covariates and transition to higher AMD severity groups
Analyses of non-dietary covariates by progression status are summarized in Table 3 [37]. Progression was more common among older subjects, those with ≤high school education, current smokers, if the fellow eye was advanced, and if other family members were affected with AMD. With every unit increase in GRS, there was an increased incidence of transitioning to higher severity groups [hazard ratio (HR): 2.40; 95% confidence interval (CI): 1.96, 2.93; P < 0.001], adjusting for nondietary covariates. Similar incidence for transitioning was also seen for GRS tertile 3 compared with that for tertile 1 (HR: 2.38; 95% CI: 1.89, 3.00; P < 0.001), adjusting for non-dietary covariates.
TABLE 3.
Associations between non-dietary covariates and AMD transitions from severity group 2 or group 3 to higher severity groups over 5 years (616 events/2697 eyes), adjusting for other covariates1.
| Variables | Non-progressing eyes (n = 2081), n (%) | Progressing eyes (n = 616), n (%) | Model 12, HR (95% CI) | P | Model 23, HR (95% CI) | P |
|---|---|---|---|---|---|---|
| Non-genetic factors | ||||||
| Age (years) | ||||||
| Mean (SD), range | 69.1 (5.2), 55–81 | 70.5 (5.1), 56–81 | 1.05 (1.03, 1.06) | <0.001 | 1.05 (1.03, 1.07) | <0.001 |
| 55 to 64 | 75 (3.6) | 17 (2.8) | 1.00 (Ref) | 1.00 (Ref) | ||
| 65 to 74 | 1110 (53.3) | 246 (39.9) | 1.57 (1.22, 2.01) | <0.001 | 1.69 (1.30, 2.12) | <0.001 |
| ≥75 | 896 (43.1) | 353 (57.3) | 1.90 (1.44, 2.50) | <0.001 | 2.04 (1.52, 2.73) | <0.001 |
| P-trend | <0.001 | <0.001 | ||||
| Sex | ||||||
| Female | 1179 (56.7) | 332 (53.9) | 1.00 (Ref) | 1.00 (Ref) | ||
| Male | 902 (43.3) | 284 (46.1) | 0.94 (0.81, 1.10) | 0.43 | 0.97 (0.80, 1.16) | 0.71 |
| Race | ||||||
| Other | 63 (3.0) | 11 (1.8) | 1.00 (Ref) | 1.00 (Ref) | ||
| Non-Hispanic White | 2018 (97.0) | 605 (98.2) | 1.45 (0.82, 2.59) | 0.20 | 1.07 (0.53, 2.16) | 0.85 |
| Education | ||||||
| ≤High school | 693 (33.3) | 238 (38.6) | 1.00 (Ref) | 1.00 (Ref) | ||
| >High School | 1388 (66.7) | 378 (61.4) | 0.84 (0.71, 0.98) | 0.03 | 0.90 (0.76, 1.06) | 0.21 |
| Smoking | ||||||
| Never | 963 (46.3) | 253 (41.0) | 1.00 (Ref) | 1.00 (Ref) | ||
| Past | 1027 (49.3) | 309 (50.2) | 1.12 (0.95, 1.32) | 0.17 | 1.10 (0.92, 1.32) | 0.28 |
| Current | 91 (4.4) | 54 (8.8) | 2.13 (1.60, 2.83) | <0.001 | 1.67 (1.22, 2.30) | 0.002 |
| Caloric intake (kcal/d) | ||||||
| Mean (SD), range | 1510.09 (567.56), 600.36–3994.63 | 1557.82 (574.07), 622.64–3869.29 | 1.19 (1.00, 1.41)4 | 0.05 | 1.08 (0.90, 1.30)4 | 0.42 |
| AREDS treatment group | ||||||
| Placebo | 491 (23.6) | 149 (24.2) | 1.00 (Ref) | 1.00 (Ref) | ||
| Active5 | 1590 (76.4) | 467 (75.8) | 0.97 (0.81, 1.16) | 0.74 | 0.95 (0.78, 1.15) | 0.61 |
| Multivitamin intake | ||||||
| No | 1089 (52.3) | 315 (51.1) | 1.00 (Ref) | 1.00 (Ref) | ||
| Yes | 992 (47.7) | 301 (48.9) | 1.02 (0.87, 1.19) | 0.83 | 0.98 (0.83, 1.15) | 0.78 |
| Fellow eye status at baseline | ||||||
| Non-advanced | 1887 (90.7) | 445 (72.2) | 1.00 (Ref) | 1.00 (Ref) | ||
| Advanced | 194 (9.3) | 171 (27.8) | 2.90 (2.44, 3.45) | <0.001 | 2.37 (1.97, 2.87) | <0.001 |
| AMD family history | ||||||
| No family member affected | 1503 (72.2) | 390 (63.3) | 1.00 (Ref) | 1.00 (Ref) | ||
| 1 family member affected | 401 (19.3) | 146 (23.7) | 1.39 (1.15, 1.68) | <0.001 | 1.18 (0.97, 1.45) | 0.10 |
| ≥2 family members affected | 177 (8.5) | 80 (13.0) | 1.60 (1.23, 2.01) | <0.001 | 1.38 (1.07, 1.78) | 0.02 |
| P-trend6 | <0.001 | 0.006 | ||||
| Genetic risk score7 | ||||||
| GRS_continuous | ||||||
| Mean (SD), range | 1.60 (0.46), 0.26–3.11 | 1.84 (0.45), 0.28–2.99 | 2.83 (2.35, 3.41) | <0.001 | 2.40 (1.96, 2.93) | <0.001 |
| GRS tertiles | ||||||
| GRS tertile 1 | 775 (37.2) | 124 (20.1) | 1.00 (Ref) | 1.00 (Ref) | ||
| GRS tertile 2 | 718 (34.5) | 182 (29.5) | 1.53 (1.22, 1.92) | <0.001 | 1.39 (1.10, 1.77) | 0.006 |
| GRS tertile 3 | 588 (28.3) | 310 (50.3) | 2.87 (2.32, 3.55) | <0.001 | 2.38 (1.89, 3.00) | <0.001 |
| P-trend8 | <0.001 | <0.001 | ||||
Abbreviations: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CI, confidence interval; GRS, genetic risk score; HR, hazard ratio; SD, standard deviation.
Progression defined as eye in AMD severity group 2 or group 3 at baseline progressing to higher severity groups at 2 consecutive visits over a 5 year follow-up period.
Model 1: Cox proportional hazard analyses adjusted for age groups and baseline AMD severity group.
Model 2: multivariate Cox proportional hazard analyses adjusted for baseline AMD severity group in addition to all non-dietary and genetic covariates in the table.
Relative risk normalized per 1340 kcal/d (average caloric intake of females and males combined).
Active group consists of subjects treated by antioxidants only, zinc only, or a combination of antioxidants and zinc.
Estimate indicates an increased incidence of progression with each additional family member affected with AMD.
GRS calculated as sum of β coefficients multiplied by number of minor alleles for 12 genetic variants [37], after adjusting for age, sex, and race.
Estimate indicates an increased incidence of progression with increase in GRS tertile.
Association between foods/nutrients and progression to higher AMD severity groups
Results for intake of individual food groups and nutrients by quintiles (mean and ranges), adjusting for other non-dietary covariates are provided in Table 4. Adjusting for age groups and baseline AMD severity group (Table 4, model 1), significant protective trends were seen with a higher consumption of green leafy vegetables, fish, LZ, and ω-3 FA compared to lower consumption (P-trends ranged from 0.005 to 0.03). After adjusting for other non-dietary covariates (Table 4, model 2), the protective effect of higher consumption of green leafy vegetables on transition to higher severity groups persisted [HR for weekly consumption of ≥2.7 servings compared with none (quintile 5 compared with quintile 1): 0.72; 95% CI: 0.56, 0.92; P-trend = 0.02]. Effects in a protective direction were also seen for intake of 2 servings of fish per week compared with less than once per month (HR quintile 4 compared with quintile 1: 0.80; 95% CI: 0.61, 1.05) and >2 servings of fish per week compared with less than once per month (HR quintile 5 compared with quintile 1: 0.81; 95% CI: 0.61, 1.07; P-trend = 0.04). Supporting these associations, there were effects in a protective direction for the biologically active components of these foods; high intake of LZ of >2 mg/d compared with <1 mg/d (HR quintile 5 compared with quintile 1: 0.72; 95% CI: 0.55, 0.95; P-trend = 0.07) and high ω-3 FA intake > 0.7 g/wk compared with <0.28 g/wk (HR quintile 5 compared with quintile 1: 0.82; 95% CI: 0.62, 1.07; P-trend = 0.08), although the trend in a protective direction for higher ω-3 FA intake was not statistically significant in this model.
TABLE 4.
Associations between dietary and nutritional factors and AMD transitions from severity group 2 or group 3 to higher severity groups over 5 years (616 events/2697 eyes), adjusting for other covariates1.
| Food group and nutrient quintiles2, mean intake (range) | Non-progressing eyes, n (%) | Progressing eyes, n (%) | Model 13, HR (95% CI) | P-trend4 | Model 25, HR (95% CI) | P-trend4 |
|---|---|---|---|---|---|---|
| Food groups | ||||||
| Green leafy vegetables (servings/wk)6 | ||||||
| Quintile 1: 0.00 (0.00–0.00) | 625 (30.0) | 215 (34.9) | 1.00 (Ref) | 0.005 | 1.00 (Ref) | 0.02 |
| Quintile 2: 0.12 (0.12–0.12) | 114 (5.5) | 33 (5.4) | 0.82 (0.57, 1.16) | 0.87 (0.59, 1.27) | ||
| Quintile 3: 0.32 (0.23–0.53) | 447 (21.5) | 135 (21.9) | 0.88 (0.72, 1.08) | 0.87 (0.71, 1.11) | ||
| Quintile 4: 0.77 (0.53–1.17) | 455 (21.9) | 133 (21.6) | 0.85 (0.69, 1.05) | 0.88 (0.71, 1.10) | ||
| Quintile 5: 2.68 (1.05–21.0) | 440 (21.1) | 100 (16.2) | 0.71 (0.56, 0.89) | 0.72 (0.56, 0.92) | ||
| Fish (servings/wk) | ||||||
| Quintile 1: 0.24 (0.00–0.53) | 388 (18.6) | 129 (21.0) | 1.00 (Ref) | 0.008 | 1.00 (Ref) | 0.04 |
| Quintile 2: 0.75 (0.50–1.05) | 427 (20.5) | 140 (22.7) | 0.99 (0.78, 1.25) | 1.03 (0.81, 1.31) | ||
| Quintile 3: 1.27 (1.00–1.58) | 402 (19.3) | 140 (22.7) | 1.03 (0.82, 1.30) | 1.10 (0.87, 1.39) | ||
| Quintile 4: 1.95 (1.50–2.50) | 430 (20.7) | 100 (16.2) | 0.74 (0.58, 0.96) | 0.80 (0.61, 1.05) | ||
| Quintile 5: 3.88 (2.33–12.25) | 434 (20.9) | 107 (17.4) | 0.79 (0.62, 1.01) | 0.81 (0.61, 1.07) | ||
| Nutrients | ||||||
| Lutein/zeaxanthin (mg/d) | ||||||
| Quintile 1: 0.73 (0.24–0.99) | 405 (19.5) | 133 (21.6) | 1.00 (Ref) | 0.009 | 1.00 (Ref) | 0.07 |
| Quintile 2: 1.09 (0.89–1.29) | 408 (19.6) | 133 (21.6) | 1.05 (0.83, 1.32) | 0.94 (0.74, 1.21) | ||
| Quintile 3: 1.40 (1.18–1.65) | 409 (19.7) | 131 (21.3) | 0.97 (0.77, 1.22) | 1.00 (0.78, 1.27) | ||
| Quintile 4: 1.88 (1.54–2.32) | 415 (20.0) | 124 (20.1) | 0.96 (0.76, 1.22) | 1.00 (0.78, 1.30) | ||
| Quintile 5: 3.25 (2.17–12.01) | 444 (21.3) | 95 (15.4) | 0.72 (0.55, 0.92) | 0.72 (0.55, 0.95) | ||
| ω-3 fatty acids (g/wk) | ||||||
| Quintile 1: 0.14 (0.00–0.28) | 404 (19.4) | 134 (21.8) | 1.00 (Ref) | 0.03 | 1.00 (Ref) | 0.08 |
| Quintile 2: 0.35 (0.21–0.49) | 413 (19.8) | 127 (20.6) | 0.95 (0.75, 1.20) | 1.00 (0.78, 1.28) | ||
| Quintile 3: 0.49 (0.35–0.70) | 405 (19.5) | 136 (22.1) | 1.03 (0.82, 1.29) | 1.09 (0.86, 1.39) | ||
| Quintile 4: 0.70 (0.49–0.98) | 428 (20.6) | 111 (18.0) | 0.81 (0.64, 1.04) | 0.87 (0.67, 1.13) | ||
| Quintile 5: 1.82 (0.77–12.04) | 431 (20.7) | 108 (17.5) | 0.80 (0.63, 1.02) | 0.82 (0.62, 1.07) | ||
Abbreviations: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CI, confidence interval; GRS, genetic risk score; HR, hazard ratio.
Progression defined as eye in AMD severity group 2 or group 3 at baseline progressing to higher severity groups at 2 consecutive visits over a 5-year follow-up period.
Quintiles are defined as sex-specific food and nutrient quintiles. Therefore, some of the quintile boundaries may overlap.
Model 1: Cox proportional hazard analyses adjusted for age and AMD baseline severity group.
P-trend indicates increase in protective effect per quintile intake increase.
Model 2: multivariate Cox proportional hazard analyses adjusted for age groups, sex, race, education, smoking status, caloric intake, multivitamin use, AREDS treatment group, fellow eyes status, AMD baseline severity group, family history of AMD and GRS (continuous).
One medium serving of green leafy vegetables may include (but not limited to) ½ cup of spinach (raw or cooked), greens (cooked), mustard greens, turnip greens, collards.
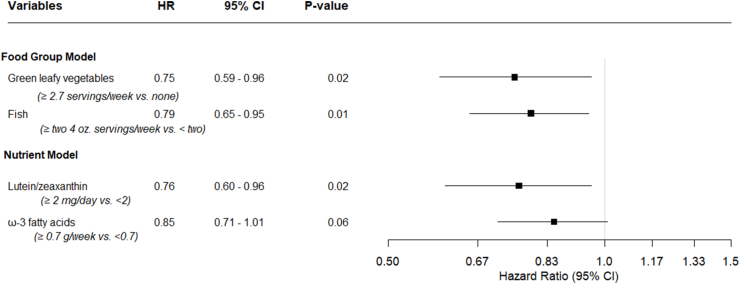
Results of the multivariate Cox proportional hazards models for the joint contributions of both foods and nutrients to AMD transitions are displayed in Figure 3 and Table 5. In the multivariate food model jointly adjusted for green leafy vegetables and fish, the HR for consumption of ≥2.7 servings of green leafy vegetables per week compared with none was 0.75 (95% CI: 0.59, 0.96; P = 0.02), and the HR for ≥2 servings of fish per week compared with <2 was 0.79 (95% CI: 0.65, 0.95; P = 0.01), after adjusting for other variables. In the nutrient model, jointly adjusted for LZ and ω-3 FA, the HR for consuming ≥2 mg LZ/d compared with <2 was 0.76 (95% CI: 0.60, 0.96; P = 0.02), and the HR for ω-3 FA consumption ≥0.7 g/wk compared with <0.7 g was 0.85 (95% CI: 0.71, 1.01; P = 0.06), after adjusting for other variables. Furthermore, eyes of subjects who had higher intake for both dietary food groups had a 41% lower incidence rate than eyes of subjects with lower intake of both green leafy vegetables and fish (HR: 0.59; 95% CI: 0.44, 0.79; P < 0.001). Eyes of subjects with dietary nutrient intake of ≥2 mg/d of LZ and ≥0.7 g/wk ω-3 FAs compared with <2 mg/d LZ and <0.7 g/wk ω-3 FAs had a 36% lower incidence rate of progressing to higher severity groups (HR: 0.64; 95% CI: 0.49, 0.84; P = 0.001).
FIGURE 3.
Multivariate Cox proportional hazards analyses identifying nutritional factors related to AMD transitions from baseline severity group 2 or 3 to higher AMD severity groups over a 5 year follow-up period, adjusted for the other food group/nutrient in respective models, in addition to age groups, sex, race, education, smoking status, caloric intake, multivitamin use, AREDS treatment groups, fellow eye status, baseline AMD severity group, a family history of AMD, and GRS (continuous). One medium serving of green leafy vegetables may include (but not limited to) ½ cup of spinach (raw or cooked), greens (cooked), mustard greens, turnip greens, collards. AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CI, confidence interval; GRS, genetic risk score; HR, hazard ratio.
TABLE 5.
Multivariate Cox proportional hazards analyses of associations between nutritional factors and AMD transitions (616 progressing eyes of 2697 eyes over 5 years of follow-up)1.
| Food group model | HR1 (95% CI); P | Nutrient model | HR2 (95% CI); P |
|---|---|---|---|
| Green leafy vegetables2 (≥2.7 servings/wk vs. none) | 0.75 (0.59, 0.96); 0.02 | Lutein/zeaxanthin (≥2 mg/d vs. <2) | 0.76 (0.60, 0.96); 0.02 |
| Fish (≥two 4 oz servings/wk vs. <2) | 0.79 (0.65, 0.95); 0.01 | ω-3 fatty acids (≥0.7 g/wk vs. <0.7) | 0.85 (0.71, 1.01); 0.06 |
| Contrasting adherence to diet with ≥2.7 servings/wk of green leafy vegetables and ≥two 4 oz servings of fish/wk compared with no green leafy vegetables and <2 servings of fish/wk3 | 0.59 (0.44, 0.79); <0.001 | Contrasting adherence to dietary intake of ≥2 mg/d of LZ and ≥0.7 g/wk ω-3 fatty acids compared with <2 mg/d LZ and <0.7 g/wk ω-3 fatty acids3 | 0.64 (0.49, 0.84); 0.001 |
Abbreviations: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; CI, confidence interval; GRS, genetic risk score; HR, hazard ratio; LZ, lutein/zeaxanthin.
Multivariate Cox proportional hazards analyses identifying nutritional factors related to AMD transitions from baseline severity group 2 or 3 to higher AMD severity groups, adjusted for the other food group/nutrient in respective models, in addition to age groups, sex, race, education, smoking status, caloric intake, multivitamin use, AREDS treatment groups, fellow eye status, baseline AMD severity group, a family history of AMD, and GRS (continuous).
One medium serving of green leafy vegetables may include (but not limited to) ½ cup of spinach (raw or cooked), greens (cooked), mustard greens, turnip greens, collards.
Contrasts estimated using linear combination of β estimates from each model.
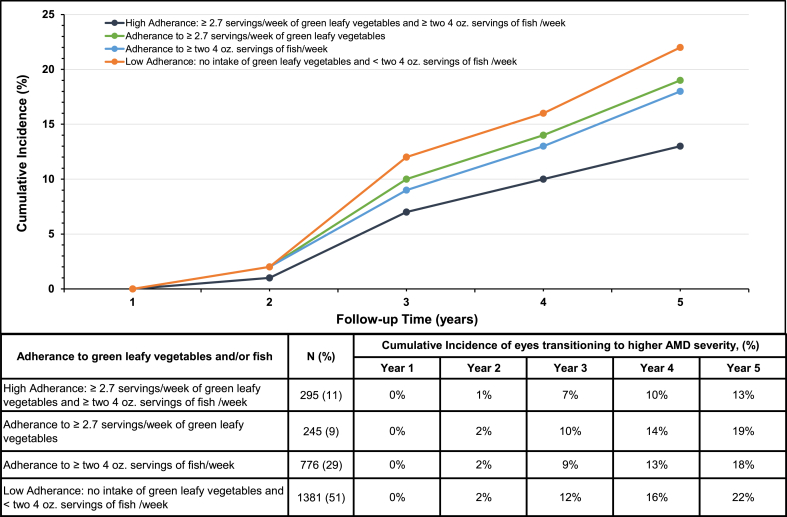
Figure 4 displays directly adjusted cumulative incidence curves for the risk of transitioning to higher AMD severity, stratified by different levels of dietary intake of green leafy vegetables and/or fish. Results indicate a 22% (95% CI: 20%, 24%) risk of transitioning to higher severity in those with low adherence to consumption of both green leafy vegetables and fish compared with 13% (95% CI: 9%, 17%) risk for those with high adherence to both food groups at the end of 5 years of follow-up. Adherence to higher intake of only 1 of these food groups was associated with 18%–19% risk of transitioning. These results suggest 41% [(22%–13%)/22%] of outcomes could be prevented by sufficient intake of green leafy vegetables and fish over 5 years.
FIGURE 4.
Cumulative incidence curves for probability of eyes transitioning to higher AMD severity, stratified by adherence to green leafy vegetables and/or fish consumption. Results adjusted for age groups, sex, race, education, smoking status, caloric intake, multivitamin use, AREDS treatment groups, fellow eye status, baseline AMD severity group, a family history of AMD, and GRS (continuous). N indicates number of eyes. AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; GRS, genetic risk score.
Discussion
Main results
Results indicate beneficial associations between dietary intake of green leafy vegetables, fish, and nutrients LZ and ω-3 FAs and subsequent transitions from early or intermediate AMD to higher AMD severity groups. We found that moderate consumption of these individual nutritional components were each independently associated with a 15%–25% lower incidence rate of progressing to higher AMD severity, compared with no or low consumption. Furthermore, individuals consuming both ≥2.7 servings/wk of green leafy vegetables and ≥two 4 oz fish/wk had a lower incidence rate of progressing to higher AMD severity over 5 years compared with individuals with lower intake, independent of other risk factors. In addition, cumulative incidence of transitioning over a 5 year period was higher among individuals who did not adhere to these dietary recommendations and could be reduced by 41% among individuals who had high consumption of both food groups. Our study provides novel insights about progression from the earlier stages of AMD to higher AMD severity and the long-term impact of dietary behaviors on the course of a chronic disease, independent of demographics, lifestyle, baseline macular status, and genetic susceptibility.
Our approach builds upon rigorous statistical procedures in longitudinal studies where the eye is the unit of analysis and the Cox proportional hazards model is used to determine progression [42,43]. In addition, we present an alternative method of analysis for longitudinal AMD data, similar to methods used for longitudinal analyses of diabetic retinopathy severity [[44], [45], [46]]. Analogous to the AMD severity staging, the Early Treatment Diabetic Retinopathy Study scale accounts for different stages of the disease before progressing to end-stage advanced proliferative retinopathy [47]. In this study, we used the AMD severity groups to define outcomes to both mild and moderate non-advanced stages as well as advanced stages with GA and NV. Traditional survival models in previous prospective cohort studies analyzing predictive factors including nutritional factors from dietary intake in AMD have had a greater focus on progression to AAMD [11,33,40,[48], [49], [50], [51], [52], [53]], neglecting the significance of changes in earlier stages of AMD and the dynamic nature of AMD progression. To address this gap, our study used an innovative approach by incorporating changes in both eyes across early and intermediate stages of AMD and confirming these changes over 2 consecutive visits. This modeling approach allowed us to efficiently use ocular longitudinal data from all severity groups, especially because some eyes transition to non-advanced stages, but do not progress to advanced AMD during the study period. By recognizing the nuanced evolution of the disease, our model provides a more detailed understanding of the factors influencing AMD progression. This approach enhances the accuracy of predictions and contributes important insights for informed clinical decision-making, paving the way for more effective public health interventions. Our findings, therefore, offer a distinctive broader conceptualization of AMD progression and promote advancements in patient care.
Previous associations of dietary intake of green leafy vegetables and fish and AMD progression
The observed protective associations between nutritional factors and transitions between stages of AMD are driven mainly by the consumption of green leafy vegetables and fish rich in LZ and ω-3 FAs. Analysis of the individual food groups and dietary patterns incorporating green leafy vegetables and fish such as the Mediterranean diet have also identified protective effects on progression to advanced stages of AMD [11,33,40,[48], [49], [50], [51], [52], [53], [54]]. Insights from our previous analysis examining 2-step drusen size progression, genetic susceptibility, and diet quality revealed that a medium/high adherence to a healthful nutrient-rich diet, including fruits, vegetables, legumes, and fish, significantly mitigated the enlargement of drusen, a hallmark of AMD [36]. Drusen size is incorporated in the staging of non-advanced AMD in addition to RPE abnormalities. In this article, we broaden the focus on progression through non-advanced stages of AMD and highlight the targeted benefits of the foods and their active biologic components during these stages.
Dietary intake of LZ and AMD progression
Macular pigments in the fovea are abundant in lutein and zeaxanthin carotenoids. These pigments play a crucial role in safeguarding macular health [55]. The body does not naturally synthesize LZ, and hence, it is important to incorporate them into one’s diet through food or supplementation to support ocular health. The first study to show the beneficial effects of higher intake of lutein and zeaxanthin nutrients in the diet was a case–control study of exudative AMD published in 1994 [8]. Specifically, spinach intake of 5–6+ servings/wk was associated with an 86% reduced risk of advanced neovascular AMD (odds ratio: 0.14; 95% CI: 0.01, 1.20; P-trend < 0.001), and dietary LZ intake of 6 mg/d compared with <1 mg/d was associated with a 57% reduced risk (odds ratio: 0.43; 95% CI: 0.20, 0.70; P-trend < 0.001). Subsequent case–control and longitudinal studies also reported protective associations of dietary carotenoid intake on AAMD [53,[56], [57], [58], [59], [60], [61]] with results summarized in reviews [62]. The Nurses’ Health Study and Health Professionals Follow-Up study in 2015 found an inverse protective association of LZ intake on progression to AAMD but not intermediate AMD [60]. In our study, incidence of transitioning from early and intermediate AMD to higher severity groups decreased by 24% for a daily dietary LZ intake of ≥2 mg (median intake = 6 mg/d) across 5 years, after controlling for other factors including ω-3 FA.
Dietary intake of ω-3 FAs in early/intermediate AMD and progression to AAMD
ω-3 FAs are a class of polyunsaturated fatty acids, including the long-chain fatty acids EPA and DHA. In the retina, ω-3 FAs are essential for maintaining the integrity and fluidity of the photoreceptor membranes [63]. The first analyses to suggest a beneficial effect of ω-3 FAs on AMD were reported more than two decades ago [10,64,65]. Many studies have since also suggested a protective effect of ω-3 FAs on early and AAMD [11,15,26,40,48,49,[66], [67], [68], [69]]. However, literature is sparse concerning the effect of dietary ω-3 FAs on transitioning to higher non-advanced and advanced stages of AMD severity, adjusting for covariates included in this study. In our analysis, we observed a notable 15% reduction in the incidence of progression of AMD to more severe stages associated with a weekly dietary intake of 0.7 g of ω-3 FAs. This reduction persisted even after accounting for other covariates including LZ, although the protective trend did not reach statistical significance.
Supplementary intake of LZ and/or ω-3 FAs in early/intermediate stages and progression to AAMD
The AREDS2 randomized controlled trial analyzed the long-term effect of supplemental LZ and ω-3 FAs in addition to the original AREDS formulation on progression to AAMD in participants with bilateral or unilateral intermediate drusen. The primary study results at the 5 year closeout did not demonstrate efficacy of supplementation of LZ, ω-3 FAs, or a combination in addition to the original AREDS supplements [70]. However, when subjects were stratified by initial dietary LZ quintiles, there were significant effects within quintile 1 (low intake) for additional LZ supplementation compared with no LZ supplementation (HR: 0.74; P = 0.01), but not within quintiles 2–5, suggesting LZ supplementation might be protective only among subjects with low dietary LZ intake. After 10 years of follow-up in AREDS2 [71], an inverse association was found with LZ supplementation (HR: 0.88) but not with supplementation of ω-3 FAs (HR: 0.97) for progression to AAMD. The multivariate results from our 5 year prospective longitudinal analyses, including non-advanced and advanced events, show stronger protective effects for dietary intake of LZ and ω-3 FAs on slowing AMD progression. Our findings underscore the advantages of maintaining healthy dietary habits and highlight how even modest alterations in behavior can significantly affect transitions between AMD stages.
Strengths and limitations
The strengths of this study include incorporation of transitions through both earlier stages as well as development of AAMD. Because there is variability of assessment of AMD severity scales, another strength of the study was the requirement that transitions be confirmed at 2 consecutive visits (usually 6 months apart) to be counted as an end point, which reduced misclassification. Because similar results were observed for effects of the nutritional factors on progression from early to intermediate and intermediate to advanced AMD, combining longitudinal data from the two non-advanced stages of AMD increased the number of end points and increased power. One limitation of the study is that we examined dietary intake at baseline. However, restricting the follow-up period to 5 years reduced the possible effect of confounding by changes in diet over longer periods.
Conclusions
Exploring the transitions from early and intermediate AMD to higher severity stages and the contribution of diet and nutrition to these transitions enhances early prevention and holistic care. Results obtained when incorporating nutritional factors, after controlling for other non-genetic and genetic factors, provide actionable insights to enrich diets with foods such as dark green leafy vegetables (e.g.: spinach—raw or cooked, kale, collards, mustard greens, turnip greens), foods rich in LZ (e.g.: peas, summer squash, corn, pumpkin, brussels sprouts, broccoli, asparagus, lettuce, carrots, egg yolks) [72] and broiled/baked fatty fishes (e.g.: salmon, sardines, mackerel, tuna, and trout) rich in EPA/DHA [73]. Benefits of healthy nutrient-rich food choices include gaining antioxidant benefits and reduction of inflammatory biomarkers including those associated with aging and other chronic diseases such as C-reactive protein [13,18,74,75]. In addressing the challenges of a disease primarily affecting the elderly, dietary modifications tailored to the aging process are essential. Challenges associated with access to quality food products, safe food handling, nutrition counseling, food security, drug–nutrition interactions and growing disparities in diet quality also need to be considered in the elderly population [[76], [77], [78], [79]].
Clinical relevance
The recent concept of “food is medicine” integrates intake of nutrient-rich healthful foods to overall well-being [80], and increasing fruit and vegetable consumption has been shown to improve cardiometabolic health outcomes [81]. Physicians should emphasize the benefits of dietary and lifestyle modifications of patients during the earlier stages of AMD to slow progression of this chronic disease. Efforts should be made to instill and reinforce these habits, ensuring their sustainability throughout an individual’s lifetime. The implication for physicians is to adopt a more holistic approach in patient care, focusing not only on advanced stages but also on the earlier transitions. This study contributes to evidence improving ophthalmic practice by guiding eye care professionals to intervene proactively during the earlier stages of AMD, delaying progression to non-advanced and advanced stages, thus leading to better patient outcomes, preserving vision, and improving overall quality of life.
Author contributions
The authors’ responsibilities were as follows – JMS: designed the research, wrote the article, and had primary responsibility for final content; DD: performed statistical analyses and wrote the article; BR: designed the research, performed statistical analysis, and wrote the article; and all authors: have revised and read and approved the final manuscript.
Conflict of interest
JMS has equity interests in Gemini Therapeutics and Apellis Pharmaceuticals and has been a consultant for Laboratoires Thea. DD and BR declare no conflict of interest.
Funding
Supported in part by National Institutes of Health, National Eye Institute grants R01-EY011309, R01-EY028602; and R01-EY022445; the American Macular Degeneration Foundation; and the Macular Degeneration Center of Excellence, University of Massachusetts Chan Medical School, Department of Ophthalmology and Visual Sciences. The sponsor or funding organization had no role in the design or conduct of this research.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2024.08.019.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rein D.B., Wittenborn J.S., Burke-Conte Z., Gulia R., Robalik T., Ehrlich J.R., et al. Prevalence of age-related macular degeneration in the US in 2019. JAMA Ophthalmol. 2022;140(12):1202–1208. doi: 10.1001/jamaophthalmol.2022.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.Y., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet. Glob. Health. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 3.Kramarow E.A., Tejada-Vera B. National vital statistics reports [Internet] 2023;72(14) https://www.cdc.gov/nchs/data/nvsr/nvsr72/nvsr72-14.pdf [cited 2024 Feb 1]. Available from: [PubMed] [Google Scholar]
- 4.Mangione C.M., Gutierrez P.R., Lowe G., Orav E.J., Seddon J.M. Influence of age-related maculopathy on visual functioning and health-related quality of life. Am. J. Ophthalmol. 1999;128(1):45–53. doi: 10.1016/S0002-9394(99)00169-5. [DOI] [PubMed] [Google Scholar]
- 5.Curcio C.A., Medeiros N.E., Millican C.L. Photoreceptor loss in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1996;37(7):1236–1249. [PubMed] [Google Scholar]
- 6.Owsley C., Swain T.A., McGwin G., Clark M.E., Kar D., Crosson J.N., et al. How vision is impaired from aging to early and intermediate age-related macular degeneration: insights from ALSTAR2 baseline. Transl. Vis. Sci. Technol. 2022;11(7):17. doi: 10.1167/tvst.11.7.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nigalye A.K., Hess K., Pundlik S.J., Jeffrey B.G., Cukras C.A., Husain D. Dark adaptation and its role in age-related macular degeneration. J. Clin. Med. 2022;11(5):1358. doi: 10.3390/jcm11051358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seddon J.M., Ajani U.A., Sperduto R.D., Hiller R., Blair N., Burton T.C., et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994;272(18):1413–1420. doi: 10.1001/jama.272.18.1413. [DOI] [PubMed] [Google Scholar]
- 9.Seddon J.M., Ajani U.A., Mitchell B.D. Familial aggregation of age-related maculopathy. Am. J. Ophthalmol. 1997;123(2):199–206. doi: 10.1016/S0002-9394(14)71036-0. [DOI] [PubMed] [Google Scholar]
- 10.Seddon J.M., Rosner B., Sperduto R.D., Yannuzzi L., Haller J.A., Blair N.P., et al. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001;119(8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 11.Seddon J.M., Cote J., Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 2003;121(12):1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seddon J.M., Cote J., Davis N., Rosner B. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch. Ophthalmol. 2003;121(6):785–792. doi: 10.1001/archopht.121.6.785. [DOI] [PubMed] [Google Scholar]
- 13.Seddon J.M., Gensler G., Milton R.C., Klein M.L., Rifai N. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291(6):704–710. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 14.Klein R.J., Zeiss C., Chew E.Y., Tsai J.Y., Sackler R.S., Haynes C., et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddon J.M., George S., Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of age-related macular degeneration. Arch. Ophthalmol. 2006;124(7):995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 16.Maller J., George S., Purcell S., Fagerness J., Altshuler D., Daly M.J., et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat. Genet. 2006;38(9):1055–1059. doi: 10.1038/ng1873. [DOI] [PubMed] [Google Scholar]
- 17.Seddon J.M., George S., Rosner B., Klein M.L. CFH gene variant, Y402H, and smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum. Hered. 2006;61(3):157–165. doi: 10.1159/000094141. [DOI] [PubMed] [Google Scholar]
- 18.Seddon J.M., Gensler G., Klein M.L., Milton R.C. C-reactive protein and homocysteine are associated with dietary and behavioral risk factors for age-related macular degeneration. Nutrition. 2006;22(4):441–443. doi: 10.1016/j.nut.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Seddon J.M., Francis P.J., George S., Schultz D.W., Rosner B., Klein M.L. Association of CFH Y402H and LOC387715 A69S with progression of age-related macular degeneration. JAMA. 2007;297(16):1793–1800. doi: 10.1001/jama.297.16.1793. [DOI] [PubMed] [Google Scholar]
- 20.Maller J.B., Fagerness J.A., Reynolds R.C., Neale B.M., Daly M.J., Seddon J.M. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat. Genet. 2007;39(10):1200–1201. doi: 10.1038/ng2131. [DOI] [PubMed] [Google Scholar]
- 21.Fagerness J.A., Maller J.B., Neale B.M., Reynolds R.C., Daly M.J., Seddon J.M. Variation near complement factor I is associated with risk of advanced AMD. Eur. J. Hum. Genet. 2009;17(1):100–104. doi: 10.1038/ejhg.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raychaudhuri S., Iartchouk O., Chin K., Tan P.L., Tai A.K., Ripke S., et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 2011;43(12):1232–1236. doi: 10.1038/ng.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobrin L., Reynolds R., Yu Y., Fagerness J., Leveziel N., Bernstein P.S., et al. ARMS2/HTRA1 locus can confer differential susceptibility to the advanced subtypes of age-related macular degeneration. Am. J. Ophthalmol. 2011;151(2):345–352.e3. doi: 10.1016/j.ajo.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y., Reynolds R., Rosner B., Daly M.J., Seddon J.M. Prospective assessment of genetic effects on progression to different stages of age-related macular degeneration using multistate Markov models. Invest. Ophthalmol. Vis. Sci. 2012;53(3):1548–1556. doi: 10.1167/iovs.11-8657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seddon J.M., Yu Y., Miller E.C., Reynolds R., Tan P.L., Gowrisankar S., et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet. 2013;45(11):1366–1370. doi: 10.1038/ng.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds R., Rosner B., Seddon J.M. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013;120(5):1020–1028. doi: 10.1016/j.ophtha.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddon J.M., Reynolds R., Yu Y., Rosner B. Three new genetic loci (R1210C in CFH, variants in COL8A1 and RAD51B) are independently related to progression to advanced macular degeneration. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0087047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddon J.M. Macular degeneration epidemiology: nature-nurture, lifestyle factors, genetic risk, and gene-environment interactions—the Weisenfeld Award Lecture. Invest. Ophthalmol. Vis. Sci. 2017;58(14):6513–6528. doi: 10.1167/iovs.17-23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMahon O., Hallam T.M., Patel S., Harris C.L., Menny A., Zelek W.M., et al. The rare C9 P167S risk variant for age-related macular degeneration increases polymerization of the terminal component of the complement cascade. Hum. Mol. Genet. 2021;30(13):1188–1199. doi: 10.1093/hmg/ddab086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seddon J.M., De D., Casazza W., Cheng S.Y., Punzo C., Daly M., et al. Risk and protection of different rare protein-coding variants of complement component C4A in age-related macular degeneration. Front. Genet. 2023;14 doi: 10.3389/fgene.2023.1274743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seddon J.M., Reynolds R., Maller J., Fagerness J.A., Daly M.J., Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest. Ophthalmol. Vis. Sci. 2009;50(5):2044–2053. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seddon J.M., Reynolds R., Yu Y., Rosner B. Validation of a prediction algorithm for progression to advanced macular degeneration subtypes. JAMA Ophthalmol. 2013;131(4):448–455. doi: 10.1001/jamaophthalmol.2013.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merle B.M.J., Silver R.E., Rosner B., Seddon J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am. J. Clin. Nutr. 2015;102(5):1196–1206. doi: 10.3945/ajcn.115.111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seddon J.M., Silver R.E., Kwong M., Rosner B. Risk prediction for progression of macular degeneration: 10 common and rare genetic variants, demographic, environmental, and macular covariates. Invest. Ophthalmol. Vis. Sci. 2015;56(4):2192–2202. doi: 10.1167/iovs.14-15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seddon J.M., Rosner B. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am. J. Ophthalmol. 2019;198:223–261. doi: 10.1016/j.ajo.2018.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merle B.M.J., Rosner B., Seddon J.M. Genetic susceptibility, diet quality, and two-step progression in drusen size. Invest. Ophthalmol. Vis. Sci. 2020;61(5):17. doi: 10.1167/iovs.61.5.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seddon J.M., Widjajahakim R., Rosner B. Rare and common genetic variants, smoking, and body mass index: progression and earlier age of developing advanced age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2020;61(14):32. doi: 10.1167/iovs.61.14.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddon J.M., De D., Rosner B. Family history of age-related macular degeneration and genetics predict progression to advanced age-related macular degeneration adjusting for macular status, demographic, and lifestyle factors. Am. J. Ophthalmol. 2023;255:74–86. doi: 10.1016/j.ajo.2023.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rein D.B., Wittenborn J.S., Zhang P., Sublett F., Lamuda P.A., Lundeen E.A., et al. The economic burden of vision loss and blindness in the United States. Ophthalmology. 2022;129(4):369–378. doi: 10.1016/j.ophtha.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Sangiovanni J.P., Agrón E., Meleth A.D., Reed G.F., Sperduto R.D., Clemons T.E., et al. ω-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2009;90(6):1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis M.D., Gangnon R.E., Lee L.Y., Hubbard L.D., Klein B.E.K., Klein R., et al. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glynn R.J., Rosner B. Regression methods when the eye is the unit of analysis. Ophthalmic. Epidemiol. 2012;19(3):159–165. doi: 10.3109/09286586.2012.674614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ying G.S., Maguire M.G., Glynn R.J., Rosner B. Tutorial on biostatistics: longitudinal analysis of correlated continuous eye data. Ophthalmic. Epidemiol. 2021;28(1):3–20. doi: 10.1080/09286586.2020.1786590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan S., Dehghani C., Pritchard N., Edwards K., Russell A.W., Malik R.A., et al. Ophthalmic and clinical factors that predict four-year development and worsening of diabetic retinopathy in type 1 diabetes. J. Diabetes Complications. 2018;32(1):67–74. doi: 10.1016/j.jdiacomp.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 45.TODAY Study Group Development and progression of diabetic retinopathy in adolescents and young adults with type 2 diabetes: results from the TODAY study. Diabetes Care. 2021;45(5):1049–1055. doi: 10.2337/dc21-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marcus D.M., Silva P.S., Liu D., Aiello L.P., Antoszyk A., Elman M., et al. Association of predominantly peripheral lesions on ultra-widefield imaging and the risk of diabetic retinopathy worsening over time. JAMA Ophthalmol. 2022;140(10):946–954. doi: 10.1001/jamaophthalmol.2022.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fundus photographic risk factors for progression of diabetic retinopathy, ETDRS report number 12, Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(Suppl 5):823–833. doi: 10.1016/S0161-6420(13)38014-2. [DOI] [PubMed] [Google Scholar]
- 48.Augood C., Chakravarthy U., Young I., Vioque J., de Jong P.T.V.M., Bentham G., et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am. J. Clin. Nutr. 2008;88(2):398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- 49.Souied E.H., Delcourt C., Querques G., Bassols A., Merle B., Zourdani A., et al. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology. 2013;120(8):1619–1631. doi: 10.1016/j.ophtha.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Wang J.J., Buitendijk G.H.S., Rochtchina E., Lee K.E., Klein B.E.K., van Duijn C.M., et al. Genetic susceptibility, dietary antioxidants, and long-term incidence of age-related macular degeneration in two populations. Ophthalmology. 2014;121(3):667–675. doi: 10.1016/j.ophtha.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 51.Merle B.M.J., Colijn J.M., Cougnard-Grégoire A., de Koning-Backus A.P.M., Delyfer M.N., Kiefte-de Jong J.C., et al. Mediterranean diet and incidence of advanced age-related macular degeneration: the EYE-RISK consortium. Ophthalmology. 2019;126(3):381–390. doi: 10.1016/j.ophtha.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Keenan T.D., Agrón E., Mares J., Clemons T.E., van Asten F., Swaroop A., et al. Adherence to the Mediterranean diet and progression to late age-related macular degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology. 2020;127(11):1515–1528. doi: 10.1016/j.ophtha.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 53.Agrón E., Mares J., Clemons T.E., Swaroop A., Chew E.Y., Keenan T.D.L., et al. Dietary nutrient intake and progression to late age-related macular degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology. 2021;128(3):425–442. doi: 10.1016/j.ophtha.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hogg R.E., Woodside J.V., McGrath A., Young I.S., Vioque J.L., Chakravarthy U., et al. Mediterranean diet score and its association with age-related macular degeneration: the European Eye Study. Ophthalmology. 2017;124(1):82–89. doi: 10.1016/j.ophtha.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein P.S., Li B., Vachali P.P., Gorusupudi A., Shyam R., Henriksen B.S., et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye. Res. 2016;50:34–66. doi: 10.1016/j.preteyeres.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho E., Seddon J.M., Rosner B., Willett W.C., Hankinson S.E. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch. Ophthalmol. 2004;122(6):883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- 57.van Leeuwen R., Boekhoorn S., Vingerling J.R., Witteman J.C.M., Klaver C.C.W., Hofman A., et al. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005;294(24):3101–3107. doi: 10.1001/jama.294.24.3101. [DOI] [PubMed] [Google Scholar]
- 58.Tan J.S.L., Wang J.J., Flood V., Rochtchina E., Smith W., Mitchell P. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115(2):334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 59.Seddon J.M., Reynolds R., Rosner B. Associations of smoking, body mass index, dietary lutein, and the LIPC gene variant rs10468017 with advanced age-related macular degeneration. Mol. Vis. 2010;16:2412–2424. [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J., Cho E., Willett W.C., Sastry S.M., Schaumberg D.A. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015;133(12):1415–1424. doi: 10.1001/jamaophthalmol.2015.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delcourt C., Carrière I., Delage M., Barberger-Gateau P., Schalch W. POLA Study Group, Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest. Ophthalmol. Vis. Sci. 2006;47(6):2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- 62.Chapman N.A., Jacobs R.J., Braakhuis A.J. Role of diet and food intake in age-related macular degeneration: a systematic review. Clin. Exp. Ophthalmol. 2019;47(1):106–127. doi: 10.1111/ceo.13343. [DOI] [PubMed] [Google Scholar]
- 63.Querques G., Forte R., Souied E.H. Retina and omega-3. J. Nutr. Metab. 2011;2011 doi: 10.1155/2011/748361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seddon J.M., Ajani U.A., Sperduto R.D., Yannuzzi L., Burton T., Haller J.A., et al. Dietary fat and risk for advanced age-related macular degeneration. Arch. Ophthalmol. 2001;119(8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 65.Mares-Perlman J.A., Brady W.E., Klein R., VandenLangenberg G.M., Klein B.E., Palta M. Dietary fat and age-related maculopathy. Arch. Ophthalmol. 1995;113(6):743–748. doi: 10.1001/archopht.1995.01100060069034. [DOI] [PubMed] [Google Scholar]
- 66.Chua B., Flood V., Rochtchina E., Wang J.J., Smith W., Mitchell P. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch. Ophthalmol. 2006;124(7):981–986. doi: 10.1001/archopht.124.7.981. [DOI] [PubMed] [Google Scholar]
- 67.Christen W.G., Schaumberg D.A., Glynn R.J., Buring J.E. Dietary ω-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch. Ophthalmol. 2011;129(7):921–929. doi: 10.1001/archophthalmol.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lawrenson J.G., Evans J.R. Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2015;2015(4) doi: 10.1002/14651858.CD010015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joachim N.D.L., Mitchell P., Kifley A., Wang J.J. Incidence, progression, and associated risk factors of medium drusen in age-related macular degeneration: findings from the 15-year follow-up of an Australian cohort. JAMA Ophthalmol. 2015;133(6):698–705. doi: 10.1001/jamaophthalmol.2015.0498. [DOI] [PubMed] [Google Scholar]
- 70.Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 71.Chew E.Y., Clemons T.E., Agrón E., Domalpally A., Keenan T.D.L., Vitale S., et al. Long-term outcomes of adding lutein/zeaxanthin and ω-3 fatty acids to the AREDS supplements on age-related macular degeneration progression: AREDS2 report 28. JAMA Ophthalmol. 2022;140(7):692–698. doi: 10.1001/jamaophthalmol.2022.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.FoodData Central [Internet]. [cited 11 July, 2024]. Available from: https://fdc.nal.usda.gov/fdc-app.html#/?component=1123.
- 73.Office of Dietary Supplements—omega-3 fatty acids [Internet]. [cited 2024 Feb 5]. Available from: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer/.
- 74.Seddon J.M., George S., Rosner B., Rifai N. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch. Ophthalmol. 2005;123(6):774–782. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 75.Seddon J.M., Gensler G., Rosner B. C-reactive protein and CFH, ARMS2/HTRA1 gene variants are independently associated with risk of macular degeneration. Ophthalmology. 2010;117(8):1560–1566. doi: 10.1016/j.ophtha.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shlisky J., Bloom D.E., Beaudreault A.R., Tucker K.L., Keller H.H., Freund-Levi Y., et al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv. Nutr. 2017;8(1):17–26. doi: 10.3945/an.116.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leung C.W., Wolfson J.A. Food insecurity among older adults: 10-year national trends and associations with diet quality. J. Am. Geriatr. Soc. 2021;69(4):964–971. doi: 10.1111/jgs.16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhao H., Andreyeva T. Diet quality and health in older Americans. Nutrients. 2022;14(6):1198. doi: 10.3390/nu14061198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J., Mozaffarian D. Trends in diet quality among U.S. adults from 1999 to 2020 by race, ethnicity, and socioeconomic disadvantage. Ann. Intern. Med. 2024;177(7):841–850. doi: 10.7326/M24-0190. [DOI] [PubMed] [Google Scholar]
- 80.Volpp K.G., Berkowitz S.A., Sharma S.V., Anderson C.A.M., Brewer L.C., Elkind M.S.V., et al. Food is medicine: a presidential advisory from the American Heart Association. Circulation. 2023;148(18):1417–1439. doi: 10.1161/CIR.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hager K., Du M., Li Z., Mozaffarian D., Chui K., Shi P., et al. Impact of produce prescriptions on diet, food security, and cardiometabolic health outcomes: a multisite evaluation of 9 produce prescription programs in the United States, Circ. Cardiovasc. Qual. Outcomes. 2023;16(9) doi: 10.1161/CIRCOUTCOMES.122.009520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon reasonable request.