Abstract
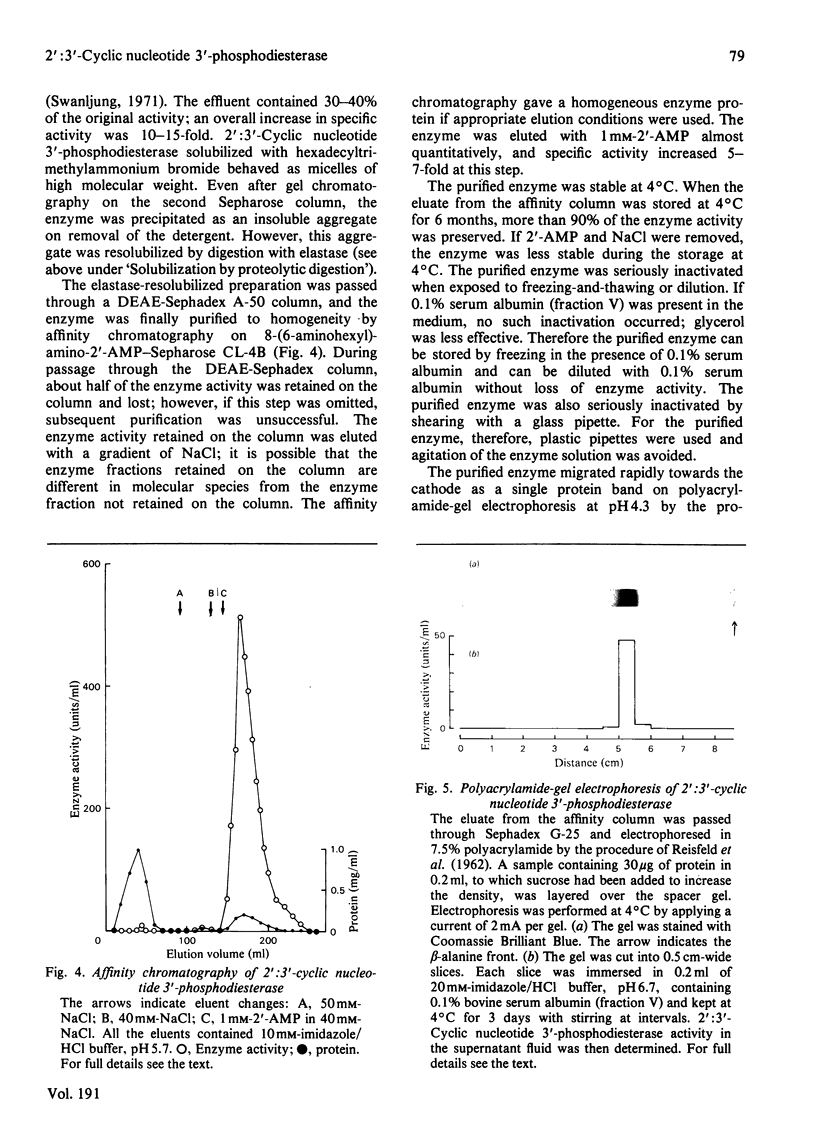
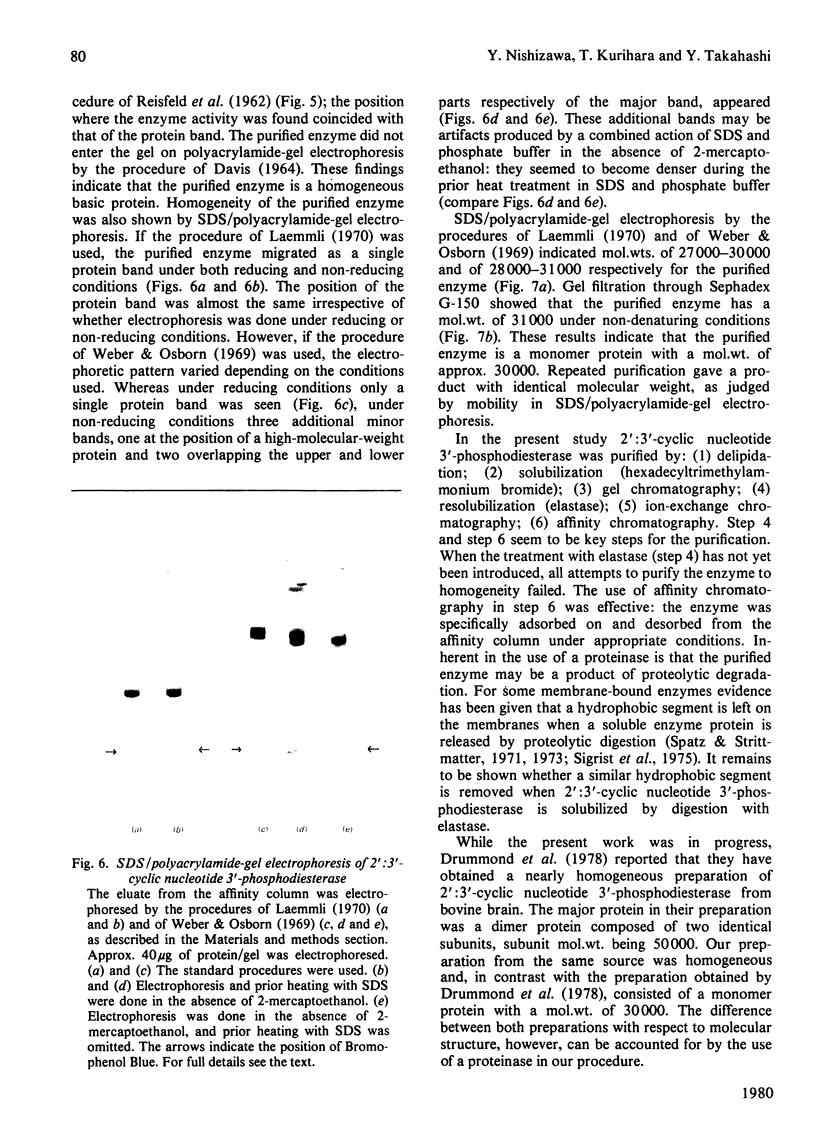
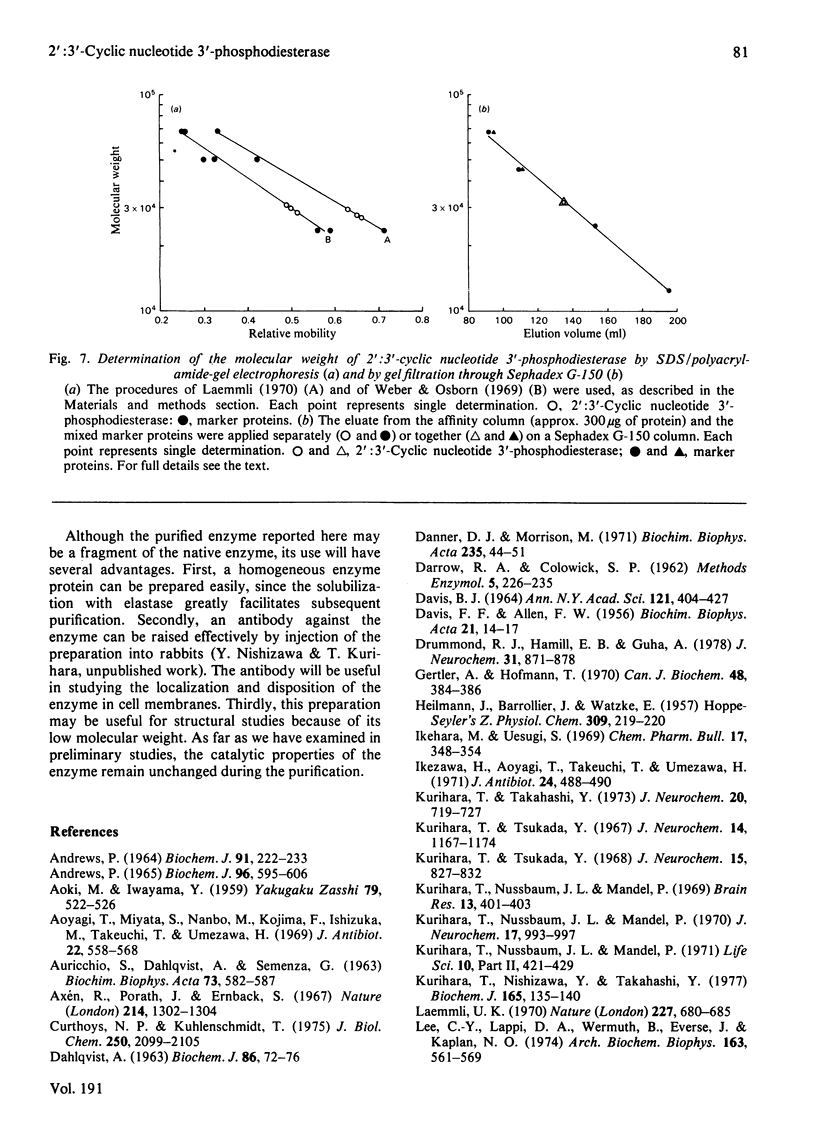
1. A spectrophotometric assay of 2':3'-cyclic nucleotide 3'-phosphodiesterase (EC 3.1.4.37) based on the use of an acid-base indicator and a buffer having identical pKa values is described. The assay is simple and rapid; it was particularly convenient for monitoring the enzyme activity at various stages of purification. 2. Several proteinases were examined for their ability to solubilize 2':3'-cyclic nucleotide 3'-phosphodiesterase from delipidated brain white matter. Trypsin (EC 3.4.21.4) and elastase (EC 3.4.21.11) appeared to be more effective than the other proteinases examined. Trypsin, however, caused inactivation; elastase was therefore chosen to solubilize 2':3'-cyclic nucleotide 3'-phosphodiesterase. When a partially purified preparation of 2':3'-cyclic nucleotide 3'-phosphodiesterase was treated with elastase, 2':3'-cyclic nucleotide 3'-phosphodiesterase was solubilized nearly quantitatively. Elastatinal, a specific inhibitor of elastase, specifically inhibited the solubilization with elastase. 3. 2':3'-cyclic nucleotide 3'-phosphodiesterase was purified from bovine brain white matter by: (i) delipidation; (ii) solubilization with hexadecyltrimethylammonium bromide; (iii) gel chromatography on Sepharose; (iv) ethanol precipitation and resolubilization by digestion with elastase; (v) chromatography on DEAE-Sephadex; (vi) affinity chromatography on 8-(6-aminohexyl)amino-2'-AMP-Sepharose. 4. The purified enzyme migrated as a single protein band on polyacrylamide-gel electrophoresis at pH 4.3 and on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis; the estimated mol.wt. in the latter electrophoresis was 27000-31000. Gel filtration of the purified enzyme through Sephadex G-150 indicated a mol.wt. of 31000. Therefore the purified enzyme is a monomer protein with a mol.wt. of approx. 30000.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN F. W., DAVIS F. F. A specific phosphodiesterase from beef pancreas. Biochim Biophys Acta. 1956 Jul;21(1):14–17. doi: 10.1016/0006-3002(56)90088-9. [DOI] [PubMed] [Google Scholar]
- AURICCHIO S., DAHLQVIST A., SEMENZA G. SOLUBILIZATION OF THE HUMAN INTESTINAL DISACCHARIDASES. Biochim Biophys Acta. 1963 Aug 6;73:582–587. doi: 10.1016/0006-3002(63)90329-9. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T., Miyata S., Nanbo M., Kojima F., Matsuzaki M. Biological activities of leupeptins. J Antibiot (Tokyo) 1969 Nov;22(11):558–568. doi: 10.7164/antibiotics.22.558. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Curthoys N. P., Kuhlenschmidt T. Phosphate-independent glutaminase from rat kidney. Partial purification and identity with gamma-glutamyltranspeptidase. J Biol Chem. 1975 Mar 25;250(6):2099–2105. [PubMed] [Google Scholar]
- DAHLQVIST A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963 Jan;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Morrison M. Isolation of the thyroid peroxidase complex. Biochim Biophys Acta. 1971 Apr 14;235(1):44–51. doi: 10.1016/0005-2744(71)90031-3. [DOI] [PubMed] [Google Scholar]
- Drummond R. J., Hamill E. B., Guha A. Purification and comparison of 2',3'-cyclic nucleotide 3'-phosphohydrolases from bovine brain and spinal cord. J Neurochem. 1978 Oct;31(4):871–878. doi: 10.1111/j.1471-4159.1978.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Gertler A., Hofmann T. Acetyl-L-alanyl-L-alanyl-L-alanine methyl ester: a new highly specific elastase substrate. Can J Biochem. 1970 Mar;48(3):384–386. doi: 10.1139/o70-061. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Ikehara M., Uesugi S. Studies on nucleosides and nucleotides. 38. Synthesis of 8-bromoadenosine nucleotides. Chem Pharm Bull (Tokyo) 1969 Feb;17(2):348–354. doi: 10.1248/cpb.17.348. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Nishizawa Y., Takahashi Y. The use of non-aqueous chloroform/methanol extraction for the delipidation of brain with minimal loss of enzyme activities. Biochem J. 1977 Jul 1;165(1):135–140. doi: 10.1042/bj1650135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Nussbaum J. L., Mandel P. 2',3'-cyclic nucleotide 3'-phosphohydrolase in brains of mutant mice with deficient myelination. J Neurochem. 1970 Jul;17(7):993–997. doi: 10.1111/j.1471-4159.1970.tb02252.x. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Nussbaum J. L., Mandel P. 2',3'-cyclic nucleotide 3'-phosphohydrolase in purified myelin from brain of Jimpy and normal young mice. Life Sci II. 1971 Apr 22;10(8):421–429. doi: 10.1016/0024-3205(71)90303-1. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Nussbaum J. L., Mandel P. 2',3'-cyclic nucleotide 3'-phosphohydrolase in the brain of the "Jimpy" mouse, a mutant with deficient myelination. Brain Res. 1969 Apr;13(2):401–403. doi: 10.1016/0006-8993(69)90299-6. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Takahashi Y. Potentiometric and colorimetric methods for the assay of 2',3'-cyclic nucleotide 3'-phosphohydrolase. J Neurochem. 1973 Mar;20(3):719–727. doi: 10.1111/j.1471-4159.1973.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Tsukada Y. The regional and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphohydrolase in the central nervous system. J Neurochem. 1967 Dec;14(12):1167–1174. doi: 10.1111/j.1471-4159.1967.tb06164.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maestracci D. Enzymic solubilization of the human intestinal brush border membrane enzymes. Biochim Biophys Acta. 1976 May 21;433(3):469–481. doi: 10.1016/0005-2736(76)90274-1. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D., Baratti J. The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta. 1973 Sep 15;321(1):282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Mosbach K. AMP and NAD as "General Ligands". Methods Enzymol. 1974;34:229–242. doi: 10.1016/s0076-6879(74)34019-0. [DOI] [PubMed] [Google Scholar]
- NAUGHTON M. A., SANGER F. Purification and specificity of pancreatic elastase. Biochem J. 1961 Jan;78:156–163. doi: 10.1042/bj0780156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A. S., Anwar R. A. The specificity of purified porcine pancreatic elastase. Biochem J. 1969 Aug;114(1):11–17. doi: 10.1042/bj1140011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Sigrist H., Ronner P., Semenza G. A hydrophobic form of the small-intestinal sucrase-isomaltase complex. Biochim Biophys Acta. 1975 Oct 17;406(3):433–446. doi: 10.1016/0005-2736(75)90022-x. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Takesue S., Omura T. Solubilization of NADH-cytochrome b5 reductase from liver microsomes by lysosomal digestion. J Biochem. 1970 Feb;67(2):259–266. doi: 10.1093/oxfordjournals.jbchem.a129249. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Okura A., Morishima H., Takeuchi T. Letter: Elastatinal, a new elastase inhibitor produced by actinomycetes. J Antibiot (Tokyo) 1973 Dec;26(12):787–789. doi: 10.7164/antibiotics.26.787. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]