Abstract
Background:
Evidence suggests that long-term exposure to air pollution may increase the risk of dementia and related cognitive outcomes. A major source of air pollution is automotive traffic, which is modifiable by technological and regulatory interventions.
Objectives:
We examined associations of four traffic-related air pollutants with rates of cognitive decline in a cohort of older adults.
Methods:
We analyzed data from the Chicago Health and Aging Project (CHAP), a longitudinal (1993–2012) community-based cohort study of older adults that included repeated assessments of participants’ cognitive performance. Leveraging previously developed air pollution models, we predicted participant-level exposures to the tailpipe pollutants oxides of nitrogen () and nitrogen dioxide (), plus the nontailpipe pollutants copper and zinc found in coarse particulate matter [PM with aerodynamic diameter to () and , respectively], over the 3 y prior to each participant’s baseline assessment. Using generalized estimating equations, we estimated covariate-adjusted associations of each pollutant with rates of cognitive decline. We probed the robustness of our results via several sensitivity analyses, including alterations to the length of the exposure assessment window and exploring the influence of pre- and post-baseline selection bias.
Results:
Using data from 6,061 participants, estimated associations of these pollutant exposures with cognitive decline were largely inconsistent with large adverse effects. For example, a standard deviation () increment in corresponded to a slightly slower rate of cognitive decline [e.g., mean difference in change in global score, 0.010 standard unit/5 y, 95% confidence interval (CI): , 0.036]. The results of most of our sensitivity analyses were in generally similar to those of our main analyses, but our prebaseline selection bias results suggest that our analytic results may have been influenced by differential survivorship into our study sample.
Discussion:
In this large prospective cohort study, we did not observe compelling evidence that long-term TRAP exposure is associated with cognitive decline. https://doi.org/10.1289/EHP14585
Introduction
The hypothesis that long-term exposure to air pollution increases the risk of dementia and dementia-related outcomes continues to receive support from epidemiological evidence.1–22 If air pollution elevates these risks, interventions could be implemented to reduce the population burden of exposures. Policy interventions can have particularly wide reach, imparting benefits to whole populations without the need for individual-level interventions or changes to behavior.
Understanding the cognitive effects of air pollution from specific sources may be especially useful for informing interventions. Automotive traffic-related air pollution (TRAP) is one such compelling source because human exposure to TRAP is common, particularly in urban settings.23 TRAP includes the products of fuel combustion (“tailpipe pollutants”) and dust generated by the wear and tear of brakes and tires, along with resuspended road material and soil (“nontailpipe pollutants”). The health effects of nontailpipe pollutants are of growing interest because many pollutants from tailpipe emissions have decreased over time.23 In comparison with tailpipe particles, those in the nontailpipe mixture are larger and have a higher metallic content.23 This mixture includes coarse fraction particulate matter (PM; particles in aerodynamic diameter) produced by brake and tire wear that is rich in copper and zinc, respectively.1
In general, exposure to TRAP is believed to adversely affect cognitive health both directly through effects on the brain and indirectly through effects on vascular health and other organ systems.23–25 Although numerous epidemiological studies have estimated associations of TRAP exposures with cognitive performance and dementia,1,5,6,8,12–14,17,18,21,26 these studies have almost entirely focused on tailpipe emissions and ignored nontailpipe emissions. Furthermore, three recent systematic reviews judged the evidence to be largely inconsistent1,23,27 and generally lacking studies of TRAP in relation to cognitive decline. This inconsistent evidence may be important because cognitive decline characterizes years-long cognitive function changes that precede and then characterize cognitive impairment and dementia.28 Evidence of an effect of TRAP exposure during adulthood on cognitive decline would more directly tie this exposure to the neurodegenerative process underlying dementia.
Selection bias also presents challenges to validly estimating TRAP’s effects on dementia-related outcomes. In particular, TRAP exposure is associated with increased mortality and morbidity,23,24,29,30 which both affect who enrolls in studies of late-life health and who continues participating after enrolling. Given that cognitive function is also associated with these determinants of selection, it is possible that the resulting study samples of older adults may be less susceptible than expected to TRAP’s adverse cognitive effects.30–32 The possible influence of selection bias is commonly acknowledged, but attempts to adjust for or quantify its potential impact have been less common.
In this study, we aimed to investigate associations of long-term exposures to two tailpipe and two nontailpipe traffic-related air pollutants with rates of cognitive change during older adulthood, advancing the evidence on how TRAP exposure affects dementia risk. We hypothesized that higher long-term exposure to TRAP would be adversely associated with rates of cognitive change. We propose that exposure over long periods—e.g., exposure accrued over years rather than days—is etiologically relevant to cognitive decline in older adulthood. Even if short bouts of exposure exert small effects on cognition, the influence of sustained exposure is presumably larger, in addition to being more suitable for evaluation in the setting of an observational study of cognitive decline. We also explored the potential influence of selection bias on our effect estimates.
Methods
Study Population
Our study was set in the Chicago Health and Aging Project (CHAP), a population-based longitudinal study (1993–2012) of older adults living in four adjacent neighborhoods on the south side of Chicago, Illinois.30,33 The founding purpose of the CHAP was to be a longitudinal study of common late-life chronic conditions, particularly those that increase the risk of dementia, in a cohort of Black and White older adults who were at least 65 y of age.33 The CHAP recruited an initial cohort of participants in the period 1993–1996, enrolling 6,157 adults, comprising 79% of all age-eligible persons in the study’s catchment area according to a community census; they also enrolled a small subset () who were 61–64.9 y of age. An additional 4,644 participants who later became age-eligible were recruited in successive cohorts, leading to a total study population of 10,802 participants by 2012.30,33 All CHAP participants were interviewed in their homes, both at baseline and all follow-up cycles (i.e., visits), which occurred roughly every 3 y post baseline. These interviews involved cognitive assessments and questionnaires about demographics, health, and health-related behaviors. This study was approved by the institutional review boards of Rush University Medical Center, Chicago, Illinois; the University of Michigan, Ann Arbor, Michigan; the University of Washington, Seattle, Washington; and Boston University, Boston, Massachusetts. All CHAP participants provided written informed consent.
Exposure Assessment
We estimated participants’ exposure to four TRAP species. The two tailpipe-generated pollutants, oxides of nitrogen () and nitrogen dioxide (), are both markers of fossil fuel exhaust. The two nontailpipe-generated pollutants were coarse copper particulate matter in diameter (), an indicator of vehicle brake wear, and coarse zinc PM in diameter (), an indicator of vehicle tire wear.34,35 Data on nearly all participants’ residential addresses (98%) were sufficiently complete and correct to allow geocoding to an exact location.
Exposure to ambient and .
and concentrations were predicted at each residential address by spatiotemporal models developed for the Chicago area as part of the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) project.36,37 In brief, these spatiotemporal models were optimized via maximum likelihood methods and incorporated hundreds of variables, including geographic features and distances from major transportation routes (e.g., roads, highways, airports, ports, and railroads).38–40 The models additionally incorporated data from a community-monitoring campaign (which featured repeated sampling from 6 fixed site monitors and 113 outdoor home samples) and included a long-term spatial mean, temporal trends with spatially varying coefficients, and a spatiotemporal residual. The resulting models had a 10-fold cross-validation of 0.87 and were finely resolved both temporally (with predictions specific to 2-wk intervals) and spatially (with predictions at precise residential locations).39
Exposure to ambient coarse copper and zinc PM.
and concentrations were predicted at residential addresses by a spatial model developed for Chicago as part of the MESA Coarse Study.41 This model employed universal kriging for and concentrations in the Chicago area and incorporated data from a two-season monitoring campaign in 2009, plus a suite of covariates similar to those used in the and models to estimate spatial variations to precise residential locations.37 The cross-validation of the estimation models was 0.81 for and 0.80 for .
Assessment of Cognitive Function
During interviews, all CHAP participants underwent a brief cognitive assessment that consisted of four cognitive tests: the Symbol Digit Modalities Test,42 which measured perceptual speed; the East Boston Memory Test,43 which measured both immediate and delayed episodic memory (two separate scores); and the Mini-Mental State Examination,44 which measured several cognitive functions, including orientation, memory, language, and visual construction. We transformed each test score to z-scores, using baseline raw scores as the source for the means and standard deviations (SDs).
For analyses, we considered both global and domain-specific rates of change in cognitive function (i.e., cognitive decline). A global cognitive score was created by generating a composite z-score from averaging the z-scores from all four tests, and then this z-score was transformed to become standard normally distributed via the baseline composite z-score’s mean and SD.32,45–47 Processing speed was defined as the Symbol Digit Modalities Test z-score, whereas episodic memory was defined as the standard-normalized average of the z-transformed immediate and delayed scores on the East Boston Memory Test.
Measurement of covariates.
Our analyses of TRAP effects on cognitive decline included the following time-fixed covariates that were measured at the time that participants enrolled in the CHAP: sex/gender, race, years of education, and neighborhood socioeconomic status at the time of CHAP enrollment. Sex/gender was recorded by CHAP investigators as either male or female. Participants reported their race according to the options given on the 1990 US Census (White, Black, American Indian/Alaska Native, or Asian/Pacific Islander). Nearly all participants (99.7%) identified as either White or Black; due to the very small number of participants who identified as Indian/Alaska Native or Asian/Pacific Islander, we categorized participants as either Black or not Black. This race variable in our models was meant to serve as a proxy for historical and structural inequalities stemming from racism. Participants also reported the years of formal education that they had completed ( y, 9–11 y, 12–16 y, or y). Neighborhood socioeconomic status at the time of CHAP enrollment was based on a composite area-based socioeconomic score developed by Diez Roux et al.48 This score was based on participants’ US census block groups and was the average of the block group–specific z-scores for the following block-specific measurements: a) median household income, b) median home value, c) percentage of households with income from interest, dividends, or rent, d) percentage of adults who completed college, and e) percentage of employed persons 16 y of age or older in executive, managerial, or professional specialty occupations. Higher scores indicated higher neighborhood socioeconomic status. Originally, the score created by Diez Roux et al. included an indicator for the percentage of adults with a high school diploma as well; however, our score did not include this variable because it was not distributed as expected across composite scores within the CHAP census-block groups. This index has been applied in several CHAP-based studies.30,49
We additionally included time-fixed covariates that were measured at participants’ analytic baseline visit, which could have been identical to or after their CHAP enrollment visit (see “Measures of long-term exposure to TRAP and defining analytic baseline” below): age, smoking status, year, and community noise level. Age at analytic baseline was defined as the number of continuous years (i.e., fractional years were permitted) between a participant’s birthday and the date of their analytic baseline visit. Smoking status was ascertained by asking participants whether they currently, formerly, or never smoked cigarettes. Analytic baseline year was defined as the year of each participant’s analytic baseline, which also served as the end point for their predicted 3-y and exposure levels as described in the section, “Measures of long-term exposure to TRAP and defining analytic baseline.” We predicted community noise levels for each participant based on their place of residence. The noise prediction model predictions incorporated data from A-weighted noise samples from 136 unique locations around the Chicago area between 2006 and 2007.50 These samples were collected during daytime, non–rush hour periods. The model predicted community noise levels at any location by incorporating geographic information (e.g., proximity from major roadways).46,50,51 The for this model was 0.7 using 10-fold cross-validation.50 Finally, we included time since baseline as a time-varying covariate; for each participant, this variable was calculated as the time (in continuous years) from their analytic baseline visit to each of their follow-up visits.
In the sensitivity analyses for which we estimated inverse probability-of-continuation weights, the models of continuation included the following time-fixed covariates: sex/gender, race, years of education, smoking status. These covariates were all measured at participants’ analytic baseline and defined as they were for the exposure effects analyses. The models of continuation also included baseline diabetes mellitus status (history of diabetes mellitus vs. no history) and TRAP exposure level (measured according to the methods described earlier; the TRAP species included varied according to the TRAP species of interest). In addition, these models included the following time-varying covariates: age, global cognitive function score, social network score, physical disability, self-rated health, and alcohol consumption. At each cycle, age was defined as the sum of a participant’s age at their analytic baseline and the time that had elapsed since their analytic baseline visit. Global cognitive score was defined as above (a composite z-score). Social network score was the sum of the number of children, relatives, and friends with whom the participant had at least monthly face-to-face contact, plus the number of neighbors with whom they had a “friendly talk” at least weekly. Physical disability was measured by the Nagi total physical function score, which captures performance on basic physical activities of upper and lower extremity function, each on a 0–5 point scale52; individual scores were summed to create an overall index score, where higher values indicated less physical disability. Participants rated their health as “poor,” “fair,” “good,” or “excellent” based on their perception of their health at the time of their interviews. Finally, alcohol consumption was recorded as a categorical variable (none, up to one drink/day, one or more drinks/day) based on how much alcohol participants reported consuming at the time of their interviews.
Statistical Analyses
Measures of long-term exposure to TRAP and defining analytic baseline.
We designed the temporal dimensions of this study to balance the need for measures of long-term exposure to TRAP and the need for a follow-up period of sufficient duration and cognitive assessment frequency to observe effects on cognitive change. The period covered by the spatiotemporal TRAP prediction models began in January 1999, and CHAP enrollment and follow-up data collection were ongoing through October 2012 (Figure S1). Approximately 25% of the CHAP participants entered the cohort after 2002, and, as described above, cognitive function was assessed every 3 y. Estimating TRAP exposure over a 3-y window prior to a designated “analytical baseline” cognitive assessment provided both a multiyear exposure measure along with a large number of participants with longitudinal cognitive assessments after this exposure window.
We estimated participants’ exposure to TRAP within a 3-y window based on a procedure previously used in this cohort.30 First, we assigned every participant an analytic baseline visit date. The analytic baseline visit date for participants who enrolled in the CHAP prior to 1 January 1999 was defined to be their first follow-up visit on or after 1 January 2002 (i.e., their first CHAP visit at least 3 y after the start of the period covered by the and prediction models). For participants who entered the CHAP after 1 January 1999, we assigned their analytic baseline visit date based on their enrollment date and their response to a question about how long they had lived at their current residence. For these participants, we subtracted the length of time they reported living at their current residence from the date of their CHAP enrollment date, which we call their “residence index date.” We then assigned these participants an analytic baseline visit date as one of the following, whichever came later: a) their first CHAP visit occurring on or after 1 January 2002 or b) their first CHAP visit occurring at least 3 y after their residence index date.
In our analyses, participants contributed cognitive assessment data from their analytic baseline visit onward. As such, we excluded data collected prior to participants’ analytic baseline visit; we also excluded participants who moved outside of the CHAP geographic area before their analytic baseline visit date or who were missing any covariate information at their analytic baseline visit. Among those who remained, we then estimated each participant’s exposure to and over the 3 y prior to their analytic baseline visit date, incorporating residential mobility within the period as warranted. Even though the prediction models for and concentrations were specific only to 2009, we applied these models to estimating each participant’s exposure during that same 3-y window, incorporating residential mobility during participants’ 3-y exposure period by weighting the location-specific exposure predictions by the percentage of time spent in each residential location within participants’ 3-y windows. In the Supplement, we provide more detailed examples of how TRAP exposure windows were assigned (Figure S2 and Table S1).
To explore the temporal stability of the 3-y and exposure estimates, we calculated Spearman rank correlations between predicted annual concentrations of these pollutants for all available years (1999–2012) at all CHAP geocoded locations. We estimated the Spearman rank correlations between each pair of 3-y TRAP exposure measures (i.e., anchored to participants’ analytic baselines). We also generated two sets of statistics describing the temporal variation (absolute and rank order) of and concentrations in the study area. First, for each pollutant, we computed the mean year-specific (1999–2012) concentrations at all CHAP residential locations. Second, we computed the Spearman correlations between each year-specific predicted annual concentration.
Association of TRAP with rate of cognitive change.
The association of TRAP with difference in rates of cognitive change was evaluated via multivariable-adjusted generalized estimating equations (GEE), with identity links and a working exchangeable correlation structure, of repeatedly measured cognitive scores.53 A total of twelve models were fit corresponding to our four TRAP exposures (, , , and ) and three cognitive measures (global cognition, processing speed, and episodic memory). All models adjusted for the following variables deemed to be putative confounders of the TRAP-cognitive change association (Figure 1): baseline age, race, years of education, neighborhood socioeconomic status, community noise level, and time since analytic baseline. All models also adjusted for sex/gender and smoking status as precision variables; we also included interactions of time since analytic baseline with all confounders and precision variables. The parameter of interest was the coefficient for the interaction between TRAP exposure and time since analytic baseline, which is interpreted as the mean difference in mean rate of cognitive change per 1-unit increment in TRAP. Because and were predicted from spatiotemporal models (unlike and , which were predicted from spatial models), we additionally adjusted for the and analyses baseline calendar year.23 We multiplied all estimated differences by 5 and by the SD of each TRAP measure, obtaining estimated differences in change in cognitive performance over 5 y per 1-SD increase in predicted TRAP exposure. To obtain 95% confidence intervals (CIs) around our estimates, we used 1,000 nonparametric bootstrap replications of our models. All analyses were conducted in R (version 4.3.2; R Development Core Team).
Figure 1.
Directed acyclic graph describing the assumed relationships between TRAP exposure, cognitive decline, and the covariates included in our primary outcome models. Note: TRAP, traffic-related air pollution.
Sensitivity and Additional Analyses
We conducted several analyses of the sensitivity of our estimated associations to assumptions about differential selection and exposure measurement.
Sensitivity to differential selection.
Following their cognitive assessments at analytical baseline and prior to its conclusion in 2012, 1,699 individuals died, and 1,103 individuals discontinued participating in the CHAP for other reasons. We addressed the potential influence of post-baseline attrition on our estimated associations by applying inverse probability-of-continuation weights to our analytic models.30,32 These weights were constructed by modeling the probability of continuing to participate in the CHAP (i.e., not dying or dropping out) at a particular visit conditional on the values of covariates measured at the prior visit, computing predicted probabilities of continuation for each participant, and taking the inverse of these probabilities. We distinguished between continuing due to not dying and continuing due to not dropping out, conditional on not dying, by fitting two separate pooled logistic regression models to obtain two cause-specific inverse probability-of-continuation weights. Then we constructed an overall inverse probability-of-continuation weight as the product of these two weights.30,32 Our models were based on prior work in the CHAP that identified key predictors of continuation30,32,45 and included the following covariates: sex, race, age, years of educational attainment, smoking status, alcohol consumption within the previous 2 wk, Nagi disability score, self-rated health, self-reported diabetes mellitus status at the analytic baseline visit, social network score, global cognition score, and the relevant TRAP exposure of interest.
The fitted models of continuation due to not dropping out had several features indicating that their predictions could be substantially inaccurate. For example, the C-statistics for our four models predicting the probability of not dropping out (each model differed only in terms of which of our four TRAP species of interest was included) were , suggesting that these models did not adequately discriminate between those who dropped out vs. did not (Table S2). In comparison, the four fitted models of continuation due to not dying performed far better (C-statistics of , indicating moderately good discrimination; Table S2). We also found that whereas associations of covariates with continuation due to not dying, from our fitted models, were approximately in the expected directions, the associations from our fitted models of continuation due to not dropping out yielded counter-to-expected results (e.g., older age was associated with lower risk of dropping out; Tables S3–S6). Nonetheless, weighting on the basis of attrition solely due to mortality ignores the potential influence of differential drop-out. Therefore, we generated three sets of continuation-weighted analyses: a) weighted based on the inverse probability of continuation due to not dying; b) these same analyses with follow-up censored when a participant reached age 90, to reduce violations of the positivity assumption,54 which become more likely as participants reach very old age; c) and weighted based on both the inverse probability of continuation due to death and the inverse probability of continuation due to not dropping out.
All estimated inverse probability-of-continuation weights were stabilized by multiplying each participant’s wave-specific unstabilized inverse probability-of-continuation weights by the conditional probability of remaining alive and uncensored up to that wave, given participants’ age, sex, race, years of educational attainment, self-reported diabetes mellitus status at the analytical baseline visit, and the relevant TRAP exposure of interest, as previously done in weight-based analyses in this cohort.32
Sensitivity to exposure measurement.
We also evaluated a longer exposure window. We chose a 3-y window for our primary analyses to strike a balance between the maximizing directly estimated exposure time and allowing sufficient post-baseline follow-up time to observe cognitive decline. We repeated our analyses using the subset of our analytic sample who had sufficient data available to estimate exposures over a 5-y window (; 86.6% of the primary analytic sample). For comparison, we also analyzed 3-y exposures using the same people and follow-up visits used in the analyses of 5-y exposures.
Addressing differential prebaseline selection into CHAP.
Given TRAP exposure’s adverse effects on mortality, we also explored the influence of differential selection into the CHAP on our effect estimates (i.e., bias arising from prebaseline mortality of susceptible individuals). We used the effect of on rate of cognitive change as an example because the effect of on mortality has undergone extensive study.23 This exploration, modified from the simulation-based approach of Mayeda et al.,55 estimates the bias on an effect estimate that results from scenarios in which the exposure of interest affects survival to enrollment age, and an unmeasured factor also affects not only survival, but also the outcome of interest (Figure 2). By restricting a cohort to those who survive to enrollment, a biased association between exposure and the outcome may result, a form of the phenomenon referred to as collider bias.31 Mayeda et al. provide complete technical details55; in short, this simulation approach consists of three fundamental steps. First, a large population (e.g., ) of pseudo-participants are generated, and each is assigned a) an exposure value, b) a value of an unmeasured factor (U) that affects both survival and rates of cognitive change, and c) a rate of cognitive change (a decline trajectory) based on linear mixed effects models with random intercepts and slopes. Second, each pseudo-participant is assigned a vital status (“nonsurvivor” or “survivor”) at study enrollment, using a probability model for survival that takes into account the values of exposure and U that were generated in the prior step. Finally, the association between exposure and cognitive change is estimated among a random sample of surviving pseudo-participants. This entire process is repeated many times (e.g., thousands of times), and prebaseline selection bias is estimated by comparing the true causal effect of TRAP exposure on cognitive change [from step 1(c)] with the average estimated associations (from the last step) across all repeated simulations.
Figure 2.
Conceptual directed acyclic graph underlying a simulation-based bias analysis for TRAP exposure on cognitive decline. Parameters next to arrows correspond to parameters in the prebaseline selection bias simulation. Note: TRAP, traffic-related air pollution.
In our simulations, we assumed that higher TRAP exposure lowered the probability of survival to study enrollment. We also assumed that individuals possessed varying levels of U, which we conceptualized as a normally distributed random variable, with higher values of U increasing the probability of prebaseline survival and slowing cognitive decline; in some of simulations, we included an interaction term between U and TRAP exposure, with U counteracting some of the adverse effect of TRAP exposure on cognitive decline. Following Mayeda et al., we developed scenarios in which TRAP and U influenced survival independently on a multiplicative scale, as well as scenarios in which their effects on survival were supramultiplicative. These latter scenarios were expected to yield larger biases.31,55 We also based our simulations on a hypothetical study that enrolled participants once they turned a certain age: an “age 65 study” and an “age 75 study.”
Although our simulation study followed the basic structure proposed by Mayeda et al., we expanded on their approach in three ways. First, we allowed vital status to vary by demographic characteristics. Because life tables suggest that Black men, Black women, White men, and White women have different life expectancies after birth,56 we fit separate survival models in step 2 for these four groups instead of a single survival model as in Mayeda et al. Second, the simulation design choices of Mayeda et al. were not informed by any real-world study. In our simulations, we assigned pseudo-participants a TRAP level based on the distribution of observed levels in the CHAP, which we stratified by race (i.e., there were distributions that were unique to White pseudo-participants and to Black pseudo-participants). The use of separate models for Black and White pseudo-participants was motivated by data in CHAP showing that the average exposure for Black participants () was higher than that of White participants (), and to further tailor our bias analyses to the CHAP, we encoded this exposure difference in our simulations. Finally, instead of simulating a dichotomous exposure, we parameterized our TRAP exposure variable as a three-category variable (“low,” “medium,” and “high”). These categories were based on tertiles of the overall (i.e., not race-stratified) distribution of in the CHAP, which retained the racial disparity in TRAP exposure levels seen in our exploratory analyses.
In total, we ran 18 simulation studies in which we systematically altered the association between TRAP exposure and survival, the association between U and survival, and the strength of a multiplicative interaction between TRAP exposure and U on survival. These studies were equally split between “age 65 studies” and “age 75 studies.” (See Supplementary Material for the R code used to conduct the simulation studies.)
Results
Our analytic sample included 6,061 participants who had complete 3-y TRAP exposure and baseline covariate information (Figure 3). These participants contributed a total of 13,275 observations [ observations, with 1,926 (31.7%) contributing only a single observation].
Figure 3.
Flowchart describing the creation of our analytic dataset based on participants who enrolled in the Chicago Health and Aging Project between 1993 and 2012.
Three-year average concentrations of most of the TRAP species that we investigated were higher among participants who identified as Black than among those identifying as non-Black, and who attained fewer years of formal education, or lived in areas with lower neighborhood socioeconomic status at analytic baseline (Table 1). Three-year , , and exposure levels were positively correlated with each other; all three exposures were inversely correlated with exposure level (Table 2). In supplementary materials, we include correlations between TRAP and community noise levels (Table S9).
Table 1.
Median and interquartile range of TRAP concentrations near participants’ homes in the Chicago Health and Aging Project (1999–2012), averaged over the 3 y prior to analytic baseline, by category of demographic and clinical characteristics.
| (%) | exposure (ppb) median (IQR) | exposure (ppb) median (IQR) | exposure () median (IQR) | exposure () median (IQR) | |
|---|---|---|---|---|---|
| Overall sample | 6,061 (100.0) | 40.8 (36.7–45.3) | 18.5 (16.9–20.1) | 7.9 (6.7–9.6) | 22.6 (21.0–24.3) |
| Baseline age (quartile) | |||||
| 61–68 y old | 1,644 (27.1) | 42.0 (37.4–45.7) | 18.9 (17.1–20.3) | 8.1 (7.2–9.6) | 22.4 (21.0–24.1) |
| 68–74 y of age | 1,479 (24.4) | 41.0 (36.2–45.2) | 18.6 (17.0–20.2) | 8.0 (7.0–9.8) | 22.5 (20.8–24.0) |
| 74–80 y of age | 1,562 (25.8) | 41.0 (37.1–45.5) | 18.6 (17.0–20.2) | 7.9 (6.7–9.7) | 22.7 (21.0–24.2) |
| 80–104 y of age | 1,376 (22.7) | 38.9 (36.3–44.1) | 17.8 (16.7–19.6) | 7.3 (6.2–9.0) | 22.9 (21.2–25.2) |
| Sex | |||||
| Male | 2,223 (36.7) | 40.8 (36.8–45.2) | 18.4 (16.9–20.1) | 7.9 (6.8–9.6) | 22.5 (20.9–24.2) |
| Female | 3,838 (63.3) | 40.8 (36.7–45.3) | 18.6 (17.0–20.1) | 7.9 (6.8–9.6) | 22.7 (21.0–24.4) |
| Race | |||||
| Black | 3,906 (64.4) | 43.9 (40.5–46.8) | 19.5 (18.3–20.8) | 8.9 (7.8–10.2) | 22.5 (20.7–23.9) |
| White | 2,155 (35.6) | 37.0 (34.6–38.6) | 16.9 (15.7–17.6) | 6.4 (5.4–7.1) | 22.9 (21.2–25.9) |
| Years of formal education | |||||
| 0–8 | 584 (9.6) | 44.1 (40.9–47.1) | 19.5 (18.4–21.0) | 8.8 (7.6–10.2) | 22.7 (20.6–24.1) |
| 9–12 | 2,771 (45.7) | 41.7 (37.3–45.5) | 18.9 (17.2–20.3) | 8.0 (6.8–9.6) | 22.6 (20.7–24.3) |
| 13–16 | 2,087 (34.4) | 39.4 (36.3–44.5) | 18.0 (16.7–19.8) | 7.7 (6.6–9.4) | 22.6 (21.1–24.4) |
| 17 or more | 619 (10.2) | 37.2 (33.9–40.6) | 17.0 (15.6–18.6) | 7.2 (6.6–8.6) | 22.7 (21.2–24.4) |
| Community noise levels [tertile, dB(A)] | |||||
| 51.1–54.6 | 2,022 (33.4) | 41.7 (37.6–45.4) | 18.9 (17.3–20.3) | 7.8 (6.8–9.2) | 22.5 (20.8–24.2) |
| 54.6–56.2 | 2,020 (33.3) | 39.0 (36.0–43.9) | 17.8 (16.5–19.3) | 7.6 (6.6–9.1) | 22.3 (21.0–24.1) |
| 56.2–70.0 | 2,019 (33.3) | 41.3 (37.1–46.7) | 19.0 (17.0–20.7) | 8.4 (6.9–10.3) | 23.0 (21.1–24.6) |
| Neighborhood socioeconomic status z-score (tertile) | |||||
| to | 2,034 (33.6) | 44.5 (41.4–47.2) | 19.7 (18.7–20.8) | 9.0 (7.8–10.3) | 22.6 (21.1–23.6) |
| to 1.1 | 2,007 (33.1) | 42.2 (37.8–46.0) | 19.0 (17.4–20.4) | 8.4 (6.5–9.9) | 22.3 (20.2–24.4) |
| 1.1–10.7 | 2,020 (33.3) | 37.1 (34.4–39.1) | 16.9 (15.6–17.8) | 6.8 (6.2–7.6) | 23.0 (21.4–26.3) |
| Smoking history | |||||
| Current | 704 (11.6) | 41.9 (38.0–46.1) | 18.9 (17.2–20.4) | 8.1 (7.1–9.7) | 22.5 (20.8–24.0) |
| Former | 2,534 (41.8) | 40.6 (36.6–45.1) | 18.4 (16.8–20.1) | 7.8 (6.7–9.6) | 22.6 (21.0–24.3) |
| Never | 2,823 (46.6) | 40.5 (36.7–45.2) | 18.5 (16.9–20.1) | 7.8 (6.7–9.5) | 22.7 (21.0–24.4) |
| Alcohol consumption (drinks/day) | |||||
| 0 | 3,813 (62.9) | 41.9 (37.3–45.7) | 18.9 (17.3–20.3) | 8.2 (7.1–9.8) | 22.6 (20.9–24.3) |
| 0–1 | 1,917 (31.6) | 39.0 (36.2–44.2) | 17.7 (16.4–19.6) | 7.4 (6.4–9.0) | 22.7 (21.1–24.4) |
| 331 (5.5) | 38.4 (35.7–43.0) | 17.4 (16.2–19.1) | 7.3 (6.5–8.5) | 22.6 (21.2–24.4) | |
| Nagi physical disability scorea | |||||
| 0 to 17 | 2,682 (44.3) | 40.5 (36.7–44.9) | 18.4 (16.9–20.0) | 7.7 (6.6–9.4) | 22.7 (21.0–24.5) |
| 18 to 20 | 3,379 (55.7) | 41.1 (36.7–45.4) | 18.6 (17.0–20.2) | 8.0 (6.8–9.6) | 22.6 (21.0–24.2) |
| Self-rated health | |||||
| Excellent | 1,322 (21.8) | 38.8 (36.0–43.9) | 17.7 (16.4–19.6) | 7.4 (6.4–9.1) | 22.7 (21.2–24.5) |
| Good | 2,999 (49.5) | 40.8 (36.7–45.3) | 18.5 (17.0–20.1) | 7.8 (6.7–9.6) | 22.6 (21.0–24.3) |
| Fair | 1,451 (23.9) | 42.1 (37.6–45.8) | 19.0 (17.4–20.4) | 8.2 (7.2–9.7) | 22.6 (20.8–24.2) |
| Poor | 289 (4.8) | 42.3 (37.6–45.5) | 19.1 (17.4–20.3) | 8.2 (7.2–9.9) | 22.5 (20.6–24.0) |
| History of diabetes mellitus | |||||
| Yes | 1,406 (23.2) | 42.3 (37.6–45.8) | 19.0 (17.3–20.3) | 8.3 (7.2–9.8) | 22.6 (21.0–24.2) |
| No | 4,655 (76.8) | 40.2 (36.5–45.0) | 18.3 (16.8–20.0) | 7.8 (6.6–9.5) | 22.6 (21.0–24.4) |
| Global cognition z-score (tertile) | |||||
| to 0.18 | 2,021 (33.3) | 42.7 (38.0–46.0) | 19.2 (17.5–20.4) | 8.3 (7.3–9.9) | 22.7 (20.8–24.3) |
| 0.18–0.71 | 2,020 (33.3) | 41.4 (37.0–45.5) | 18.7 (17.1–20.2) | 7.9 (6.9–9.6) | 22.7 (21.0–24.3) |
| 0.71–1.76 | 2,021 (33.3) | 38.5 (35.7–43.5) | 17.7 (16.3–19.5) | 7.3 (6.4–9.0) | 22.5 (21.0–24.4) |
Note: IQR, interquartile range; TRAP, traffic-related air pollution.
Higher scores indicate less physical impairment and disability.
Table 2.
Spearman rank correlations between estimated TRAP concentrations, averaged over 3 y prior to participants’ () analytic baseline in the Chicago Health and Aging Project (1999–2012).
| 1.00 | 0.91 | 0.63 | ||
| 0.91 | 1.00 | 0.71 | ||
| 0.63 | 0.71 | 1.00 | ||
| 1.00 |
Note: PM, particulate matter; TRAP, traffic-related air pollution.
Differences in Rates of Cognitive Change
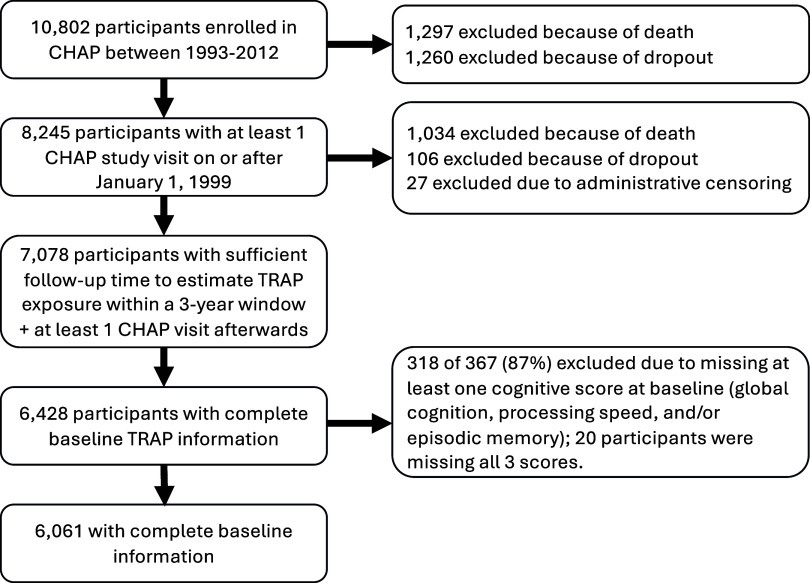
Overall, estimated mean differences in 5-y rates of cognitive change with increasing 3-y TRAP exposure were small and qualitatively mixed (Figure 4). From the primary analyses, a 1-SD increase in () was associated with a difference in mean 5-y rate of change in global cognition score of 0.010 SD units (95% CI: , 0.036), a difference in mean 5-y rate of change in episodic memory of SD units (95% CI: , 0.035), and a difference in mean 5-y rate of change in processing speed of 0.022 SD units (95% CI: , 0.052). With respect to , we observed that a 1-SD increase () was associated with small negative differences in mean 5-y rate of change in all three cognitive domains. Associations between and 5-y rates of change in global cognition and episodic memory scores were found to be small and positive; however, the association between a 1-SD increase () in and 5-y rate of change in processing speed was negative and more pronounced ( SD units, 95% CI: , 0.008). The associations between and mean 5-y rates of change in global cognition and episodic memory were similar to those observed with , but in contrast to , we observed that a 1-SD increase () in was associated with a mean 5-y rate of change in processing speed that was positive (0.011 SD units, 95% CI: , 0.031).
Figure 4.
Adjusted differences in the mean 5-y rate of change in global cognition, processing speed, and episodic memory per 1-SD increment in TRAP exposure, estimated from our primary analysis (). Note: All models adjusted for baseline age, sex, race, study time, educational attainment, smoking status, community noise level, neighborhood socioeconomic status and cross-products between these variables and study time. The parameter of interest was the interaction between TRAP exposure and study time. Models for and additionally adjusted for the calendar year of the baseline visit. In addition, although CHAP collected data on its participants from 1993 to 2012, model-based predicted values of and for CHAP participants were available from 1999 to 2012, whereas model-based predicted values for and were available for 2009 only. To partially address this misalignment of exposure and outcome ascertainment, we used the procedure described in the main text to assign 3-y TRAP exposures to each participant in our analytic sample. We modeled associations between TRAP and rates of cognitive change, correcting for potential post-baseline attrition bias by incorporating inverse probability-of-continuation weights into our GEE models. CHAP, Chicago Health and Aging Project; GEE, generalized estimating equations; PM, particulate matter; SD, standard deviation; TRAP, traffic-related air pollution.
The analytic sample for sensitivity analyses involving continuation weights (either due to not dying or jointly due to not dying and not dropping out) was identical to that for our primary analyses (6,061 participants, 16,067 observations). Samples sizes of the other sensitivity analyses were smaller. For analyses in which we censored participant follow-up once the participant became 90 y of age or older ( participants, 12,731 observations), participants contributed a observations. For sensitivity analyses restricted to those participants with sufficient follow-up time to estimate a 5-y TRAP exposure, our sample size was 5,251 (12,227 observations), and participants contributed a observations to these analyses.
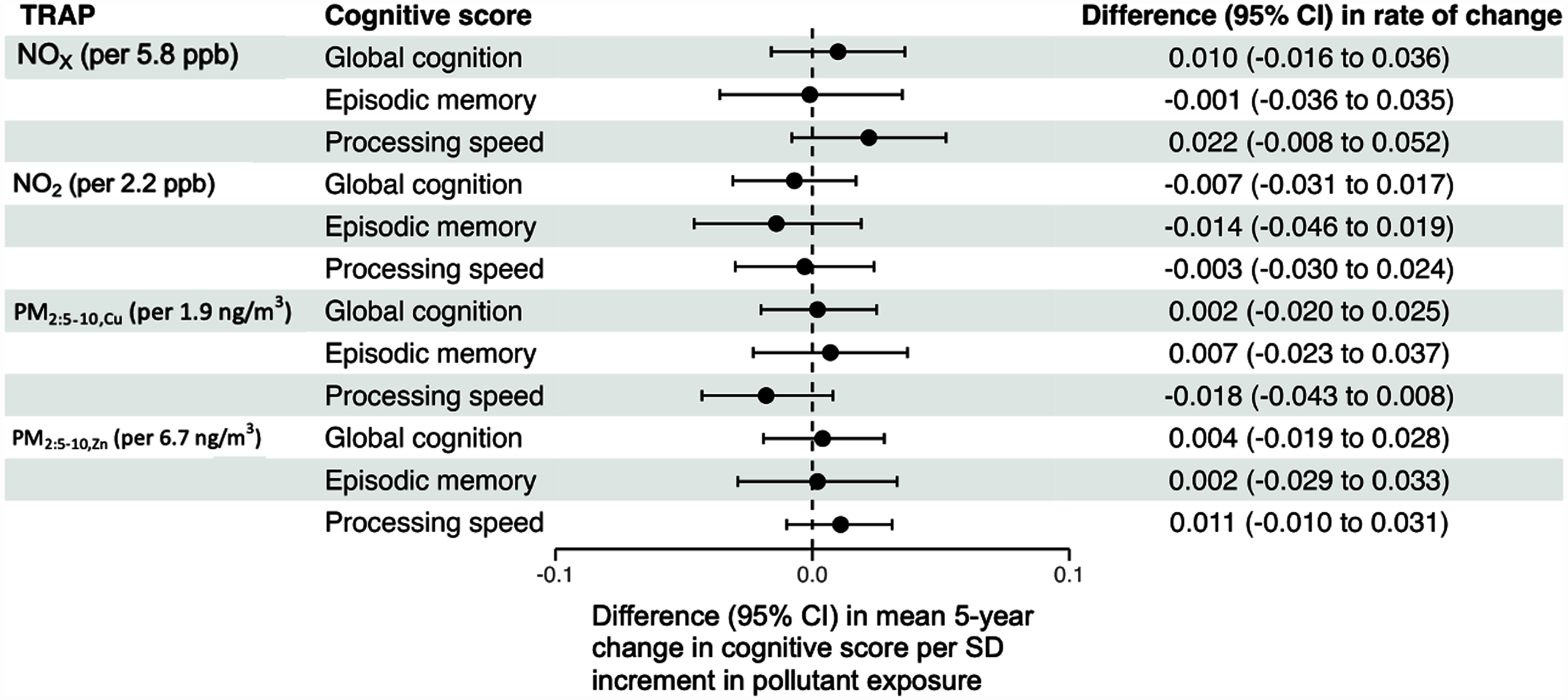
In our sensitivity analyses involving tailpipe-related TRAP (Figure 5), we observed mixed results. The application of continuation weights due to not dying led to small shifts in the estimated associations between our four TRAP species of interest and rates of change across three cognitive domains, with half of these estimates shifting toward more positive values (i.e., TRAP is less harmful) and half shifting toward more negative values (i.e., TRAP is more harmful). Applying continuations weights to analyses where we censored follow-up time after participants turned 90 y of age led to more consistent negative shifts in estimated associations, whereas applying uncensored overall continuation weights (i.e., weights based on the product of the inverse probability of not dying and not dropping out estimated from all available participant follow-up time) yielded estimated associations that were shifted in a similar way to how they were shifted after applying (uncensored) continuation weights due to not dying. Restricting to the subset of participants eligible for a 5-y TRAP exposure window also in general yielded similar results to those of our primary analyses for both the analyses using a 5-y or a 3-y exposure window. However, we found that with respect to both and , using a 5-y exposure window resulted in noticeably more negative estimated associations with respect to rates of change in processing speed.
Figure 5.
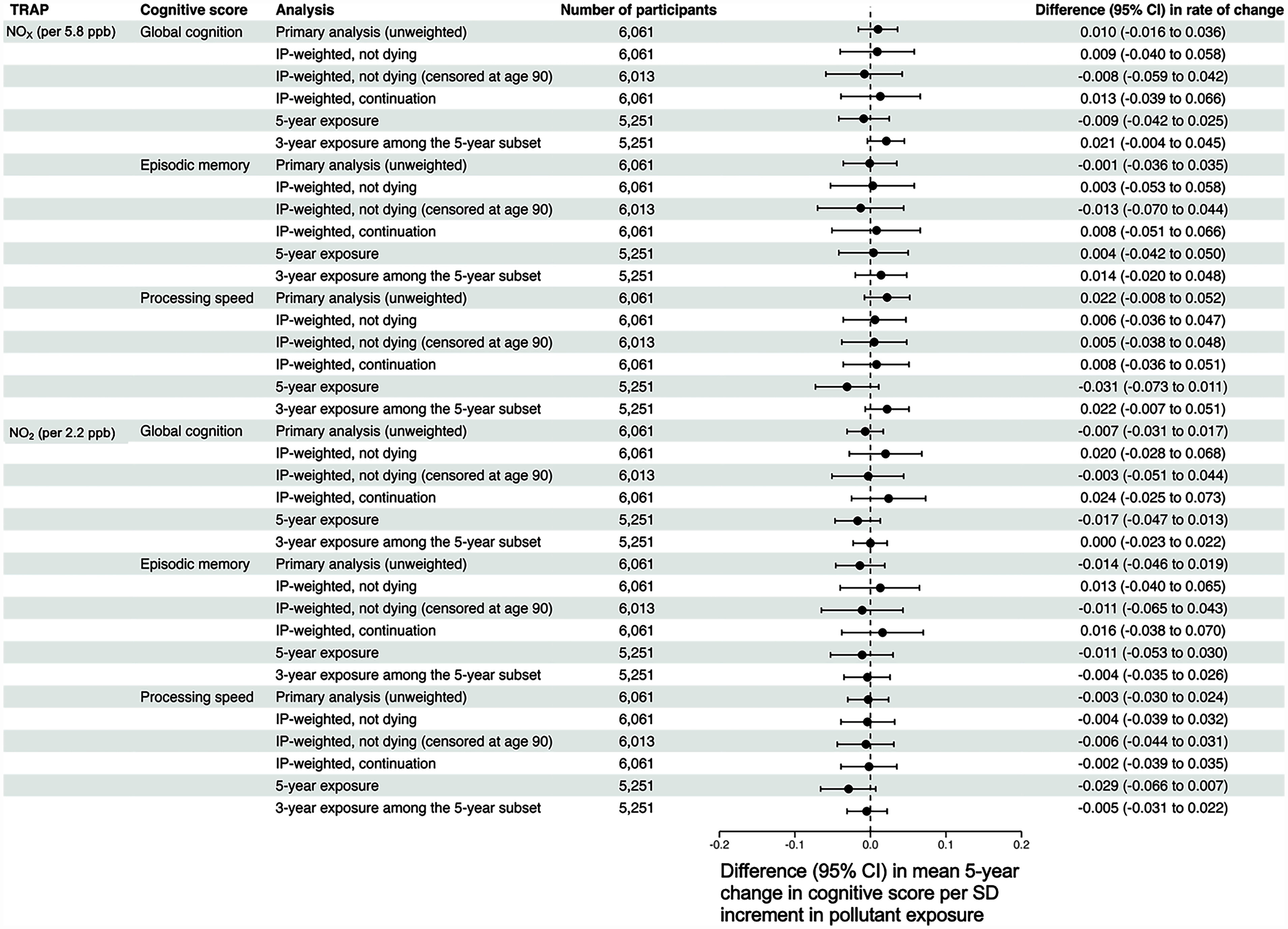
Adjusted differences in the mean 5-y rate of change in global cognition, processing speed, and episodic memory per 1-standard deviation increment in tailpipe-related traffic-related air pollution exposure, estimated from sensitivity analyses. Note: All models adjusted for baseline age, sex, race, study time, educational attainment, smoking status, community noise level, neighborhood socioeconomic status, calendar year of the baseline visit, and cross-products between these variables and study time. In addition, although the CHAP collected data on its participants from 1993 to 2012, model-based predicted values for and were available for 2009 only. To partially address this misalignment of exposure and outcome ascertainment, we used the procedure described in the main text to assign 3-y TRAP exposures to each participant in our analytic sample. To explore the impact of potential post-enrollment selection biases and our choice of a 3-y exposure window, we conducted the following sensitivity analyses: a) incorporating stabilized inverse probability of continuation due to not dying weights into our outcome models; b) incorporating stabilized inverse probability of continuation due to not dying weights into our outcome models, censoring follow-up time once a participant became at least 90 y of age; c) incorporating stabilized inverse probability of continuation weights, which were defined as the product of the inverse probability of continuation due to not dying and the inverse probability of continuation due to not dropping out of the study; d) restricting to those eligible for a 5-y exposure window [i.e., those who had at least one CHAP visit 5 y after their working start date described in the main text, and estimating 5-y and 3-y TRAP exposure among those eligible for 5-y exposure windows (“5-year exposure” and “3-year exposure among the 5-year exposure subset,” respectively)]. For all sensitivity analyses, we assumed as with our primary analyses that 2009 predicted and concentrations reflect, at least in rank ordering, what would have been observed for each CHAP participant at their analytic baseline visit. CHAP, Chicago Health and Aging Project; PM, particulate matter; TRAP, traffic-related air pollution.
With respect to our sensitivity analyses involving nontailpipe-related TRAP (Figure 6), we found that the application of weights and changing the exposure window again did not yield results that were qualitatively different from those of our primary analysis.
Figure 6.
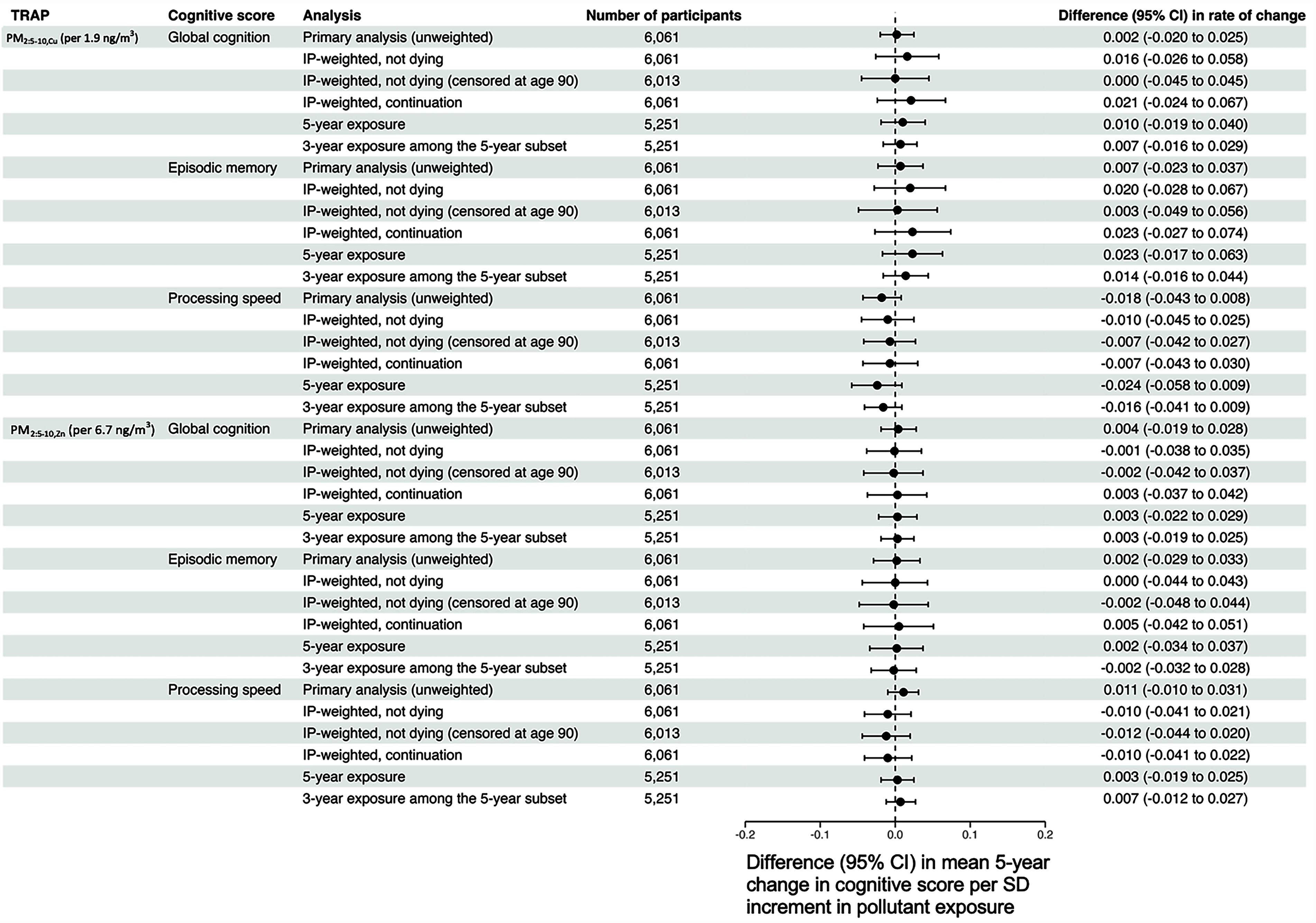
Adjusted differences in the mean 5-y rate of change in global cognition, processing speed, and episodic memory per 1-SD increment in nontailpipe-related traffic-related air pollution exposure, estimated from sensitivity analyses. Note: All models adjusted for baseline age, sex, race, study time, educational attainment, smoking status, community noise level, neighborhood socioeconomic status, calendar year of the baseline visit, and cross-products between these variables and study time. Although the CHAP collected data on its participants from 1993 to 2012, model-based predicted values for and were available for 2009 only. To partially address this misalignment of exposure and outcome ascertainment, we used the procedure described in the main text to assign 3-y TRAP exposures to each participant in our analytic sample. To explore the impact of potential post-enrollment selection biases and our choice of a 3-y exposure window, we conducted the following sensitivity analyses: a) incorporating stabilized inverse probability of continuation due to not dying weights into our outcome models; b) incorporating stabilized inverse probability of continuation due to not dying weights into our outcome models, censoring follow-up time once a participant became at least 90 y of age; c) incorporating stabilized inverse probability of continuation weights, which were defined as the product of the inverse probability of continuation due to not dying and the inverse probability of continuation due to not dropping out of the study; d) restricting to those eligible for a 5-y exposure window [i.e., those who had at least one CHAP visit 5 y after their working start date described in the main text, and estimating 5-y and 3-y TRAP exposure among those eligible for 5-y exposure windows (“5-year exposure” and “3-year exposure among the 5-year exposure subset,” respectively)]. For all sensitivity analyses, we assumed as with our primary analyses that 2009 predicted and concentrations reflect, at least in rank ordering, what would have been observed for each CHAP participant at their analytic baseline visit. CHAP, Chicago Health and Aging Project; PM, particulate matter; SD, standard deviation; TRAP, traffic-related air pollution.
Finally, from our quantitative bias analysis simulation of prebaseline selection bias, we identified some scenarios in which the estimated effect of TRAP on change in global cognition was biased upward from a true inverse (deleterious) value. In these simulation analyses, we assumed that the true difference in the mean difference in the annual rate of cognitive change was per change in categorical TRAP level (e.g., “low” to “medium” or “medium” to “high” exposure level). When our data-generating models did not include supramultiplicative interactions between TRAP exposure and U, we found estimated differences in mean rates of cognitive change per change in categorical TRAP exposure level between and (Table 3). In practical terms, these correspond to a scenario in which the estimated difference in mean rate of cognitive change would be approximately correct both qualitatively and quantitatively and to a scenario where one would incorrectly estimate a mean difference of approximately zero. When supramultiplicative interactions were added to the data-generating models, we observed estimated mean differences between and 0.005. As such, in other words, in all simulations, the estimated cognitive change coefficient was biased upward from the true value, but in the simulations with supramultiplicative interactions between TRAP and U were introduced, the resulting bias universally led to both quantitively and qualitatively incorrect effect estimates (i.e., based on the direction of the estimated effect, one could mistakenly conclude that TRAP exposure is beneficial, when in truth it is harmful).
Table 3.
Results of a quantitative analysis of prebaseline selection bias according to the causal structure in Figure 4 across 2,000 simulated cohorts modeled after participants in our sample of Chicago Health and Aging Project participants ( in each simulated cohort).
| (bias %) age 65 y | (bias %) age 75 y | |||||
|---|---|---|---|---|---|---|
| 1.05 | 1.25 | 0.9 | 1.0 | 1.0 | () | () |
| 1.25 | 1.75 | 0.7 | 1.0 | 1.0 | 0.000 () | () |
| 1.25 | 1.75 | 0.8 | 1.0 | 1.0 | () | () |
| 1.50 | 2.25 | 0.7 | 1.0 | 1.0 | () | () |
| 1.50 | 2.25 | 0.8 | 1.0 | 1.0 | () | () |
| 1.25 | 1.75 | 0.7 | 0.9 | 0.7 | 0.003 () | 0.002 () |
| 1.25 | 1.75 | 0.8 | 0.9 | 0.7 | 0.003 () | 0.002 () |
| 1.50 | 2.25 | 0.7 | 0.8 | 0.5 | 0.005 () | 0.005 () |
| 1.50 | 2.25 | 0.8 | 0.8 | 0.5 | 0.005 () | 0.005 () |
The parameters to correspond to log OR, and therefore, to correspond to ORs. An OR of 1.0 is the null value, indicating no association (or in the case of interactions, no interaction). We assumed that the probability of death was given by the following equation in agreement with Figure 2 in the main text:
where I is an indicator function and denotes the pseudo-individual’s TRAP exposure, with 1 corresponding to the first/lowest tertile of exposure (i.e., “low”), 2 corresponding to the second tertile of exposure (i.e., “medium”), and 3 corresponding to the third/highest tertile of exposure (i.e., “high”). The values of through are chosen as part of the simulation design (i.e., they are user-specified), and a root finding procedure is applied to estimate the that gives approximately the correct marginal probability of death, as given by life tables in Arias.56 OR, odds ratio; TRAP, traffic-related air pollution.
Discussion
This large study of urban-dwelling older adults generated evidence that did not support the hypothesis that long-term exposure to TRAP, measured via residentially located ambient concentrations of four surrogate pollutants, is associated with steeper rate of cognitive decline. Associations were small and imprecisely estimated, and they were also largely insensitive—with a few notable exceptions—to the choice of exposure window and eligible participant sample.
The absence of a clear deleterious association of TRAP with cognitive decline in our study joins a body of equivocal evidence. Prior studies of and in relation to cognitive decline, which have been set in Europe, North America, and Asia, have produced mixed results. A study that included the Washington Heights–Inwood Community Aging Project (WHICAP), a cohort of older adults living in the northern Manhattan area of New York City, New York, found evidence of an association between and faster rates of cognitive decline in that cohort.57 However, the same study failed to observe any such associations in another, smaller cohort located in the same geographic area [the Northern Manhattan Study (NOMAS)]. Most studies of and/or and cognitive decline, however, have failed to find any evidence of an association.6,58–66 With respect to and , our study is the first to examine associations between these pollutants and cognitive decline, and although this is a notable strength of our study, it prevents any comparison to prior literature.
There are several potential explanations for why our study did not find associations between TRAP and cognitive decline, assuming that such an association exists. First, it could be that there was low variability in our TRAP exposure estimates because all CHAP participants lived in one of four adjacent neighborhoods in Chicago. For example, the interquartile range (IQR) of was (Table 1), which is considerably smaller than the estimated IQR of in the WHICAP cohort in which was associated with rate of cognitive decline. At the same time, other studies have not found associations between and cognitive decline, despite having considerable variability in estimated TRAP levels, which suggests that low variability in TRAP exposure estimates cannot fully explain null associations between TRAP and cognitive decline.
Second, we may not have been able to detect an association between TRAP and cognitive decline because of insufficient follow-up time. The longest possible follow-up period in our study was from 2002 to 2012, and our participants contributed an average of 2.2 assessments over an average of 3.7 y of follow-up, with almost a third of participants only contributing a single observation. Consequently, we may have needed a longer window in which to measure rates of cognitive decline to detect the effect of TRAP, if it exists. At the same time, several studies with longer follow-up time than our study59,64,66 (up to 22 y59) have not found evidence of any association of or with cognitive decline, and in our own results, we found in sensitivity analyses that a longer exposure window (and therefore, shorter follow-up window) yielded estimated associations that were slightly stronger than those in our primary analysis. This finding suggests that although follow-up time may be important for the study of TRAP and cognitive decline, other considerations (e.g., aspects of the sample over time) may also play important roles.
Finally, our study results may have been influenced by either prebaseline or postbaseline selection bias. Although we explored the presence of postbaseline selection bias and its correction through the use of inverse probability weights, the results of our unweighted (primary) and weighted (sensitivity) analyses were only slightly different, which is consistent with either the lack of appreciable postbaseline selection bias or misspecified inverse probability-of-continuation weight models (e.g., the exclusion of one or more key drivers of postbaseline selection bias, which meant that our weights could not fully correct for the bias that was present). With respect to prebaseline selection bias, some of our simulation results were not inconsistent with considerable bias, particularly in the presence of supramultiplicative interactions between TRAP exposure and an unmeasured cause of survival and cognitive decline. However, even though we designed our simulations with the CHAP and our study in mind and made our data-generating assumptions explicit, no simulation can fully capture the complex relationships between TRAP, survival to study baseline, and cognitive decline, and as such, we must interpret these results with appropriate caution.67 For example, we implicitly assumed that TRAP exposure affected the probability of survival to age 60 or 75 y starting from a pseudo-participant’s birth; however, in reality, TRAP exposure may only affect the probability of survival during specific windows.
Our study advances the line of inquiry intro TRAP exposure and cognitive decline in several ways. First, the CHAP investigators succeeded in recruiting most age-eligible residents from their target census area and implemented design features (e.g., in-home study assessments) that reduced participant burden and helped retain participants after enrollment; most attrition was from death rather than disengagement from the study. In addition, given that more than 60% of the CHAP participants identified as Black, our study addressed the effects of TRAP on cognitive health among a racialized group in the United States that shoulders a high burden of TRAP exposure but that has long been under-included in dementia-related studies. Our study is among the few to evaluate the cognitive effects of air pollution estimates that were generated by highly resolved spatiotemporal ( and )36,39 and spatial ( and ) models that incorporated data from within-city air sampling campaigns.
Our study also addressed both pre- and postbaseline selection bias through simulation-based quantitative bias analyses and sensitivity analyses incorporating inverse probability-of-continuation weights, respectively, with no change to our overall qualitative finding of little discernible association between TRAP and cognitive decline. In studies of older adults, such tools are helpful for understanding the extent to which differential survival and participation affect study results, particularly studies that cannot practically cover the entire life course. In our simulations, we used parameter values that we believed were within plausible ranges. Prior work supports the hypothesis that TRAP exposure has a harmful effect on mortality, as reflected in our simulations.23 Regarding U, we remained agnostic to what exactly this variable might be, aside from it being an unmeasured resilience factor. Although U could be a biological variable (e.g., a genetic mutation), it could also plausibly be an often-unmeasured socioeconomic privilege variable (e.g., access to high-quality health care). This latter possibility is relevant for the plausibility of potential interactions between TRAP and U in relation to mortality. For example, socioeconomic status has been shown to be related to both mortality,68 cognitive decline,69 and risk factors for mortality or cognitive decline like stroke and diabetes mellitus.70,71 If socioeconomic vulnerability makes individuals more susceptible to TRAP’s harmful effect on mortality (or conversely, if high socioeconomic status buffers against this effect), this could be a real-world example of the TRAP–U supramultiplicative interaction assumed by our simulation study; however, more work is needed to test this hypothesis.
Another noteworthy strength of our study was our ability to examine rarely studied nontailpipe-related aspects of TRAP, like and , because the health effects of these pollutants remain poorly understood.72 Whereas tailpipe TRAP is generated through combustion-related reactions, nontailpipe TRAP is generated through physical grinding and resuspension of solid materials; these distinct generative processes mean that tailpipe- and nontailpipe-related TRAP differs not only in terms of composition, but also potential biological deposition and ultimate impact on cognitive health. Nontailpipe TRAP also contains far more metallic content than tailpipe TRAP, including metals, such as copper and zinc, that have neurotoxic potential at high doses or the presence of brain metal dyshomeostasis.73 The cognitive effects of nontailpipe TRAP may operate via excessive exposure to these metals, though it is possible that these metals are proxies for the mixture of air pollutants generated by brake and tire wear. The relevance of the features of nontailpipe pollutants to cognitive decline merits further study, particularly given that the transition of the world’s vehicle fleet to fully electric or hybrid energy sources will reduce tailpipe but not nontailpipe pollutants.23 It is also interesting that we observed an inverse correlation between and levels in our study. Although we cannot be certain, we believe that is because is a surrogate of brake wear and is a surrogate of tire wear. One can imagine that areas with a lot of “stop-and-go” traffic would be high in because of widespread brake usage, but low in because fewer vehicles reach speeds that generate substantial tire wear. Conversely, areas where vehicles travel at high speeds (e.g., highways) could see high because of tire wear, but low , because braking is infrequent or even nonexistent. Relatedly, these different traffic patterns could explain why was inversely correlated with and ; in areas with stop-and-go traffic, and , levels could be higher because vehicles are more likely to be idle, thereby allowing tailpipe-related pollutant concentrations to increase. However, future work is needed to test these speculations. Our study therefore represents an important step toward better understanding the potential cognitive health impact of TRAP, even as vehicle emissions continue to decrease.
Our study also had several important limitations. First, we assumed that all TRAP estimates reflected long-term exposure, even though we were unable to predict location-based concentrations of and prior to January 1999, and we had only a single spatial surface of predicted and concentrations that reflected spatial contrasts from 2009. Our assumptions of temporal stability were likely met in terms of rank ordering but not absolute concentration, as shown in year-specific and concentrations over the study period 1999–2012 and year-by-year correlations (Tables S7 and S8). However, it is less clear whether our assumption about the stability of and concentrations was met. As mentioned, these concentrations were predicted from highly resolved spatial models that incorporated hundreds of covariates, giving us residence-based data on these pollutants, which is rare for studies based in the United States. We believe that many of the features contributing to our predicted and concentrations remained constant over the study period, but it is unlikely that all have (e.g., traffic patterns may have changed, the weight and weight distributions of vehicles may have changed, and/or the materials used to make brake and tires may have changed). It is clear that there remains much to be known about these pollutants, and we view our study as a starting point for subsequent research that advances the temporal dimension of measuring these exposures.
In addition, positioning our 3-y TRAP estimates prior to cognitive assessments meant that our results are based on an average of 2.2 observations per CHAP participant, which limited our ability to assess rates of cognitive change over time. Although the results of our sensitivity analysis using 5-y exposure windows showed stronger effects with respect to processing speed, it is challenging to compare these findings with those from the primary analyses, because the 5-y exposure findings are based on a smaller analytic sample ( vs. 6,061 in our primary analyses). Having a 5-y exposure window also led to relatively fewer total observations per participant (30% with more than two cognitive measurements vs. 42% in our primary analytic sample; had more than three measurements vs. 8% in our primary analytic sample). As discussed above, following participants for longer periods may be needed to detect the effects of TRAP on cognitive decline. Finally, although our analyses were adjusted for self-identified race, it should be acknowledged that this variable is at best a proxy for exposure to racism of all types and degrees, including redlining and other injustices that differentially subject Black individuals in the United States to higher exposure to TRAP and higher susceptibility to poor cognitive outcomes in older adulthood.
In conclusion, the evidence from our study did not support noteworthy adverse associations of TRAP exposure with cognitive decline in a well-characterized cohort of older adults.
Supplementary Material
Acknowledgments
The authors wish to acknowledge funding from R01AG065359 [principal investigator (PI): J.W.]. R.M.A. was also funded by P30AG072978 (PI: A. McKee). The foundational CHAP investigation was supported by the National Institutes of Health grant R01AG11101 (PI: D.E.).
Conclusions and opinions are those of the individual authors and do not necessarily reflect the policies or views of EHP Publishing or the National Institute of Environmental Health Sciences.
References
- 1.Weuve J, Bennett EE, Ranker L, Gianattasio KZ, Pedde M, Adar SD, et al. 2021. Exposure to air pollution in relation to risk of dementia and related outcomes: an updated systematic review of the epidemiological literature. Environ Health Perspect 129(9):096001, PMID: 34558969, 10.1289/EHP8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer RM, Blanco MN, Li G, Adar SD, Carone M, Szpiro AA, et al. 2021. Fine particulate matter and dementia incidence in the adult changes in thought study. Environ Health Perspect 129(8):087001, PMID: 34347531, 10.1289/EHP9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aretz B, Janssen F, Vonk JM, Heneka MT, Boezen HM, Doblhammer G. 2021. Long-term exposure to fine particulate matter, lung function and cognitive performance: a prospective Dutch cohort study on the underlying routes. Environ Res 201:111533, PMID: 34153335, 10.1016/j.envres.2021.111533. [DOI] [PubMed] [Google Scholar]
- 4.Åström DO, Adolfsson R, Segersson D, Forsberg B, Oudin A. 2021. Local contrasts in concentration of ambient particulate air pollution (PM2.5) and incidence of Alzheimer’s disease and dementia: results from the Betula cohort in Northern Sweden. J Alzheimers Dis 81(1):83–85, PMID: 33749652, 10.3233/JAD-201538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M-C, Wang C-F, Lai B-C, Hsieh S-W, Chen S-C, Hung C-H, et al. 2021. Air pollution is associated with poor cognitive function in Taiwanese adults. Int J Environ Res Public Health 18(1):316, PMID: 33406674, 10.3390/ijerph18010316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duchesne J, Gutierrez L-A, Carrière I, Mura T, Chen J, Vienneau D, et al. 2022. Exposure to ambient air pollution and cognitive decline: results of the prospective three-city cohort study. Environ Int 161:107118, PMID: 35147081, 10.1016/j.envint.2022.107118. [DOI] [PubMed] [Google Scholar]
- 7.Gao Q, Zang E, Bi J, Dubrow R, Lowe SR, Chen H, et al. 2022. Long-term ozone exposure and cognitive impairment among Chinese older adults: a cohort study. Environ Int 160:107072, PMID: 34979350, 10.1016/j.envint.2021.107072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He F, Tang J, Zhang T, Lin J, Li F, Gu X, et al. 2022. Impact of air pollution exposure on the risk of Alzheimer’s disease in China: a community-based cohort study. Environ Res 205:112318, PMID: 34742710, 10.1016/j.envres.2021.112318. [DOI] [PubMed] [Google Scholar]
- 9.Ilango SD, Gonzalez K, Gallo L, Allison MA, Cai J, Isasi CR, et al. 2021. Long-term exposure to ambient air pollution and cognitive function among Hispanic/Latino adults in San Diego, California. J Alzheimers Dis 79(4):1489–1496, PMID: 33492285, 10.3233/JAD-200766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji JS, Liu L, Zeng Y, Yan LL. 2022. Effect of FOXO3 and air pollution on cognitive function: a longitudinal cohort study of older adults in China from 2000 to 2014. J Gerontol A Biol Sci Med Sci 77(8):1534–1541, PMID: 35029671, 10.1093/gerona/glac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Wang Y, Steenland K, Liu P, van Donkelaar A, Martin RV, et al. 2022. Long-term effects of PM2.5 components on incident dementia in the northeastern United States. Innovation (Camb) 3(2):100208, PMID: 35199078, 10.1016/j.xinn.2022.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mortamais M, Gutierrez L-A, de Hoogh K, Chen J, Vienneau D, Carrière I, et al. 2021. Long-term exposure to ambient air pollution and risk of dementia: results of the prospective three-city study. Environ Int 148:106376, PMID: 33484961, 10.1016/j.envint.2020.106376. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Han J, Kim SH, Suk HW, Park JE, Lee DY. 2022. Impact of long-term exposure to air pollution on cognitive decline in older adults without dementia. J Alzheimers Dis 86(2):553–563, PMID: 35094991, 10.3233/JAD-215120. [DOI] [PubMed] [Google Scholar]
- 14.Parra KL, Alexander GE, Raichlen DA, Klimentidis YC, Furlong MA. 2022. Exposure to air pollution and risk of incident dementia in the UK Biobank. Environ Res 209:112895, PMID: 35149105, 10.1016/j.envres.2022.112895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran J, Zhang Y, Han L, Sun S, Zhao S, Shen C, et al. 2021. The joint association of physical activity and fine particulate matter exposure with incident dementia in elderly Hong Kong residents. Environ Int 156:106645, PMID: 34015665, 10.1016/j.envint.2021.106645. [DOI] [PubMed] [Google Scholar]
- 16.Semmens EO, Leary CS, Fitzpatrick AL, Ilango SD, Park C, Adam CE, et al. 2023. Air pollution and dementia in older adults in the Ginkgo Evaluation of Memory Study. Alzheimers Dement 19(2):549–559, PMID: 35436383, 10.1002/alz.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zare Sakhvidi MJ, Yang J, Lequy E, Chen J, de Hoogh K, Letellier N, et al. 2022. Outdoor air pollution exposure and cognitive performance: findings from the enrolment phase of the CONSTANCES cohort. Lancet Planet Health 6(3):e219–e229, PMID: 35278388, 10.1016/S2542-5196(22)00001-8. [DOI] [PubMed] [Google Scholar]
- 18.Shi L, Steenland K, Li H, Liu P, Zhang Y, Lyles RH, et al. 2021. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat Commun 12(1):6754, PMID: 34799599, 10.1038/s41467-021-27049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan KJ, Ran X, Wu F, Chang C-CH, Sharma R, Jacobsen E, et al. 2021. Ambient fine particulate matter exposure and incident mild cognitive impairment and dementia. J Am Geriatr Soc 69(8):2185–2194, PMID: 33904156, 10.1111/jgs.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan J, Li N, Wang X, Chen G, Yan L, Wang L, et al. 2021. Associations of particulate matter with dementia and mild cognitive impairment in China: a multicenter cross-sectional study. Innovation (Camb) 2(3):100147, PMID: 34557784, 10.1016/j.xinn.2021.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Grande G, Stafoggia M, Ljungman P, Laukka EJ, Eneroth K, et al. 2022. Air pollution as a risk factor for cognitive impairment no dementia (CIND) and its progression to dementia: a longitudinal study. Environ Int 160:107067, PMID: 35032863, 10.1016/j.envint.2021.107067. [DOI] [PubMed] [Google Scholar]
- 22.Yao Y, Wang K, Xiang H. 2022. Association between cognitive function and ambient particulate matters in middle-aged and elderly Chinese adults: evidence from the China Health And Retirement Longitudinal Study (CHARLS). Sci Total Environ 828:154297, PMID: 35288137, 10.1016/j.scitotenv.2022.154297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.HEI (Health Effects Institute) Panel on the Health Effects of Long-Term Exposure to Traffic-Related Air Pollution. 2022. Systematic Review and Meta-Analysis of Selected Health Effects of Long-Term Exposure to Traffic-Related Air Pollution. Special Report 23. Boston, MA: Health Effects Institute.
- 24.US EPA (US Environmental Protection Agency). 2016. Integrated Science Assessment (ISA) for Oxides of Nitrogen – Health Criteria (Final Report, 2016). EPA/600/R-15/068. Washington, DC: US EPA. [Google Scholar]
- 25.Adar SD, D’Souza J, Mendelsohn-Victor K, Jacobs DR, Cushman M, Sheppard L, et al. 2015. Markers of inflammation and coagulation after long-term exposure to coarse particulate matter: a cross-sectional analysis from the multi-ethnic study of atherosclerosis. Environ Health Perspect 123(6):541–548, PMID: 25616153, 10.1289/ehp.1308069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerin E, Barnett A, Shaw JE, Martino E, Knibbs LD, Tham R, et al. 2021. From urban neighbourhood environments to cognitive health: a cross-sectional analysis of the role of physical activity and sedentary behaviours. BMC Public Health 21(1):2320–15, PMID: 34949175, 10.1186/s12889-021-12375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. 2019. Air pollution and dementia: a systematic review. J Alzheimers Dis 70(s1):S145–S163, PMID: 30775976, 10.3233/JAD-180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett DA, Yu L, De Jager PL. 2014. Building a pipeline to discover and validate novel therapeutic targets and lead compounds for Alzheimer’s disease. Biochem Pharmacol 88(4):617–630, PMID: 24508835, 10.1016/j.bcp.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian Y, Li H, Rosenberg A, Li Q, Sarnat J, Papatheodorou S, et al. 2021. Long-term exposure to low-level NO2 and mortality among the elderly population in the southeastern United States. Environ Health Perspect 129(12):127009, PMID: 34962424, 10.1289/EHP9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weuve J, Kaufman JD, Szpiro AA, Curl C, Puett RC, Beck T, et al. 2016. Exposure to traffic-related air pollution in relation to progression in physical disability among older adults. Environ Health Perspect 124(7):1000–1008, PMID: 27022889, 10.1289/ehp.1510089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernan MA, Hernandez-Diaz S, Robins JM. 2004. A structural approach to selection bias. Epidemiology 15(5):615–625, PMID: 15308962, 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 32.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. 2012. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology 23(1):119–128, PMID: 21989136, 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. 2003. Design of the Chicago Health and Aging Project (CHAP). J Alzheimers Dis 5(5):349–355, PMID: 14646025, 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 34.HEI Panel on the Health Effects of Traffic-Related Air Pollution. 2010. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. HEI Special Report 17. Boston, MA: Health Effects Institute.
- 35.US EPA. 2009. Integrated Science Assessment for Particulate Matter. EPA/600/R-15/068. Washington, DC: US Environmental Protection Agency. [PubMed] [Google Scholar]
- 36.Cohen MA, Adar SD, Allen RW, Avol E, Curl CL, Gould T, et al. 2009. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ Sci Technol 43(13):4687–4693, PMID: 19673252, 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mercer LD, Szpiro AA, Sheppard L, Lindström J, Adar SD, Allen RW, et al. 2011. Comparing universal kriging and land-use regression for predicting concentrations of gaseous oxides of nitrogen (NOx) for the multi-ethnic study of atherosclerosis and air pollution (MESA air). Atmos Environ (1994) 45(26):4412–4420, PMID: 21808599, 10.1016/j.atmosenv.2011.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. 2011. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ 45(36):6593–6606, 10.1016/j.atmosenv.2011.04.073. [DOI] [Google Scholar]
- 39.Keller JP, Olives C, Kim S-Y, Sheppard L, Sampson PD, Szpiro AA, et al. 2015. A unified spatiotemporal modeling approach for predicting concentrations of multiple air pollutants in the Multi-Ethnic Study of Atherosclerosis and Air Pollution. Environ Health Perspect 123(4):301–309, PMID: 25398188, 10.1289/ehp.1408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gill EA, Curl CL, Adar SD, Allen RW, Auchincloss AH, O’Neill MS, et al. 2011. Air pollution and cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Prog Cardiovasc Dis 53(5):353–360, PMID: 21414470, 10.1016/j.pcad.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang K, Larson TV, Gassett A, Szpiro AA, Daviglus M, Burke GL, et al. 2014. Characterizing spatial patterns of airborne coarse particulate (PM10-2.5) mass and chemical components in three cities: the Multi-Ethnic Study of Atherosclerosis. Environ Health Perspect 122(8):823–830, PMID: 24642481, 10.1289/ehp.1307287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith A. 1982. Symbol Digit Modalities Test Manual - Revised. Torrance, CA: Western Psychological Services. [Google Scholar]
- 43.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. 1991. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. Int J Neurosci 57(3–4):167–178, PMID: 1938160, 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. 1975. Mini-mental state: a practical method for grading the state of patients for the clinician. J Psychiatr Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- 45.Weuve J, Barnes LL, Mendes de Leon CF, Rajan KB, Beck T, Aggarwal NT, et al. 2018. Cognitive aging in black and white Americans: cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 29(1):151–159, PMID: 28863046, 10.1097/EDE.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weuve J, D’Souza J, Beck T, Evans DA, Kaufman JD, Rajan KB, et al. 2021. Long‐term community noise exposure in relation to dementia, cognition, and cognitive decline in older adults. Alzheimers Dement 17(3):525–533, PMID: 33084241, 10.1002/alz.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. 2012. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172(3):219–227, PMID: 22332151, 10.1001/archinternmed.2011.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. 2001. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345(2):99–106, PMID: 11450679, 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 49.Rajan KB, Wilson RS, Weuve J, Barnes LL, Evans DA. 2015. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology 85(10):898–904, PMID: 26109713, 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen RW, Davies H, Cohen MA, Mallach G, Kaufman JD, Adar SD. 2009. The spatial relationship between traffic-generated air pollution and noise in 2 US cities. Environ Res 109(3):334–342, PMID: 19193368, 10.1016/j.envres.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Souza JC, Weuve J, Brook RD, Evans DA, Kaufman JD, Adar SD. 2018. Long-term exposures to community noise and blood pressure levels and control among older adults. Hypertension 78(6):1801–1808, PMID: 34689591, 10.1161/HYPERTENSIONAHA.121.17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagi SZ. 1976. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc 54(4):439–467, PMID: 137366. [PubMed] [Google Scholar]
- 53.Zeger SL, Liang KY. 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42(1):121–130, PMID: 3719049. [PubMed] [Google Scholar]
- 54.Westreich D, Cole SR. 2010. Invited commentary: positivity in practice. Am J Epidemiol 171(6):674–677; discussion 678–681, PMID: 20139125, 10.1093/aje/kwp436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayeda ER, Filshtein TJ, Tripodis Y, Glymour MM, Gross AL. 2018. Does selective survival before study enrolment attenuate estimated effects of education on rate of cognitive decline in older adults? A simulation approach for quantifying survival bias in life course epidemiology. Int J Epidemiol 47(5):1507–1517, PMID: 30010793, 10.1093/ije/dyy124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arias E. 2015. United States Life Tables, 2011. Natl Vital Stat Rep 64(11):1–63, PMID: 26460931. [PubMed] [Google Scholar]
- 57.Kulick ER, Wellenius GA, Boehme AK, Joyce NR, Schupf N, Kaufman JD, et al. 2020. Long-term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology 94(17):e1782–e1792, PMID: 32269113, 10.1212/WNL.0000000000009314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cullen B, Newby D, Lee D, Lyall DM, Nevado-Holgado AJ, Evans JJ, et al. 2018. Cross-sectional and longitudinal analyses of outdoor air pollution exposure and cognitive function in UK Biobank. Sci Rep 8(1):12089, PMID: 30108252, 10.1038/s41598-018-30568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oudin A, Forsberg B, Lind N, Nordin S, Oudin Åström D, Sundström A, et al. 2017. Is long-term exposure to air pollution associated with episodic memory? A longitudinal study from Northern Sweden. Sci Rep 7(1):12789, PMID: 28986549, 10.1038/s41598-017-13048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tonne C, Elbaz A, Beevers S, Singh-Manoux A. 2014. Traffic-related air pollution in relation to cognitive function in older adults. Epidemiology 25(5):674–681, PMID: 25036434, 10.1097/EDE.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Younan D, Wang X, Millstein J, Petkus AJ, Beavers DP, Espeland MA, et al. 2022. Air quality improvement and cognitive decline in community-dwelling older women in the United States: a longitudinal cohort study. PLoS Med 19(2):e1003893, PMID: 35113870, 10.1371/journal.pmed.1003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee Y-G, Yoon S-J, Yoon SH, Kang SW, Jeon S, Kim M, et al. 2023. Air pollution is associated with faster cognitive decline in Alzheimer’s disease. Ann Clin Transl Neurol 10(6):964–973, PMID: 37106569, 10.1002/acn3.51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franz CE, Gustavson DE, Elman JA, Fennema-Notestine C, Hagler DJ, Baraff A, et al. 2023. Associations between ambient air pollution and cognitive abilities from midlife to early old age: modification by APOE genotype. J Alzheimers Dis 93(1):193–209, PMID: 36970897, 10.3233/JAD-221054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Younan D, Petkus AJ, Beavers DP, Espeland MA, Chui HC, et al. 2021. Ambient air pollution and long-term trajectories of episodic memory decline among older women in the WHIMS-ECHO cohort. Environ Health Perspect 129(9):097009, PMID: 34516296, 10.1289/EHP7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russ TC, Cherrie MPC, Dibben C, Tomlinson S, Reis S, Dragosits U, et al. 2021. Life course air pollution exposure and cognitive decline: modelled historical air pollution data and the Lothian Birth Cohort 1936. J Alzheimers Dis 79(3):1063–1074, PMID: 33427734, 10.3233/JAD-200910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petkus AJ, Younan D, Wang X, Beavers DP, Espeland MA, Gatz M, et al. 2021. Air pollution and the dynamic association between depressive symptoms and memory in oldest-old women. J Am Geriatr Soc 69(2):474–484, PMID: 33205418, 10.1111/jgs.16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maldonado G, Greenland S. 1997. The importance of critically interpreting simulation studies. Epidemiology 8(4):453–456, PMID: 9209864. [PubMed] [Google Scholar]
- 68.Bosworth B. 2018. Increasing disparities in mortality by socioeconomic status. Annu Rev Public Health 39:237–251, PMID: 29608870, 10.1146/annurev-publhealth-040617-014615. [DOI] [PubMed] [Google Scholar]
- 69.Koster A, Penninx BWJH, Bosma H, Kempen GIJM, Newman AB, Rubin SM, et al. 2005. Socioeconomic differences in cognitive decline and the role of biomedical factors. Ann Epidemiol 15(8):564–571, PMID: 15922627, 10.1016/j.annepidem.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, Wolfe CD. 2015. The effects of socioeconomic status on stroke risk and outcomes. Lancet Neurol 14(12):1206–1218, PMID: 26581971, 10.1016/S1474-4422(15)00200-8. [DOI] [PubMed] [Google Scholar]
- 71.Suwannaphant K, Laohasiriwong W, Puttanapong N, Saengsuwan J, Phajan T. 2017. Association between socioeconomic status and diabetes mellitus: the National Socioeconomics Survey, 2010 and 2012. J Clin Diagn Res 11(7):LC18–LC22, PMID: 28892937, 10.7860/JCDR/2017/28221.10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Adar SD, Filigrana PA, Clements N, Peel JL. 2014. Ambient coarse particulate matter and human health: a systematic review and meta-analysis. Curr Environ Health Rep 1(3):258–274, PMID: 25152864, 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lei P, Ayton S, Bush AI. 2021. The essential elements of Alzheimer’s disease. J Biol Chem 296:100105, PMID: 33219130, 10.1074/jbc.REV120.008207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



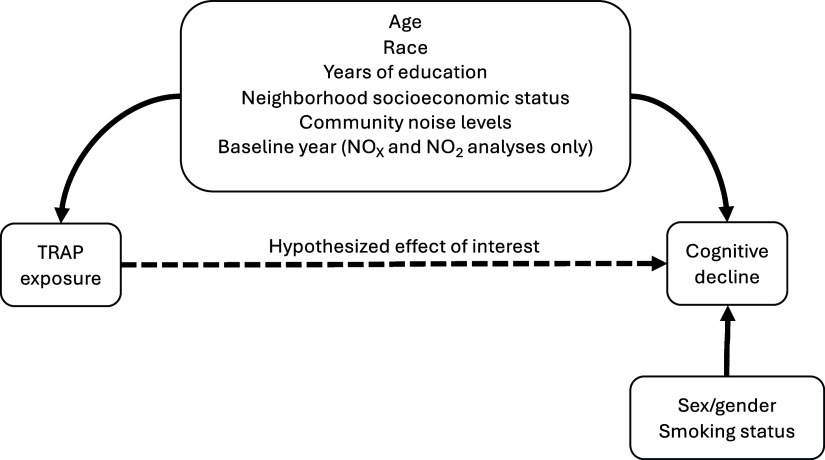
![Figure 2 is conceptual directed acyclic graph that was used for pre-baseline survival bias simulations. The graph shows that the effect of traffic-related air pollution on the probability of being alive at the age of study recruitment was categorized into three levels and modeled with two parameters corresponding to medium exposure [lowercase gamma begin subscript 1 end subscript] and high exposure [lowercase gamma begin subscript 2 end subscript] in reference to low exposure. Further, the graph shows that an unmeasured confounder (U) is hypothesized to affect the probability of being alive at the age of study recruitment through the model parameter lowercase gamma begin subscript 3 end subscript. Finally, the graph shows that for some simulations, traffic-related air pollution and U were hypothesized to jointly affect the probability of being alive at the age of study recruitment, with the relevant interaction terms being lowercase gamma begin subscript 4 end subscript and lowercase gamma begin subscript 5 end subscript.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/407d/11623384/54e2b438c8f2/ehp14585_f2.jpg)