Abstract
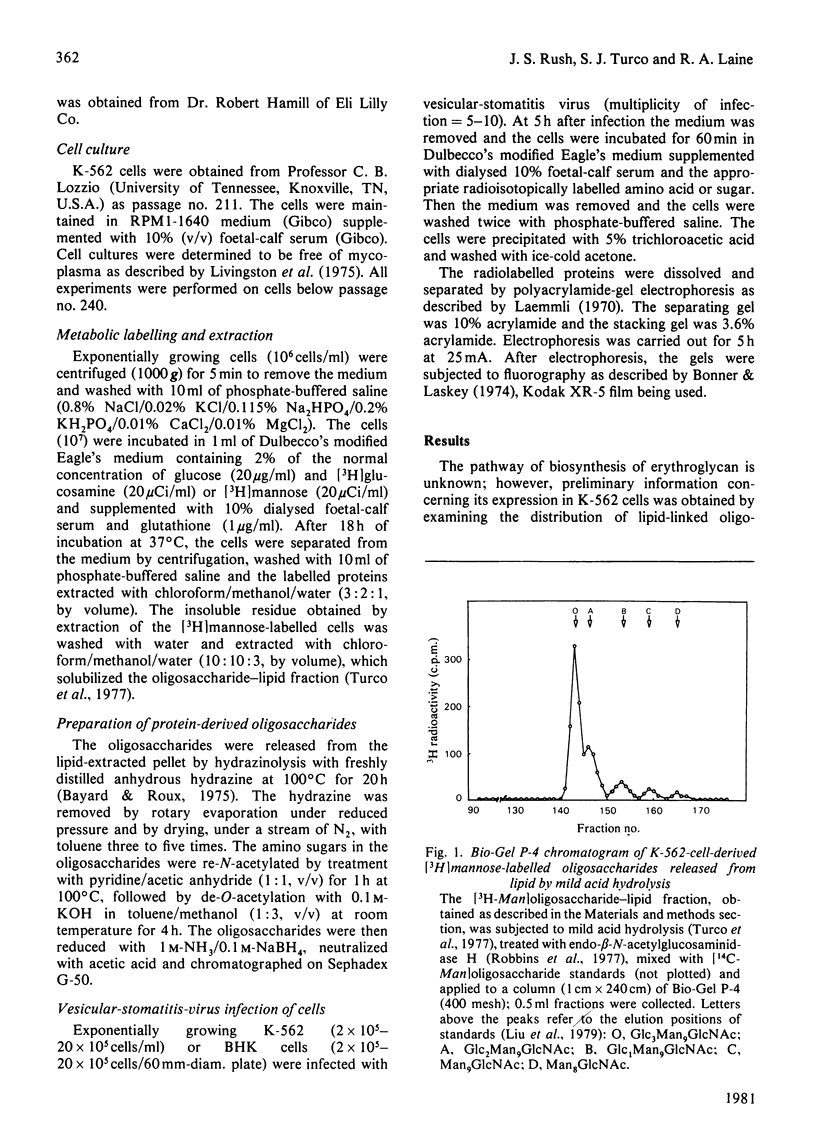
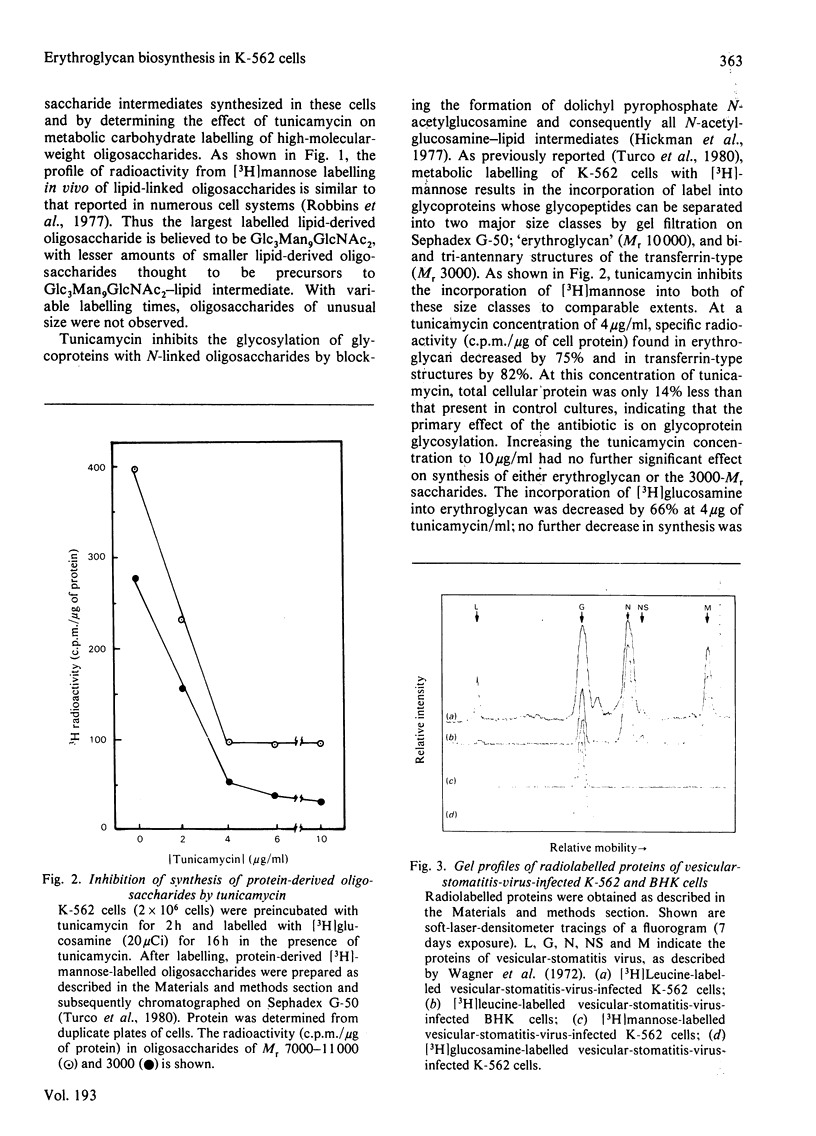
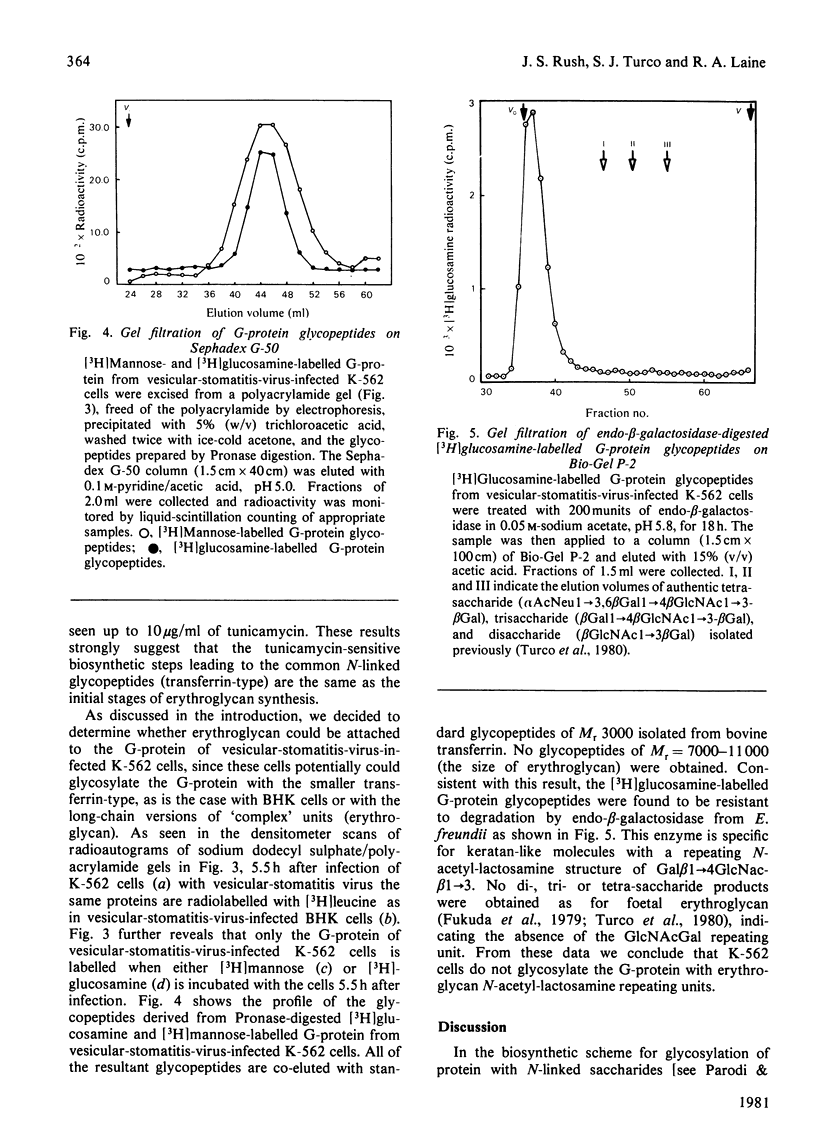
K-562 cells, which express foetal erythroglycan, are shown to synthesize the lipid-linked oligosaccharide intermediates commonly found in tissues and cultured fibroblasts. The addition of tunicamycin, which blocks the formation of these intermediates and thus of asparagine-linked oligosaccharides, inhibits the synthesis of erythroglycan (Mr 7000-11 000). Vesicular-stomatitis-virus infection of K-562 cells results in the glycosylation of the G-protein with the transferrin-type oligosaccharide (Mr 3000), but not with the larger erythroglycan. These results suggest that, in K-562 cells, the early stages of erythroglycan biosynthesis are the same as those of the transferrin-type oligosaccharides. However, maturation of the oligosaccharide is influenced by protein structure such that erythroglycan is only expressed on specific glycoproteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayard B., Roux D. Hydrazinolysis and nitrous deamination of glycoproteins. Evidence for a common inner core in carbohydrate moiety. FEBS Lett. 1975 Jul 15;55(1):206–211. doi: 10.1016/0014-5793(75)80993-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Fukuda M., Hakomori S. Cell surface modification by endo-beta-galactosidase. Change of blood group activities and release of oligosaccharides from glycoproteins and glycosphingolipids of human erythrocytes. J Biol Chem. 1979 Jun 25;254(12):5458–5465. [PubMed] [Google Scholar]
- Hickman S., Kulczycki A., Jr, Lynch R. G., Kornfeld S. Studies of the mechanism of tunicamycin in hibition of IgA and IgE secretion by plasma cells. J Biol Chem. 1977 Jun 25;252(12):4402–4408. [PubMed] [Google Scholar]
- Järnefelt J., Rush J., Li Y. T., Laine R. A. Erythroglycan, a high molecular weight glycopeptide with the repeating structure [galactosyl-(1 leads to 4)-2-deoxy-2-acetamido-glucosyl(1 leads to 3)] comprising more than one-third of the protein-bound carbohydrate of human erythrocyte stroma. J Biol Chem. 1978 Nov 25;253(22):8006–8009. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The poly(glycosyl) chains of glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur J Biochem. 1978 Dec 1;92(1):289–300. doi: 10.1111/j.1432-1033.1978.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu T., Stetson B., Turco S. J., Hubbard S. C., Robbins P. W. Arrangement of glucose residues in the lipid-linked oligosaccharide precursor of asparaginyl oligosaccharides. J Biol Chem. 1979 Jun 10;254(11):4554–4559. [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Turco S. J., Rush J. S., Laine R. A. Presence of erythroglycan on human K-562 chronic myelogenous leukemia-derived cells. J Biol Chem. 1980 Apr 25;255(8):3266–3269. [PubMed] [Google Scholar]
- Turco S. J., Stetson B., Robbins P. W. Comparative rates of transfer of lipid-linked oligosaccharides to endogenous glycoprotein acceptors in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4411–4414. doi: 10.1073/pnas.74.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


