Abstract
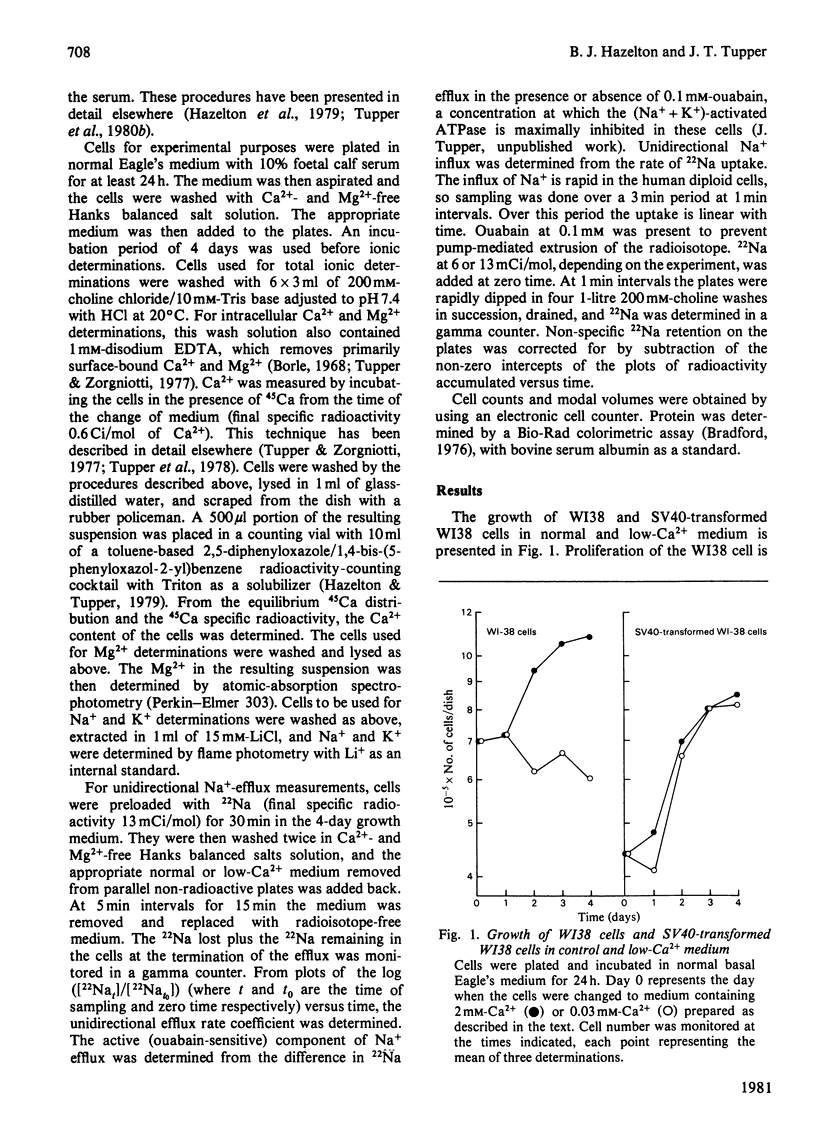
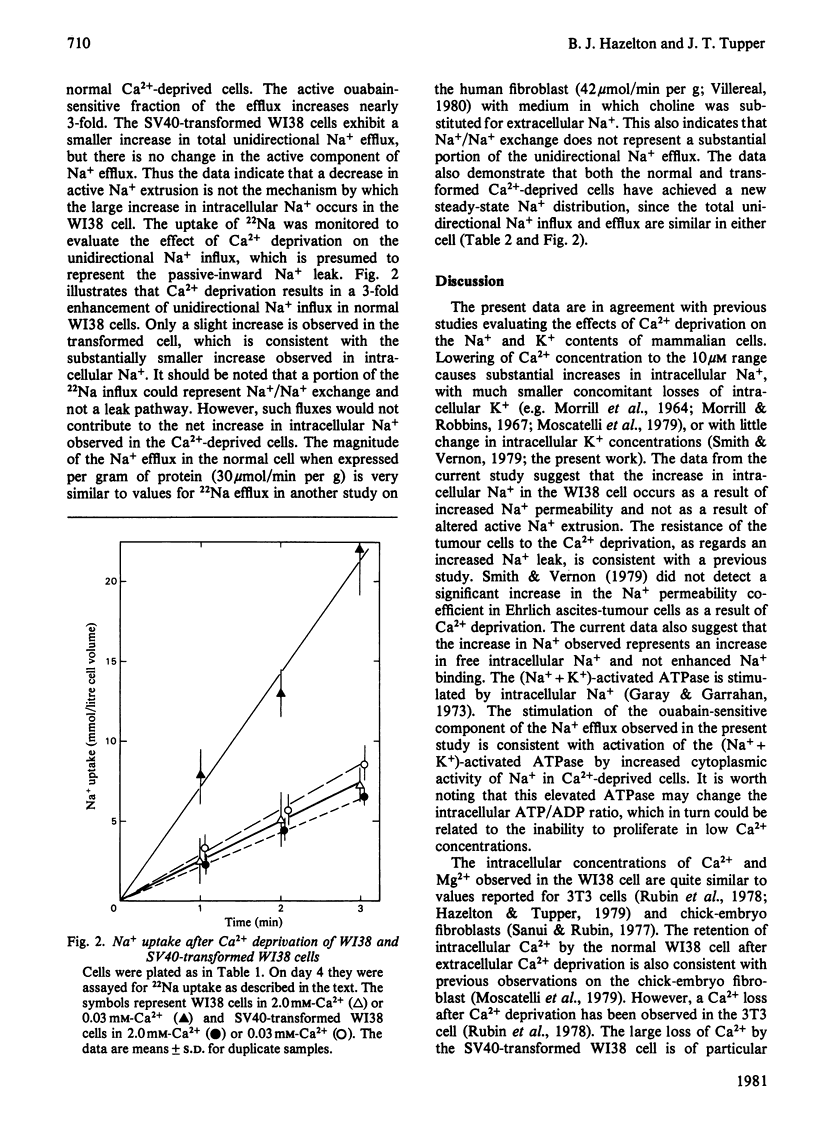
The lowering of extracellular Ca2+ concentration in the growth medium reversibly blocks normal, but not SV40-transformed WI38 diploid fibroblasts in the early G1/G0 phase of the cell cycle. This growth response is characterized by specific changes in ionic content and transport. Ca2+ deprivation (0.03 mM) has little effect on the K+ content of either normal or transformed cells. Na+ content, however, is increased nearly 2-fold in the normal cells. This increase is presumably due to a 3-fold increase in unidirectional Na+ influx in Ca2+-deprived cells. The increased intracellular Na+ also gives rise to a nearly 3-fold enhancement of the active (ouabain-sensitive) Na+ efflux. Ca2+ deprivation causes only slight increases in Na+ influx, ouabain-sensitive Na+ efflux and intracellular Na+ in the transformed cell. In contrast, the transformed cells lose nearly 60% of their intracellular Ca2+ on deprivation, whereas normal WI38 cells lose only 10%. The data suggest that the growth arrest exhibited by the normal cell but not the transformed cell may be related to different membrane-transport and permeability changes in response to Ca2+ deprivation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. P., Cuatrecasas P., Snyder S. H. Nerve growth factor receptor binding. Influence of enzymes, ions, and protein reagents. J Biol Chem. 1975 Feb 25;250(4):1427–1433. [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism in HeLa cells and the effects of parathyroid hormone. J Cell Biol. 1968 Mar;36(3):567–582. doi: 10.1083/jcb.36.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J Cell Physiol. 1977 Aug;92(2):241–247. doi: 10.1002/jcp.1040920212. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carafoli E., Crompton M. The regulation of intracellular calcium by mitochondria. Ann N Y Acad Sci. 1978 Apr 28;307:269–284. doi: 10.1111/j.1749-6632.1978.tb41957.x. [DOI] [PubMed] [Google Scholar]
- Desai K. S., Zinman B., Steiner G., Hollenberg C. H. Effect of calcium on [125I]insulin binding to rat adipocytes. Can J Biochem. 1978 Sep;56(9):843–848. doi: 10.1139/o78-129. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- GARDOS G. The function of calcium in the potassium permeability of human erythrocytes. Biochim Biophys Acta. 1958 Dec;30(3):653–654. doi: 10.1016/0006-3002(58)90124-0. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert I. G. The effects of divalent cations on the ionic permeability of cell membranes in normal and tumour tissues. Eur J Cancer. 1972 Feb;8(1):99–105. doi: 10.1016/0014-2964(72)90089-8. [DOI] [PubMed] [Google Scholar]
- Hazelton B. J., Tupper J. T. Calcium transport and exchange in mouse 3T3 and SV40-3T3 cells. J Cell Biol. 1979 Jun;81(3):538–542. doi: 10.1083/jcb.81.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelton B., Mitchell B., Tupper J. Calcium, magnesium, and growth control in the WI-38 human fibroblast cell. J Cell Biol. 1979 Nov;83(2 Pt 1):487–498. doi: 10.1083/jcb.83.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H. A., Adam G. Regulation of ion permeabilities of isolated rat liver cells by external calcium concentration and temperature. J Membr Biol. 1976 Mar 18;26(2-3):121–151. doi: 10.1007/BF01868870. [DOI] [PubMed] [Google Scholar]
- Ledbetter M. L., Lubin M. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp Cell Res. 1977 Mar 15;105(2):223–236. doi: 10.1016/0014-4827(77)90120-3. [DOI] [PubMed] [Google Scholar]
- MORRILL G. A., KABACK H. R., ROBBINS E. EFFECT OF CALCIUM ON INTRACELLULAR SODIUM AND POTASSIUM CONCENTRATIONS IN PLANT AND ANIMAL CELLS. Nature. 1964 Nov 14;204:641–642. doi: 10.1038/204641a0. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeehan W. L., Ham R. G. Calcium and magnesium ions and the regulation of multiplication in normal and transformed cells. Nature. 1978 Oct 26;275(5682):756–758. doi: 10.1038/275756a0. [DOI] [PubMed] [Google Scholar]
- Morrill G. A., Robbins E. The role of calcium in the regulation of the steady-state levels of sodium and potassium in the HeLa cell. J Gen Physiol. 1967 Mar;50(4):781–792. doi: 10.1085/jgp.50.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Sanui H., Rubin A. H. Effects of depletion of K+, Na+, or Ca2+ on DNA synthesis and cell cation content in chick embryo fibroblasts. J Cell Physiol. 1979 Oct;101(1):117–128. doi: 10.1002/jcp.1041010114. [DOI] [PubMed] [Google Scholar]
- Rubin A. H., Terasaki M., Sanui H. Magnesium reverses inhibitory effects of calcium deprivation on coordinate response of 3T3 cells to serum. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4379–4383. doi: 10.1073/pnas.75.9.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanui H., Rubin A. H. Measurement of total, intracellular and surface bound cations in animal cells grown in culture. J Cell Physiol. 1979 Aug;100(2):215–226. doi: 10.1002/jcp.1041000203. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin A. H. Membrane bound and cellular cationic changes associated with insulin stimulation of cultured cells. J Cell Physiol. 1978 Sep;96(3):265–278. doi: 10.1002/jcp.1040960302. [DOI] [PubMed] [Google Scholar]
- Sanui H., Rubin H. Correlated effects of external magnesium on cation content and DNA synthesis in culture chicken embryo fibroblasts. J Cell Physiol. 1977 Jul;92(1):23–31. doi: 10.1002/jcp.1040920104. [DOI] [PubMed] [Google Scholar]
- Smith T. C., Vernon K. D. Correlation of the effect of Ca+2 on Na+ and K+ permeability and membrane potential of Ehrlich ascites tumor cells. J Cell Physiol. 1979 Feb;98(2):359–369. doi: 10.1002/jcp.1040980212. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Del Rosso M., Hazelton B., Zorgniotti F. Serum-stimulated changes in calcium transport and distribution in mouse 3T3 cells and their modification by dibutyryl cyclic AMP. J Cell Physiol. 1978 Apr;95(1):71–84. doi: 10.1002/jcp.1040950110. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Kaufman L., Bodine P. V. Related effects of calcium and serum on the G1 phase of the human W138 fibroblast. J Cell Physiol. 1980 Jul;104(1):97–103. doi: 10.1002/jcp.1041040113. [DOI] [PubMed] [Google Scholar]
- Tupper J. T., Zografos L. Effect of imposed serum deprivation on growth of the mouse 3T3 cell. Dissociation from changes in potassium ion transport as measured from [86Rb)rubidium ion uptake. Biochem J. 1978 Sep 15;174(3):1063–1065. doi: 10.1042/bj1741063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper J. T., Zorgniotti F. Calcium content and distribution as a function of growth and transformation in the mouse 3T3 cell. J Cell Biol. 1977 Oct;75(1):12–22. doi: 10.1083/jcb.75.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]


