Abstract
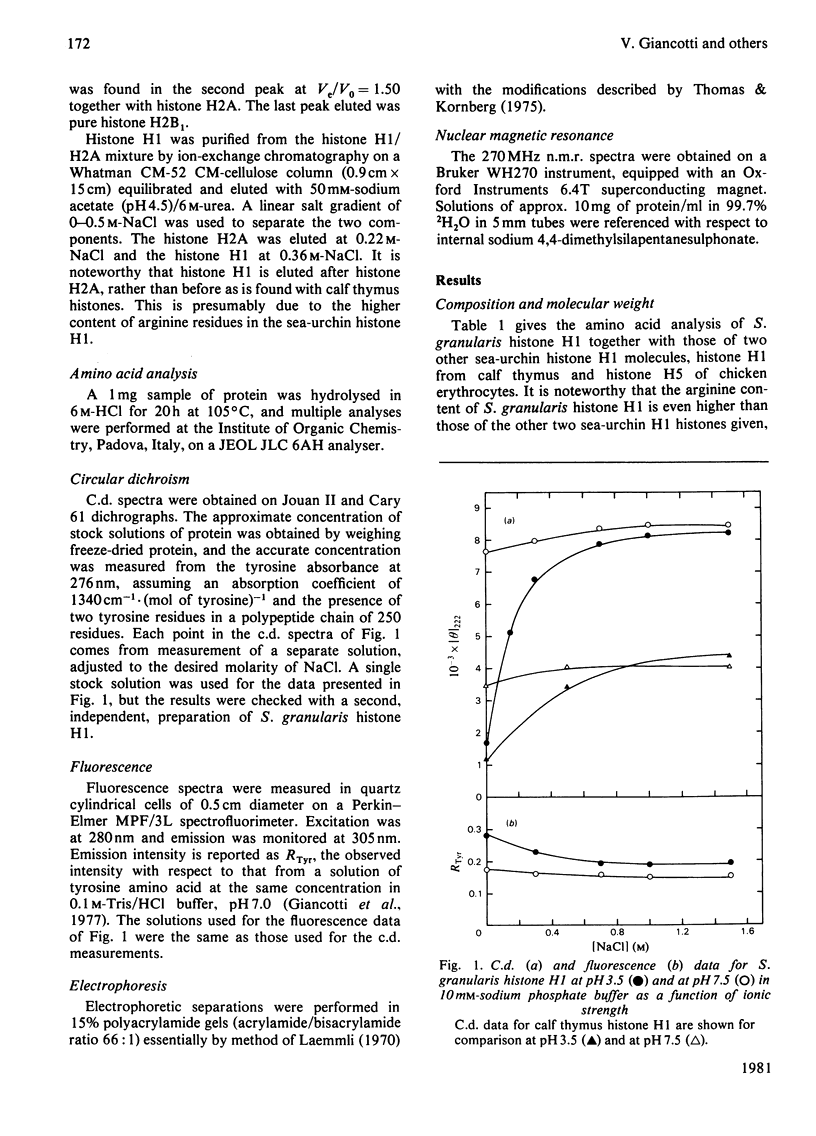
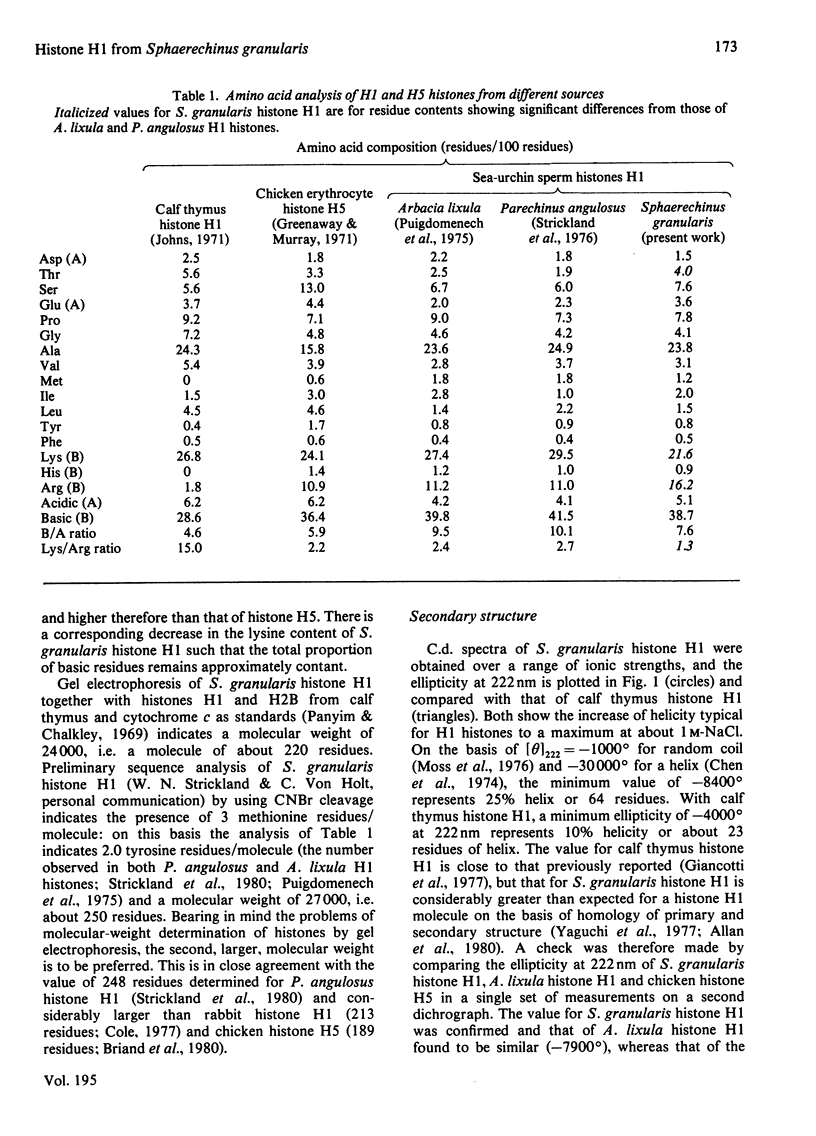
The separation and purification of histone H1 from the sperm of the sea-urchin Sphaerechinus granularis is described. Physical studies were used to compare this histone H1 molecule with H1 histones from other species. C.d. and 270 MHz n.m.r. spectroscopy indicate that, despite significant compositional differences from other sea-urchin sperm H1 histones, their secondary and tertiary structures are very similar. A large difference in helicity was, however, found between S. granularis histone H1 and calf thymus histone H1, and their n.m.r. and fluorescence spectra also differ considerably. It is concluded that secondary structure and tertiary structure have not been conserved in the evolution of the H1 histone family.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Carpenter B. G., Rattle H. W. Magnetic resonance studies of deoxyribonucleoprotein. Nature. 1973 Jan 12;241(5385):123–126. doi: 10.1038/241123a0. [DOI] [PubMed] [Google Scholar]
- Briand G., Kmiecik D., Sautiere P., Wouters D., Borie-Loy O., Biserte G., Mazen A., Champagne M. Chicken erythrocyte histone H5. IV. Sequence of the carboxy-termined half of the molecule (96 residues) and complete sequence. FEBS Lett. 1980 Apr 7;112(2):147–151. doi: 10.1016/0014-5793(80)80167-0. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Crane-Robinson C., Dancy S. E., Bradbury E. M., Garel A., Kovacs A. M., Champagne M., Daune M. Structural studies of chicken erythrocyte histone H5. Eur J Biochem. 1976 Aug 16;67(2):379–388. doi: 10.1111/j.1432-1033.1976.tb10702.x. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraci G., Noviello L. DNAase hydrolysis of chromatin DNA in intact sea urchin sperm cells. Effect of the ionic strength on the digestion parameters. Cell Differ. 1979 Jun;8(3):203–210. doi: 10.1016/0045-6039(79)90047-2. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Fonda M., Crane-Robinson C. Tyrosine fluorescence of two tryptophan-free proteins: histones H1 and H5. Biophys Chem. 1977 Apr;6(3):379–383. doi: 10.1016/0301-4622(77)85019-9. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., Murray K. Heterogeneity and polymorphism in chicken erythrocyte histone fraction V. Nat New Biol. 1971 Feb 24;229(8):233–238. doi: 10.1038/newbio229233a0. [DOI] [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitt M., Chothia C. Structural patterns in globular proteins. Nature. 1976 Jun 17;261(5561):552–558. doi: 10.1038/261552a0. [DOI] [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T., Cary P. D., Abercrombie B. D., Crane-Robinson C., Bradbury E. M. A pH-dependent interaction between histones H2A and H2B involving secondary and tertiary folding. Eur J Biochem. 1976 Dec 11;71(2):337–350. doi: 10.1111/j.1432-1033.1976.tb11120.x. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The molecular weights of vertebrate histones exploiting a modified sodium dodecyl sulfate electrophoretic method. J Biol Chem. 1971 Dec 25;246(24):7557–7560. [PubMed] [Google Scholar]
- Puigdomenech P., Palau J., Crane-Robinson C. The structure of sea-urchin-sperm histone phi 1 (H1) in chromatin and in free solution. Trypsin digestion and spectroscopic studies. Eur J Biochem. 1980 Feb;104(1):263–270. doi: 10.1111/j.1432-1033.1980.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Puigdoménech P., Cabré O., Palau J., Bradbury E. M., Crane-Robinson C. Studies on the role and mode of operation of the very-lysine-rich histones in eukaryote chromatin. The conformation of phi1 histones from marine invertebrate sperm. Eur J Biochem. 1975 Nov 1;59(1):237–243. doi: 10.1111/j.1432-1033.1975.tb02447.x. [DOI] [PubMed] [Google Scholar]
- Renz M., Nehls P., Hozier J. Involvement of histone H1 in the organization of the chromosome fiber. Proc Natl Acad Sci U S A. 1977 May;74(5):1879–1883. doi: 10.1073/pnas.74.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H., Kubai D. F. Chromosome structure. Annu Rev Genet. 1970;4:263–294. doi: 10.1146/annurev.ge.04.120170.001403. [DOI] [PubMed] [Google Scholar]
- Strickland W. N., Schaller H., Strickland M., Von Holt C. Partial amino acid sequence of histone H1 from sperm of the sea urchin, Parechinus angulosus. FEBS Lett. 1976 Jul 15;66(2):322–327. doi: 10.1016/0014-5793(76)80531-5. [DOI] [PubMed] [Google Scholar]
- Strickland W. N., Strickland M., Brandt W. F., Von Holt C., Lehmann A., Wittmann-Liebold B. The primary structure of histone H1 from sperm of the sea urchin Parechinus angulosus. 2. Sequence of the C-terminal CNBr peptide and the entire primary structure. Eur J Biochem. 1980 Mar;104(2):567–578. doi: 10.1111/j.1432-1033.1980.tb04460.x. [DOI] [PubMed] [Google Scholar]
- Suau P., Bradbury E. M., Baldwin J. P. Higher-order structures of chromatin in solution. Eur J Biochem. 1979 Jul;97(2):593–602. doi: 10.1111/j.1432-1033.1979.tb13148.x. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi M., Roy C., Dove M., Seligy V. Amino acid sequence homologies between H1 and H5 histones. Biochem Biophys Res Commun. 1977 May 9;76(1):100–106. doi: 10.1016/0006-291x(77)91673-4. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., Böhm E. L., von Holt C. Fractionation of chicken erythrocyte whole histone into the six main components by gel exclusion chromatography. Biochim Biophys Acta. 1974 Aug 8;359(2):341–345. doi: 10.1016/0005-2795(74)90233-5. [DOI] [PubMed] [Google Scholar]


