Abstract
Purpose of review
Women in Africa bear the burden of the HIV epidemic, which has been associated with the high prevalence of bacterial vaginosis (BV) in the region. However, little progress has been made in finding an effective cure for BV. Drawing on advances in microbiome-directed therapies for gastrointestinal disorders, similar live-biotherapeutic based approaches for BV treatment are being evaluated. Here, we summarize current knowledge regarding vaginal microbiota in BV, explore geographical differences in vaginal microbiota, and argue that novel BV therapeutics should be tailored specifically to meet the needs of African women.
Recent findings
Cervicovaginal microbiota dominated by Lactobacillus crispatus are optimal, although these are uncommon in African women. Besides socio-behavioural and environmental influences on the vaginal microbiota, host and microbial genetic traits should be considered, particularly those relating to glycogen metabolism. Novel microbiome-directed approaches being developed to treat BV should employ transfers of multiple microbial strains to ensure sustained colonization and BV cure.
Summary
Improving the efficacy and durability of BV treatment with microbiome-directed therapies by appropriately accounting for host and microbial genetic factors, could potentially reduce the risk of HIV infection in African women.
Keywords: female, bacterial vaginosis, HIV, inflammation, probiotics, geography, host genetics, Africa
Introduction
Women living in Africa arguably represent the highest degrees of diversity in genetics, culture, environment, diet and access to resources, yet reproductive health barriers encountered by African women remain a low priority on the global health agenda. Despite wide access to HIV testing and treatment (1), African women continue to bear the burden of the ongoing HIV epidemic (2). While risks of HIV acquisition outcomes are determined by an array of socio-behavioural, economic and biomedical factors, the attributable risk associated with each differ regionally (3). One of these risk factors is bacterial vaginosis (BV), a common dysbiosis of vaginal microorganisms in reproductive-aged women (4).
The microbiome has emerged as a crucial factor in human health, impacting susceptibility to pathogens (5), poor reproductive outcomes, cancer, metabolic diseases, allergies, autism, and obesity (6). Recognising the transformative potential of microbiome-targeted therapeutics, funding agencies in the global north developed strategic plans for investing in microbiome research (7, 8), yielding large volumes of publicly available data pertaining to wealthy industrialised nations (9). Relatively little progress has been made in understanding the relationships between microbial variability and health in other parts of the world. The context and locations of these “missing microbiomes” have major implications for disease management in African populations and the global community (10). Put simply, answering critical questions relating to the roles that geography, diet, socioeconomic status, and antibiotic use play in shaping the reproductive microbiomes of African women requires microbiome data from Africa.
The purpose of this review is to summarize what is known about the vaginal microbiome in relation to BV, to evaluate whether geographical differences exist in the composition of vaginal microbiota and host interactions with components of the microbiota, and to consider the importance of geography in developing novel BV-treatment modalities that address the unmet needs of African women.
Shifting from simplicity to complexity is bad in the vaginal niche
Resilience in ecosystems frequently correlates with diversity (11). In the reproductive tract, however, low diversity colonization with Lactobacillus species (L. crispatus, L. jensenii, L. gasseri, L. mucosae, and L. vaginalis) is considered optimal, with high diversity being associated with BV (12). Protective mechanisms used by vaginal Lactobacillus spp. to prevent colonization by other commensals and pathogens include competitive exclusion, production of lactic acid, bacteriocins, and biosurfactants (13). Lactic acid lowers vaginal pH, and enhances the structural integrity of the mucosal barrier (14). While low pH excludes competitors, Lactobacillus spp. are not acidophiles: they are simply less susceptible to acid than other bacterial species in the vagina (15). Lactobacillus spp. differ in their abilities to lower pH and inhibit other strains (16–18). Unlike other Lactobacillus spp., L. iners, is found in both optimal and dysbiotic microbial states, harbors a cytolysin (inerolysin) and does not produce D-lactic acid (12). In this ecosystem, estrogen gives Lactobacillus spp. an advantage (19).
Ecosystems frequently shift in composition in response to changes in the environment, and should be studied holistically, considering interactions between all the ecosystem's components (20); including those components present at low relative abundance. The finding that a “key” group of species - Lactobacillus spp. - dominate most healthy vaginal microbiomes suggests an important functional role in the ecosystem (20). It is commonly assumed that other non-key species are “passengers” that do not significantly alter the dynamics or function of the ecosystem, with the key species “driving” ecosystem processes such as the maintenance of species diversity and/or stability (9). However, Greenbaum et al. (20) argued that “rarer” microbial taxa occurring during optimal vaginal ecological states may influence the dynamics of the vaginal ecosystem, being “seed banks” poised for proliferation and outgrowth once environmental conditions change (menses, pregnancy, menopause).
In the absence of Lactobacillus spp. dominance, the vaginal microbiota shift to a high-diversity state, comprising a diverse assortment of strict and facultative anaerobic bacteria, including Gardnerella spp., Prevotella spp., and Fannyhessea vaginae (13, 21). These diverse anaerobes can form complex biofilms, likely comprising G. vaginalis as an “anchor species”, synergistically fostering the outgrowth of other BV-associated anaerobes (22–24). Both Prevotella and Gardnerella species produce sialidases that degrade cervicovaginal mucus, allowing better contact between the vaginal microbiota and the epithelial barrier (25, 26). BV-associated anaerobes produce a complex array of biogenic amines (such as cadaverine, putrescine, and tyramine), which slows the growth of most vaginal Lactobacillus spp. and reduces the production of lactic acid by vaginal Lactobacillus spp (27). G. vaginalis also produces cytotoxic compounds such as vaginolysin, which trigger epithelial immune responses and NF-κB activation (26). G. vaginalis may also differ geographically in terms of prevalence, genetic diversity, antimicrobial resistance profiles, and strain distribution (28, 29), that need to be considered when developing treatment protocols for BV.
Factors influencing the vaginal microbiome and BV risk
Many socio-behavioural and biomedical risk factors have been defined for BV, including menses, menstrual practices, antibiotics, sexual behaviours, contraceptives, hygiene practices, and partner characteristics [reviewed elsewhere (30)]. Vaginal practices are complex and vary regionally, based on the social and cultural norms, sometimes including intravaginal insertion of commercial products, chemicals, and/or natural products (31–34). In some regions, lubricated sex is preferred, while in others dry sex is preferred (35). Some studies have associated having new or multiple sexual partners and frequent condomless intercourse with a higher risk of BV (36, 37). Condomless intercourse and recent exposure to semen have been associated with reduced Lactobacillus spp. prevalence, increased P. bivia and G. vaginalis prevalence, and increased BV recurrence (38–41). Furthermore, condomless sex with an uncircumcised male partner may further exacerbate risk for BV (42). Female vaginal microbiota often resembles her partners’ and uncircumcised males generally have penile microbiota dominated by anaerobes such as Finegoldia, Prevotella, Dialister, and Peptoniphilus (43). Thus, male circumcision practices in various geographies and cultures may influence risk for BV.
Is BV the same globally?
While a cervicovaginal microbiota dominated by L. crispatus is considered optimal, African women appear to have more diverse bacterial communities, including those dominated by L. iners (4, 21, 42, 44, 45). These associations appear to persist when controlling for sociodemographic factors and sexual practices (46, 47), suggesting that host genetics may influence vaginal microbiome composition (48). Studies of gut microbiota have shown that the composition and function of the gut microbiome are heritable and transferable (49–53). Bubier et al. (54) summarized SNPs in >100 host genes associated with bacterial abundance in twins or from GWAS data. Of those affecting Lactobacillus spp. abundance, >50% of the SNPs were located on chromosomes 1–3 and 11, with many of the genes located on these chromosomes involved in sugar and/or lipid metabolism (Table 1).
Table 1.
Chromosomes with highest density of SNPs influencing lactobacilliaceae colonization.
| Chromosome | # SNPs | Gene | Product | Process |
|---|---|---|---|---|
| 1 | 7 | FPGT | Fucose-1-phosphate guanylyltransferase | Fructose and mannose metabolism |
| GALE | UDP-galactose-4-epimerase | Galactose metabolism | ||
| MAN1A2 | Mannosyl-oligosaccharide 1,2-alpha-mannosidase IB | |||
| SLC2A1 | Glucose transporter 1 | Glucose transport (blood-brain barrier) | ||
| SPRR1A | Cornifin-A | Squamous differentiation | ||
| SPRR1B | Cornifin-B | Squamous differentiation | ||
| 2 | 15 | GFPT1 | Glutamine-fructose-6-phosphate transaminase 1 | Enzyme participating in glutamate and amino sugars metabolism |
| GCKR | Glucokinase regulator | Sugar isomerase: regulatory protein that inhibits glucokinase in liver and pancreatic islet cells | ||
| LEPQTL1 | Leptin, serum levels of | Regulate long-term energy balance; correlate with the amount of energy reserves (triglycerides) stored in adipose tissue | ||
| COL3A1 | Collagen, type III, alpha 1 | Provide structural support to the extracellular space of connective tissues | ||
| COL4A3 | Collagen, type IV, alpha 3 | Major structural component of basement membranes | ||
| COL4A4 | Collagen, type IV, alpha 4 | Major structural component of basement membranes | ||
| COL5A2 | Collagen, type V, alpha 2 | Fibrillar collagens | ||
| MGAT5 | Mannosyl (alpha-1,6-)-glycoprotein beta-1,6-N-acetyl-glucosaminyltransferase | Involved in the synthesis of protein-bound and lipid-bound oligosaccharides | ||
| HADHA | Part of an enzyme complex called mitochondrial trifunctional protein | Mitochondrial trifunctional protein required to metabolize long-chain fatty acids | ||
| ABCG5/8 | ABC transporter proteins (sterolin-1 and -2) | Regulates sterol absorption and excretion | ||
| 3 | 14 | COL7A1 | Collagen, type VII, alpha 1 | Functions as an anchoring fibril between the dermal-epidermal junction in basement membrane |
| GMPPB | GDP-mannose pyrophosphorylase B | Enzyme catalyzes conversion of mannose-1-phosphate and GTP to GDP-mannose, involved in N-linked oligosaccharides production | ||
| ADIPOQ | Adiponectin | Protein hormone involved in regulating glucose levels and fatty acid breakdown | ||
| GYG1 | Glycogenin-1 | Involved in the biosynthesis of glycogen | ||
| BTD | Amidohydrolase biotinidase | Propionic acidemia | ||
| SI | Sucrase-isomaltase | Bifunctional glucosidase | ||
| CACT | Carnitine-acylcarnitine translocase | Enzyme responsible for passive transport of carnitine and carnitine-fatty acid complexes and across the inner mitochondrial membrane as part of the carnitine shuttle system | ||
| 11 | 10 | ACAT1 | Acetyl-Coenzyme A acetyltransferase 1 (acetoacetyl Coenzyme A thiolase) | Converts intracellular free cholesterol into cholesteryl esters for storage in lipid droplets |
| APOA4 | Apolipoprotein A-IV | Major protein of high-density lipoproteins; implicated in regulating lipid absorption and metabolism, food intake, and glucose metabolism | ||
| BGNT1 | N-acetyl-lactosaminide beta-1,3-N-acetyl-glucosaminyl transferase | Key enzyme for core-2 O-glycans biosynthesis; belongs to the family of glycosyltransferases; participates in 4 metabolic pathways: keratan sulfate biosynthesis, glycosphingolipid biosynthesis - neo-lactoseries, glycan structures - biosynthesis 1, and glycan structures - biosynthesis 2 | ||
| INS | Insulin gene | Produces insulin | ||
| CPT1A | Carnitine palmitoyltransferase 1A | Mitochondrial enzyme responsible for the formation of acyl carnitines by catalyzing transfer of acyl group of a long-chain fatty acyl-CoA from coenzyme A to l-carnitine |
Evidence that the vaginal microbiomes of monozygotic twins are more similar to each other than to their mothers or sisters (55, 56), argues for a role of host genetics in determining microbiome structure. However, it is difficult to disentangle the relative contributions of genetic and environmental factors to overall microbiome structure. For example, a meta-analysis that included 2,748 twins concluded that 31% of reproductive traits were heritable (57), while also highlighting the fact that reproductive traits were one of the trait types most influenced by the environment Nevertheless, if L. crispatus heritability is indeed influenced in part by host genetics, this may have implications for probiotic effectiveness in improving vaginal health in diverse populations (42).
Missing African vaginal strain genomic data in global databases
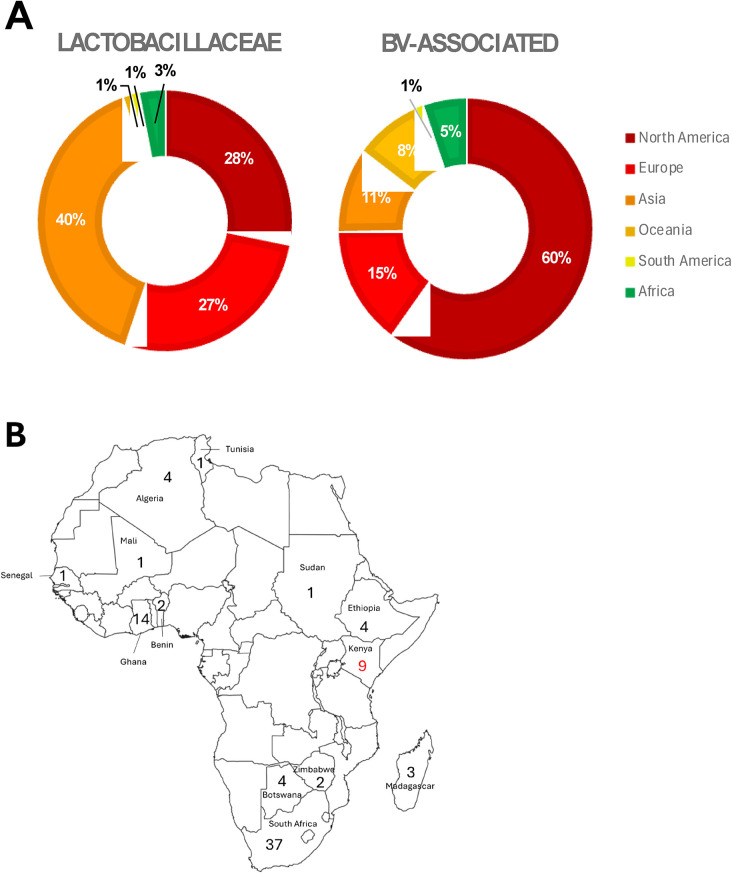
Recently, Bloom et al. (58) published an associated vaginal genome catalogue, comprising ∼1,200 Lactobacillus spp. genomes and metagenome-assembled genomes from >300 women across four continents, including Africa. Despite this useful resource, the NCBI RefSeq assembly database currently contains only 3,084 Lactobacillaceae and 185 BV-associated whole genome sequences (we focused just on Prevotella, Gardnerella and Fannyhessea only for this review; Figure 1; Supplementary Table S1). Of these, most (81% and 91% for Lactobacillaceae and BV-associated bacteria, respectively) were from samples collected in the global north and few were derived from vaginal samples.
Figure 1.
Proportion of publicly available whole genome sequences of Lactobacillaceae (n = 3084) and BV-associated organisms (BV; n = 185), according to (A) region: North America (dark red), Europe (bright red), Asia (deep orange), Oceania (light orange), South America (yellow), and Africa (green). BV-associated organisms included Prevotella spp., Gardnerella spp., and Fannyhessea spp. only. (B) Within Africa, the numbers of Lactobacillaceae genomes (black numbers) and BV-associated organism genomes (red numbers) from different countries are shown.
Better understanding these elusive missing African vaginal genomic data is and will be important moving forward. An example of this is demonstrated in a study by Lithgow et al. (59), where Lactobacillus-dominant African women were 3-fold more likely to be colonized by L. crispatus isolates lacking the gene involved in glycogen degradation, pulA, than European or North America women (60, 61). Glycogen is a key host-provided nutrient that supports vaginal lactobacilli and their fermentative lactic acid production (60). The findings of Lithgow et al. (59) may be critical for our understanding of BV in Africa, suggesting that pulA gene loss explains reductions in L. crispatus abundance, pullulanase activity and D-lactic acid levels.
Progress in improving BV treatment
BV is still diagnosed clinically based on symptoms [presence of clue cells [shed epithelial cells coated with BV-associated microbes, evident by Gram stain], vaginal pH >4.5, discharge and a “fishy” odor - known as Amsel criteria (62)] or Nugent scoring (63). However, BV is frequently asymptomatic (64), particularly in Africa (65, 66). Defining the complex ecology of BV using more sensitive molecular approaches (molecular-BV) has proved invaluable in understanding the dysbiosis and identifying new targets for therapy (21).
Symptomatic BV is treated with metronidazole or clindamycin, with treatment focusing on selectively halting the proliferation of BV-associated microorganisms to restore “optimal” vaginal microbes (67). Following treatment, vaginal microbiota shift to L. iners rather than L. crispatus dominance, primarily driven by a massive reduction in BV-associated bacterial abundance (68, 69). BV treatment outcomes following antibiotics appear to be better in women from the US than those living in Africa (70). The vaginal microbiota composition and structure prior to BV treatment is known to influence treatment outcome, such that women with more vaginal bacterial diversity pre-treatment are more likely to experience treatment failure (71). G. vaginalis resistant to metronidazole may be a factor underlying different treatment outcomes (72), particularly within biofilms (73, 74), although this has not been systematically compared geographically.
BV recurrence is frequent, with >50% women who clear BV relapsing within six months (75, 76). Some studies have shown that G. vaginalis (77) and F. vaginae (78) strains that recolonize after initial BV treatment have an increased resistance to subsequent courses of antibiotic treatment. Bannatyne et al. (79) showed that metronidazole susceptibility in G. vaginalis strains declined sequentially, with almost all isolates being sensitive after the first course of treatment, and sensitivity reducing by 20%–30% for each subsequent metronidazole treatment (79). In another cross-sectional study, 40% of P. bivia isolates, 14% of P. amnii and 58% of P. timonensis isolates were resistant to clindamycin (80). The extent to which African strains possess antibiotic resistance is yet to be determined.
Several promising novel treatment approaches are currently being investigated for BV treatment. For example, to address post-treatment re-colonization with L. iners, Zhu et al. recently showed that oleic acid and other unsaturated long-chain fatty acids, enhance metronidazole-mediated cure rates; by selectively inhibiting L. iners, while enhancing L. crispatus growth (81). Other combinatorial approaches to enhance metronidazole efficacy have been developed, such as a vaginally inserted ring product that sustainably releases either metronidazole alone (82), or with dapivirine (for HIV prevention) (83). Endolysins, enzymes produced by bacteriophages to degrade bacterial cell walls and disrupt biofilms, are being tested to treat BV, specifically targeting G. vaginalis (84). While these approaches are currently in preclinical and in vitro study phases, and their efficacies are yet to be tested in humans, they do represent promising avenues for further research to enhance current BV treatment strategies.
Vaginal microbiome transplants and lessons from the gut
Studies using faecal microbiome transplantation have shown that some donor microbiome-associated phenotypes can be transferred to recipients. Microbiome transplant between obese and lean mice (85), and between lean and obese human donors into mice (86) have demonstrated these phenomena. Faecal microbiome transplant from healthy donors to individuals with autism spectrum disorder (87) and multiple sclerosis (89) have been shown to reduce disease severity, and is now standard-of-care for patients with recurrent Clostridioides difficile infections (88). From these studies, it is evident that the clinical benefits of treatment are only sustained if there is successful stable colonization of donor microbiota within the new host (89, 90). Host genetic factors are thought to prevent successful engraftment in recipients who experience only transient colonization, suggesting these ecologically sensitive approaches should factor in a complex set of phenotypes for donor-recipient pairs to ensure successful and sustained colonization (91).
Vaginal microbiome transplantation similarly involves transfer of vaginal fluid from healthy donors with well characterized optimal vaginal microbiota to recipient women with BV (92). The feasibility of transplanting the vaginal microbiome between women to protect against BV has been implied by evidence from women who have sex with women, where both female partners have low risk of BV and relatively stable concordant vaginal microbiota (93). Vaginal microbiome transplantation was first trialled in a small cohort of women with BV in Israel in 2019 (94) with four out of five recipients of the vaginal microbiome transplant showing promising results. In this first-in-human study, donors and recipients shared similar genetic backgrounds, as well as similar socio-behavioural characteristics so the impact of genetic and cultural diversity cannot be extrapolated. Although Mitchell et al. (95) discusses the potential risks of vaginal microbiome transplantation, which necessitate strict safety precautions, a clear benefit is that the “whole” vaginal environment is transferred between donor and recipient, including exact mixtures of vaginal microbes and molecules produced by both hosts and microbes that were associated with health in the donor. This likely assists in the colonization of beneficial bacteria while working against BV-associated bacteria. Understanding the main functional components that need to be transferred to ensure the success of this approach is critical in developing new vaginal microbiome-targeted therapies.
Simplifying transplantation using probiotics/live-biotherapeutics
Probiotics were defined by the WHO as “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host” while the US FDA introduced the term “live biotherapeutic product”, defined as “a biological product that contains live organisms; is applicable to the prevention, treatment or cure of a disease or condition of humans; and is not a vaccine.” Moving from complex transplantation of entire vaginal microbial communities, the effectiveness of simple single or multi-strain live biopharmaceutical products/probiotics have been tested for treating BV: either with or without pre-treatment (“weeding”) with antibiotics (96). Evidence synthesized from >30 clinical trials that tested different probiotics for treating BV suggests that Lactobacillus strain, its origin, route of administration and pre-treatment status of participants are important determinants of treatment outcomes (96). Many trials tested single Lactobacillus strain-containing probiotics, frequently not vaginal in origin, raising questions about whether one strain would fit all the possible genetic and immunological permutations of all potential recipients (97), regardless of geography and genetics (98).
Two clinical trials have tested vaginally-delivered L. crispatus CTV-05 (LACTIN-V), after metronidazole treatment (99, 100). Both trials showed that the product was generally safe and acceptable to women, significantly decreased recurrence of BV and increased L. crispatus colonization among recipients. Short-term cure rates of 100%, and long-term cure rates of 70% were achieved, with BV by Amsel's criteria being the endpoint. However, efficacy of LACTIN-V appeared to depend on the extent to which metronidazole “cured” BV (101), particularly the extent of G. vaginalis clearance (102). Other factors that influenced efficacy of LACTIN-V included condomless sex or having menses, suggesting that semen and menstrual blood affected CTV-05 colonization (100). While these products are promising, the lack of microbiota data from Africa (in its diversity) limits evidence-based live therapeutic product formulation and subsequent clinical trials. No clinical studies have yet evaluated the effect of live biotherapeutics for BV treatment on reducing HIV infections.
Conclusion
The importance of a healthy vaginal microbiome and the potential benefits of specifically tailored probiotics that contain beneficial Lactobacillus strains is clear. The new approaches being developed aim to maintain a healthy vaginal environment following BV treatment, that may reduce HIV risk in women. It is critical to focus on Africa to reveal and harness our “missing microbes”, as these will provide the foundations upon which microbiome-centred reproductive health solutions will be built. If appropriately focused on regionally-responsive microbes, these new approaches will have the greatest probability of being sustainable and efficacious for all Africa's women.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. J-AP is funded by the Bill and Melinda Gates Foundation Calestous Juma Scientific Leadership Fellowship, for a project entitled “Vaginal Microbiome Research Consortium (VMRC)-4-Africa” (INV-037612).
Author contributions
J-AP: Conceptualization, Formal Analysis, Funding acquisition, Writing – original draft, Writing – review & editing. SN: Writing – original draft, Writing – review & editing. SG: Writing – original draft, Writing – review & editing. BK: Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. KW: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. DM: Visualization, Writing – original draft, Writing – review & editing. DP: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. MO: Writing – original draft, Writing – review & editing. KG: Writing – original draft, Writing – review & editing. MF: Writing – original draft, Writing – review & editing. A-UH: Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. HJ: Writing – original draft, Writing – review & editing. MK: Investigation, Writing – original draft, Writing – review & editing. EB: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Conflict of interest
J-AP is receiving funding from the Bill and Melinda Gates Foundation Calestous Juma Scientific Leadership Fellowship, for a project entitled “Vaginal Microbiome Research Consortium (VMRC)-4-Africa” (INV-037612) to characterize Lactobacillus crispatus isolates, in part for development of regionally responsive live-biotherapeutic products for women in Africa.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2024.1431306/full#supplementary-material
References
- 1.Chipanta D, Amo-Agyei S, Giovenco D, Estill J, Keiser O. Socioeconomic inequalities in the 90-90-90 target, among people living with HIV in 12 Sub-Saharan African countries - implications for achieving the 95-95-95 target - analysis of population-based surveys. EClinicalMedicine. (2022) 53:101652. 10.1016/j.eclinm.2022.101652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. Women and girls carry the heaviest HIV burden in sub-Saharan Africa: UNAIDS (2022). Available online at: https://www.unaids.org/en/resources/presscentre/featurestories/2022/march/20220307_women-girls-carry-heaviest-hiv-burden-sub-saharan-africa (accessed April 9, 2024).
- 3.Scorgie F, Khoza N, Delany-Moretlwe S, Velloza J, Mangxilana N, Atujuna M, et al. Narrative sexual histories and perceptions of HIV risk among young women taking PrEP in Southern Africa: findings from a novel participatory method. Soc Sci Med. (2021) 270:113600. 10.1016/j.socscimed.2020.113600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis. (2019) 46(5):304–11. 10.1097/OLQ.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 5.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. (2012) 13(4):260–70. 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther. (2022) 7(1):135. 10.1038/s41392-022-00974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NIH. Interagency strategic plan for microbiome research. Online (2018).
- 8.KTM-UK. Microbiome Strategic Roadmap (2021).
- 9.Brewster R, Tamburini FB, Asiimwe E, Oduaran O, Hazelhurst S, Bhatt AS. Surveying gut microbiome research in Africans: toward improved diversity and representation. Trends Microbiol. (2019) 27(10):824–35. 10.1016/j.tim.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortega RP. Microbiome data dominated by wealthy countries. Science. (2022) 375(6582):709. 10.1126/science.ada1336 [DOI] [PubMed] [Google Scholar]
- 11.Hickey RJ, Zhou X, Pierson JD, Ravel J, Forney LJ. Understanding vaginal microbiome complexity from an ecological perspective. Transl Res. (2012) 160(4):267–82. 10.1016/j.trsl.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.France M, Alizadeh M, Brown S, Ma B, Ravel J. Towards a deeper understanding of the vaginal microbiota. Nat Microbiol. (2022) 7(3):367–78. 10.1038/s41564-022-01083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borgogna JC, Shardell MD, Grace SG, Santori EK, Americus B, Li Z, et al. Biogenic amines increase the odds of bacterial vaginosis and affect the growth of and lactic acid production by vaginal Lactobacillus spp. Appl Environ Microbiol. (2021) 87(10):e03068-20. 10.1128/AEM.03068-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delgado-Diaz DJ, Jesaveluk B, Hayward JA, Tyssen D, Alisoltani A, Potgieter M, et al. Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression. Microbiome. (2022) 10(1):141. 10.1186/s40168-022-01337-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navarro S, Abla H, Delgado B, Colmer-Hamood JA, Ventolini G, Hamood AN. Glycogen availability and pH variation in a medium simulating vaginal fluid influence the growth of vaginal Lactobacillus species and Gardnerella vaginalis. BMC Microbiol. (2023) 23(1):186. 10.1186/s12866-023-02916-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Happel AU, Kullin B, Gamieldien H, Wentzel N, Zauchenberger CZ, Jaspan HB, et al. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog. (2020) 16(6):e1008559. 10.1371/journal.ppat.1008559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chetwin E, Manhanzva MT, Abrahams AG, Froissart R, Gamieldien H, Jaspan H, et al. Antimicrobial and inflammatory properties of South African clinical Lactobacillus isolates and vaginal probiotics. Sci Rep. (2019) 9(1):1917. 10.1038/s41598-018-38253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Veer C, Hertzberger RY, Bruisten SM, Tytgat HLP, Swanenburg J, de Kat Angelino-Bart A, et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: implications for in vivo dominance of the vaginal microbiota. Microbiome. (2019) 7(1):49. 10.1186/s40168-019-0667-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. (2014) 2(1):4. 10.1186/2049-2618-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenbaum S, Greenbaum G, Moran-Gilad J, Weintraub AY. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am J Obstet Gynecol. (2019) 220(4):324–35. 10.1016/j.ajog.2018.11.1089 [DOI] [PubMed] [Google Scholar]
- 21.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. (2011) 108(Suppl 1):4680–7. 10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradshaw CS, Brotman RM. Making inroads into improving treatment of bacterial vaginosis - striving for long-term cure. BMC Infect Dis. (2015) 15:292. 10.1186/s12879-015-1027-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sousa LGV, Pereira SA, Cerca N. Fighting polymicrobial biofilms in bacterial vaginosis. Microb Biotechnol. (2023) 16(7):1423–37. 10.1111/1751-7915.14261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy L, Jespers V, Abdellati S, De Baetselier I, Mwambarangwe L, Musengamana V, et al. A fruitful alliance: the synergy between Atopobium vaginae and Gardnerella vaginalis in bacterial vaginosis-associated biofilm. Sex Transm Infect. (2016) 92(7):487–91. 10.1136/sextrans-2015-052475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shvartsman E, Hill JE, Sandstrom P, MacDonald KS. Gardnerella revisited: species heterogeneity, virulence factors, mucosal immune responses, and contributions to bacterial vaginosis. Infect Immun. (2023) 91(5):e0039022. 10.1128/iai.00390-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anton L, Ferguson B, Friedman ES, Gerson KD, Brown AG, Elovitz MA. Gardnerella vaginalis alters cervicovaginal epithelial cell function through microbe-specific immune responses. Microbiome. (2022) 10(1):119. 10.1186/s40168-022-01317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borgogna JC, Grace SG, Holm JB, Aviles Zuniga T, Kadriu H, He X, et al. Investigating the impact of condomless vaginal intercourse and lubricant use on the vaginal metabolome: a pre-post observational study. Sex Transm Infect. (2023) 99(7):489–96. 10.1136/sextrans-2022-055667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornejo OE, Hickey RJ, Suzuki H, Forney LJ. Focusing the diversity of Gardnerella vaginalis through the lens of ecotypes. Evol Appl. (2018) 11(3):312–24. 10.1111/eva.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pillay K, Nzimande S, Naicker M, Ramsuran V, Tinarwo P, Abbai N. Prevalence of genotypes and subtypes of Gardnerella vaginalis in South African pregnant women. Infect Dis Obstet Gynecol. (2020) 2020:3176407. 10.1155/2020/3176407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis FMT, Bernstein KT, Aral SO. Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol. (2017) 129(4):643–54. 10.1097/AOG.0000000000001932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphries H, Mehou-Loko C, Phakathi S, Mdladla M, Fynn L, Knight L, et al. ‘You'll always stay right': understanding vaginal products and the motivations for use among adolescent and young women in rural KZN. Cult Health Sex. (2019) 21(1):95–107. 10.1080/13691058.2018.1453086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nsereko E, Moreland PJ, Dunlop AL, Nzayirambaho M, Corwin EJ. Consideration of cultural practices when characterizing the vaginal microbiota among African and African American women. Biol Res Nurs. (2021) 23(1):91–9. 10.1177/1099800420940788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francis SC, Baisley K, Lees SS, Andrew B, Zalwango F, Seeley J, et al. Vaginal practices among women at high risk of HIV infection in Uganda and Tanzania: recorded behaviour from a daily pictorial diary. PLoS One. (2013) 8(3):e59085. 10.1371/journal.pone.0059085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hull T, Hilber AM, Chersich MF, Bagnol B, Prohmmo A, Smit JA, et al. Prevalence, motivations, and adverse effects of vaginal practices in Africa and Asia: findings from a multicountry household survey. J Womens Health (Larchmt). (2011) 20(7):1097–109. 10.1089/jwh.2010.2281 [DOI] [PubMed] [Google Scholar]
- 35.Milford C, Beksinska M, Smit J, Deperthes B. Lubrication and vaginal sex: lubricant use and preferences in general population women and women at risk of HIV. Arch Sex Behav. (2020) 49(6):2103–16. 10.1007/s10508-020-01673-3 [DOI] [PubMed] [Google Scholar]
- 36.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. (2008) 35(1):78–83. 10.1097/OLQ.0b013e318156a5d0 [DOI] [PubMed] [Google Scholar]
- 37.Fethers KA, Fairley CK, Hocking JS, Gurrin LC, Bradshaw CS. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin Infect Dis. (2008) 47(11):1426–35. 10.1086/592974 [DOI] [PubMed] [Google Scholar]
- 38.Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-saharan Africa. Sci Rep. (2017) 7(1):11974. 10.1038/s41598-017-12198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner AN, Reese PC, Snead MC, Fields K, Ervin M, Kourtis AP, et al. Recent biomarker-confirmed unprotected vaginal sex, but not self-reported unprotected sex, is associated with recurrent bacterial vaginosis. Sex Transm Dis. (2016) 43(3):172–6. 10.1097/OLQ.0000000000000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. (2012) 4(132):132ra52. 10.1126/scitranslmed.3003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mngomezulu K, Mzobe GF, Mtshali A, Osman F, Liebenberg LJP, Garrett N, et al. Recent semen exposure impacts the cytokine response and bacterial vaginosis in women. Front Immunol. (2021) 12:695201. 10.3389/fimmu.2021.695201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta SD, Zhao D, Green SJ, Agingu W, Otieno F, Bhaumik R, et al. The microbiome composition of a man’s penis predicts incident bacterial vaginosis in his female sex partner with high accuracy. Front Cell Infect Microbiol. (2020) 10:433. 10.3389/fcimb.2020.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CM, Prodger JL, Tobian AAR, Abraham AG, Kigozi G, Hungate BA, et al. Penile anaerobic dysbiosis as a risk factor for HIV infection. mBio. (2017) 8(4):e00996–17. 10.1128/mbio.00996-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas-Robles D, Morales N, Rodriguez I, Nieves T, Godoy-Vitorino F, Alcaraz LD, et al. Changes in the vaginal microbiota across a gradient of urbanization. Sci Rep. (2020) 10(1):12487. 10.1038/s41598-020-69111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marconi C, El-Zein M, Ravel J, Ma B, Lima MD, Carvalho NS, et al. Characterization of the vaginal microbiome in women of reproductive age from 5 regions in Brazil. Sex Transm Dis. (2020) 47(8):562–9. 10.1097/OLQ.0000000000001204 [DOI] [PubMed] [Google Scholar]
- 46.Alcendor DJ. Evaluation of health disparity in bacterial vaginosis and the implications for HIV-1 acquisition in African American women. Am J Reprod Immunol. (2016) 76(2):99–107. 10.1111/aji.12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fettweis JM, Brooks JP, Serrano MG, Sheth NU, Girerd PH, Edwards DJ, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading). 2014;160(Pt 10):2272–82. 10.1099/mic.0.081034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin DH, Marrazzo JM. The vaginal microbiome: current understanding and future directions. J Infect Dis. (2016) 214(Suppl 1):S36–41. 10.1093/infdis/jiw184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe. (2016) 19(5):731–43. 10.1016/j.chom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. (2014) 159(4):789–99. 10.1016/j.cell.2014.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim MY, You HJ, Yoon HS, Kwon B, Lee JY, Lee S, et al. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut. (2017) 66(6):1031–8. 10.1136/gutjnl-2015-311326 [DOI] [PubMed] [Google Scholar]
- 52.Xie H, Guo R, Zhong H, Feng Q, Lan Z, Qin B, et al. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. (2016) 3(6):572–84.e3. 10.1016/j.cels.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587(Pt 17):4153–8. 10.1113/jphysiol.2009.174136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bubier JA, Chesler EJ, Weinstock GM. Host genetic control of gut microbiome composition. Mamm Genome. (2021) 32(4):263–81. 10.1007/s00335-021-09884-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JE, Lee S, Lee H, Song YM, Lee K, Han MJ, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One. (2013) 8(5):e63514. 10.1371/journal.pone.0063514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright ML, Fettweis JM, Eaves LJ, Silberg JL, Neale MC, Serrano MG, et al. Vaginal microbiome Lactobacillus crispatus is heritable among European American women. Commun Biol. (2021) 4(1):872. 10.1038/s42003-021-02394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polderman TJ, Benyamin B, de Leeuw CA, Sullivan PF, van Bochoven A, Visscher PM, et al. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. (2015) 47(7):702–9. 10.1038/ng.3285 [DOI] [PubMed] [Google Scholar]
- 58.Bloom SM, Mafunda NA, Woolston BM, Hayward MR, Frempong JF, Abai AB, et al. Cysteine dependence of Lactobacillus iners is a potential therapeutic target for vaginal microbiota modulation. Nat Microbiol. (2022) 7(3):434–50. 10.1038/s41564-022-01070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lithgow KV, Cochimamogulos A, Muirhead K, Konschuh S, Oluoch LM, Mugo NR, et al. Resolving glycogen and related enzymes reveals correlates of Lactobacillus crispatus dominance in a cohort of young African women. Preprint. Research Square (2022).
- 60.Jenkins DJ, Woolston BM, Hood-Pishchany MI, Pelayo P, Konopaski AN, Quinn Peters M, et al. Bacterial amylases enable glycogen degradation by the vaginal microbiome. Nat Microbiol. (2023) 8(9):1641–52. 10.1038/s41564-023-01447-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hertzberger R, May A, Kramer G, van Vondelen I, Molenaar D, Kort R. Genetic elements orchestrating Lactobacillus crispatus glycogen metabolism in the vagina. Int J Mol Sci. (2022) 23(10):5590. 10.3390/ijms23105590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. (1983) 74(1):14–22. 10.1016/0002-9343(83)91112-9 [DOI] [PubMed] [Google Scholar]
- 63.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. (1991) 29(2):297–301. 10.1128/jcm.29.2.297-301.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muzny CA, Schwebke JR. Asymptomatic bacterial vaginosis: to treat or not to treat? Curr Infect Dis Rep. (2020) 22(12):32. 10.1007/s11908-020-00740-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barnabas SL, Dabee S, Passmore JS, Jaspan HB, Lewis DA, Jaumdally SZ, et al. Converging epidemics of sexually transmitted infections and bacterial vaginosis in Southern African female adolescents at risk of HIV. Int J STD AIDS. (2018) 29(6):531–9. 10.1177/0956462417740487 [DOI] [PubMed] [Google Scholar]
- 66.Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis. (2012) 206(1):6–14. 10.1093/infdis/jis298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marrazzo JM. Vaginal biofilms and bacterial vaginosis: of mice and women. J Infect Dis. (2013) 207(10):1481–3. 10.1093/infdis/jit050 [DOI] [PubMed] [Google Scholar]
- 68.Armstrong E, Hemmerling A, Miller S, Burke KE, Newmann SJ, Morris SR, et al. Metronidazole treatment rapidly reduces genital inflammation through effects on bacterial vaginosis-associated bacteria rather than lactobacilli. J Clin Invest. (2022) 132(6):e152930. 10.1172/JCI152930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mtshali A, San JE, Osman F, Garrett N, Balle C, Giandhari J, et al. Temporal changes in vaginal Microbiota and genital tract cytokines among South African women treated for bacterial vaginosis. Front Immunol. (2021) 12:730986. 10.3389/fimmu.2021.730986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serebrenik J, Wang T, Hunte R, Srinivasan S, McWalters J, Tharp GK, et al. Differences in vaginal Microbiota, host transcriptome, and proteins in women with bacterial vaginosis are associated with metronidazole treatment response. J Infect Dis. (2021) 224(12):2094–104. 10.1093/infdis/jiab266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gustin AT, Thurman AR, Chandra N, Schifanella L, Alcaide M, Fichorova R, et al. Recurrent bacterial vaginosis following metronidazole treatment is associated with microbiota richness at diagnosis. Am J Obstet Gynecol. (2022) 226(2):225.e1–e15. 10.1016/j.ajog.2021.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muzny CA, Sobel JD. The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment. Antibiotics (Basel). (2022) 11(4):500. 10.3390/antibiotics11040500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arroyo-Moreno S, Cummings M, Corcoran DB, Coffey A, McCarthy RR. Identification and characterization of novel endolysins targeting Gardnerella vaginalis biofilms to treat bacterial vaginosis. NPJ Biofilms Microbiomes. (2022) 8(1):29. 10.1038/s41522-022-00285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment: 2018 update. Curr Opin Infect Dis. (2019) 32(1):38–42. 10.1097/QCO.0000000000000516 [DOI] [PubMed] [Google Scholar]
- 75.Bradshaw CS, Morton AN, Hocking J, Garland SM, Morris MB, Moss LM, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. (2006) 193(11):1478–86. 10.1086/503780 [DOI] [PubMed] [Google Scholar]
- 76.Javed A, Parvaiz F, Manzoor S. Bacterial vaginosis: an insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb Pathog. (2019) 127:21–30. 10.1016/j.micpath.2018.11.046 [DOI] [PubMed] [Google Scholar]
- 77.Schuyler JA, Mordechai E, Adelson ME, Sobel JD, Gygax SE, Hilbert DW. Identification of intrinsically metronidazole-resistant clades of Gardnerella vaginalis. Diagn Microbiol Infect Dis. (2016) 84(1):1–3. 10.1016/j.diagmicrobio.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 78.Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, Fidel PL, Jr, Martin DH. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis. 2004;4:5. 10.1186/1471-2334-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bannatyne RM, Smith AM. Recurrent bacterial vaginosis and metronidazole resistance in Gardnerella vaginalis. Sex Transm Infect. (1998) 74(6):455–6. [PubMed] [Google Scholar]
- 80.Petrina MAB, Cosentino LA, Rabe LK, Hillier SL. Susceptibility of bacterial vaginosis (BV)-associated bacteria to secnidazole compared to metronidazole, tinidazole and clindamycin. Anaerobe. (2017) 47:115–9. 10.1016/j.anaerobe.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu M, Frank MW, Radka CD, Jeanfavre S, Tse MW, Pacheco JA, Pierce K, et al. Vaginal Lactobacillus fatty acid response mechanisms reveal a novel strategy for bacterial vaginosis treatment. Preprint ed (2023). [DOI] [PMC free article] [PubMed]
- 82.Zhao X, Boyd P, Dallal Bashi YH, McCoy CF, Karl Malcolm R. Physicochemical considerations in the formulation development of silicone elastomer vaginal rings releasing 5-nitroimidazole drugs for the treatment of bacterial vaginosis. Int J Pharm. (2023) 644:123296. 10.1016/j.ijpharm.2023.123296 [DOI] [PubMed] [Google Scholar]
- 83.Zhao X, Boyd P, Bashi YD, Murphy DJ, McCoy CF, Coulter S, et al. Two into one does go: formulation development of a multipurpose combination vaginal ring releasing dapivirine and metronidazole for prevention of HIV infection and treatment of bacterial vaginosis. Int J Pharm. (2023) 648:123572. 10.1016/j.ijpharm.2023.123572 [DOI] [PubMed] [Google Scholar]
- 84.Landlinger C, Oberbauer V, Podpera Tisakova L, Schwebs T, Berdaguer R, Van Simaey L, et al. Preclinical data on the Gardnerella-specific endolysin PM-477 indicate its potential to improve the treatment of bacterial vaginosis through enhanced biofilm removal and avoidance of resistance. Antimicrob Agents Chemother. (2022) 66(5):e0231921. 10.1128/aac.02319-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. (2008) 3(4):213–23. 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. (2013) 341(6150):1241214. 10.1126/science.1241214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang J, Fu X, Liao X, Li Y. Effects of gut microbial-based treatments on gut microbiota, behavioral symptoms, and gastrointestinal symptoms in children with autism spectrum disorder: a systematic review. Psychiatry Res. (2020) 293:113471. 10.1016/j.psychres.2020.113471 [DOI] [PubMed] [Google Scholar]
- 88.Engen PA, Zaferiou A, Rasmussen H, Naqib A, Green SJ, Fogg LF, et al. Single-arm, non-randomized, time series, single-subject study of fecal Microbiota transplantation in multiple sclerosis. Front Neurol. (2020) 11:978. 10.3389/fneur.2020.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu ND, Crothers JW, Nguyen LTT, Kearney SM, Smith MB, Kassam Z, et al. Dynamic colonization of microbes and their functions after fecal microbiota transplantation for inflammatory bowel disease. mBio. (2021) 12(4):e0097521. 10.1128/mBio.00975-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gopalakrishnan V, Dozier EA, Glover MS, Novick S, Ford M, Morehouse C, et al. Engraftment of Bacteria after fecal microbiota transplantation is dependent on both frequency of dosing and duration of preparative antibiotic regimen. Microorganisms. (2021) 9(7):1399. 10.3390/microorganisms9071399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The super-donor phenomenon in fecal microbiota transplantation. Front Cell Infect Microbiol. (2019) 9:2. 10.3389/fcimb.2019.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma D, Chen Y, Chen T. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol Lett. (2019) 366(4):fnz025. 10.1093/femsle/fnz025 [DOI] [PubMed] [Google Scholar]
- 93.Vodstrcil LA, Walker SM, Hocking JS, Law M, Forcey DS, Fehler G, et al. Incident bacterial vaginosis (BV) in women who have sex with women is associated with behaviors that suggest sexual transmission of BV. Clin Infect Dis. (2015) 60(7):1042–53. 10.1093/cid/ciu1130 [DOI] [PubMed] [Google Scholar]
- 94.Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. (2019) 25(10):1500–4. 10.1038/s41591-019-0600-6 [DOI] [PubMed] [Google Scholar]
- 95.Yockey LJ, Hussain FA, Bergerat A, Reissis A, Worrall D, Xu J, et al. Screening and characterization of vaginal fluid donations for vaginal microbiota transplantation. Sci Rep. (2022) 12(1):17948. 10.1038/s41598-022-22873-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu S, Hugerth LW, Schuppe-Koistinen I, Du J. The right bug in the right place: opportunities for bacterial vaginosis treatment. NPJ Biofilms Microbiomes. (2022) 8(1):34. 10.1038/s41522-022-00295-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Q, Zhang L, Ross P, Zhao J, Zhang H, Chen W. Comparative genomics of Lactobacillus crispatus from the gut and vagina reveals genetic diversity and lifestyle adaptation. Genes (Basel). (2020) 11(4):360. 10.3390/genes11040360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, Perez-Munoz ME, et al. Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev. (2017) 41(Supp_1):S27–48. 10.1093/femsre/fux030 [DOI] [PubMed] [Google Scholar]
- 99.Cohen CR, Wierzbicki MR, French AL, Morris S, Newmann S, Reno H, et al. Randomized trial of lactin-V to prevent recurrence of bacterial vaginosis. N Engl J Med. (2020) 382(20):1906–15. 10.1056/NEJMoa1915254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ngugi BM, Hemmerling A, Bukusi EA, Kikuvi G, Gikunju J, Shiboski S, et al. Effects of bacterial vaginosis-associated bacteria and sexual intercourse on vaginal colonization with the probiotic Lactobacillus crispatus CTV-05. Sex Transm Dis. (2011) 38(11):1020–7. 10.1097/OLQ.0b013e3182267ac4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hemmerling A, Wierzbicki MR, Armstrong E, Cohen C. Response to antibiotic treatment of bacterial vaginosis predicts the effectiveness of LACTIN-V (Lactobacillus crispatus CTV-05) in the prevention of recurrent disease. MedRxiv (2023). [DOI] [PMC free article] [PubMed]
- 102.Armstrong E, Hemmerling A, Joag V, Huibner S, Kulikova M, Crawford E, et al. Treatment success following standard antibiotic treatment for bacterial vaginosis is not associated with pretreatment genital immune or microbial parameters. Open Forum Infect Dis. (2023) 10(1):ofad007. 10.1093/ofid/ofad007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.