ABSTRACT
Immunogenic cell death (ICD) is a distinct type of stress-induced regulated cell death that can lead to adaptive immune responses and the establishment of immunological memory. ICD exhibits both similarities and differences when compared to apoptosis and other non-apoptotic forms of regulated cell death (RCD). The interplay between ICD-mediated immunosurveillance against cancer and the ability of cancer cells to evade ICD influences the host-tumor immunological interaction. Consequently, the restoration of ICD and the development of effective strategies to induce ICD have emerged as crucial considerations in the treatment of cancer within the context of immunotherapy. To enhance comprehension of ICD in the setting of cancer, this paper examines the interconnected responsive pathways associated with ICD, the corresponding biomarkers indicative of ICD, and the mechanisms through which tumors subvert ICD. Additionally, this review explores strategies for reinstating ICD and the therapeutic potential of harnessing ICD in cancer immunotherapy.
KEYWORDS: Cancer, immunogenic cell death, cell stress, immunotherapy, nanoparticle
Introduction
The demise of cells through various crucial cell death pathways facilitates physiological homeostasis in both normal and stress-challenged conditions.1 The loss of control over single or mixed types of cell death in response to different stresses contributes to the turnover process in the context of the tumor microenvironment.2,3 Regulate cell death (RCD) can be classified as either immunogenic RCD or non-immunogenic RCD, depending on its capacity to elicit an adaptive immune response. Immunogenic RCD, including necroptosis, ferroptosis, pyroptosis, and cuproptosis, has been identified as playing pivotal roles in modulating the immunosuppressive tumor microenvironment (TME) and influencing the clinical outcomes of cancer therapeutic strategies via tuning tumor immunity.4–6
To elucidate the fundamental determinants governing the capacity of dying cells to elicit an adaptive immune response and foster the development of enduring immunological memory, the mechanisms endowing dying cells with antigenicity and adjuvanticity have been examined.7,8 Furthermore, surrogate biomarkers, including soluble DAMPs and cytokines associated with ICD, have played a pivotal role in delineating the principal molecular participants and identifying potential ICD inducers through extensive screening endeavors.9 Nevertheless, the evaluation of ICD necessitates empirical validation from a variety of in vitro and in vivo assays to certify the ability of malignant cells undergoing ICD to recruit antigen processing cells (APCs) and initiate adaptive anti-cancer immunity.10
In contrast, the microenvironment surrounding developing tumors can hinder the initiation or execution of ICD through various mechanisms.11–13 To address this deficiency, several strategies have been suggested to counteract the compromising effect of cancer on the ability of RCD to stimulate adaptive immunity. In this review, we have examined the immunosuppressive factors within the tumor microenvironment (TME) that impede ICD and proposed corresponding strategies to enhance its efficacy. Furthermore, we have highlighted the significance of ICD-based immunotherapy, as well as nanoparticle-based ICD, as prominent therapeutic approaches for activating the immune system against cancer, which in turn determines the long-term success of anticancer therapies.14–16
Immunogenic RCD and ICD
Cells, including tumor cells, can undergo various forms of death in response to different stresses, facilitating the elimination of unwanted cells by the body. According to the Nomenclature Committee on Cell Death (NCCD), cell death can be categorized into accidental cell death (ACD) and RCD based on functional characteristics.17 ACD is an uncontrolled process of cell death that occurs in response to an unexpected attack or injury. Conversely, RCD denotes a genetically encoded molecular-controlled form of autonomous and orderly cellular demise.18 According to the ability to drive antigen-specific adaptive immune response culminating in immunological memory or not, RCD can be further categorized as ICD or non-immunogenic cell death.
Apoptosis
Apoptosis, an early recognized form of RCD, was traditionally believed to be immunologically silent or even tolerogenic. During apoptosis, cells exhibit cytoplasmic membrane blebbing, nucleus condensation, fragmentation of cellular organelles and DNA, and the formation of apoptotic bodies that encapsulate the ruptured nucleus and cell debris.19 However, late apoptotic cells are ultimately engulfed by phagocytic cells of the innate immune system, without releasing pro-inflammatory cellular contents into the extracellular environment. However, in the presence of prolonged and severe endoplasmic reticulum stress (ERS), including factors such as oxidative stress, ischemia, hypoxia, disruption of calcium homeostasis, and viral infection, the immunogenicity of apoptosis can be attained.20,21 In such circumstances, apoptosis and ICD exhibit a mutually reinforcing association. Consequently, a significant area of interest in cancer therapy revolves around the induction of cancer cell-specific apoptosis within the tumor microenvironment (TME), while also ensuring its immunogenicity.22,23
Immunogenic RCD
In addition to apoptosis, a series of non-apoptotic RCD mechanisms have been gradually discovered in recent years. These include necroptosis, ferroptosis, pyroptosis, cuproptosis, PANoptosis, entosis, PARthanatos, alkaliptosis, oxeiptosis, lysosome-dependent cell death.24,25 Some of these mechanisms have also been found to have immunogenic potential and are connected to tumor immunity, promoting the enrichment of either anti-tumor effector immune cells or regulatory immune cells, ultimately leading to either tumor regression or progression, as shown in Figure 1. However, the ability of immunogenic RCD to induce adaptive immunity relies on two main factors: antigenicity and adjuvanticity, both of which are not inherently intrinsic to dying cells.
Figure 1.
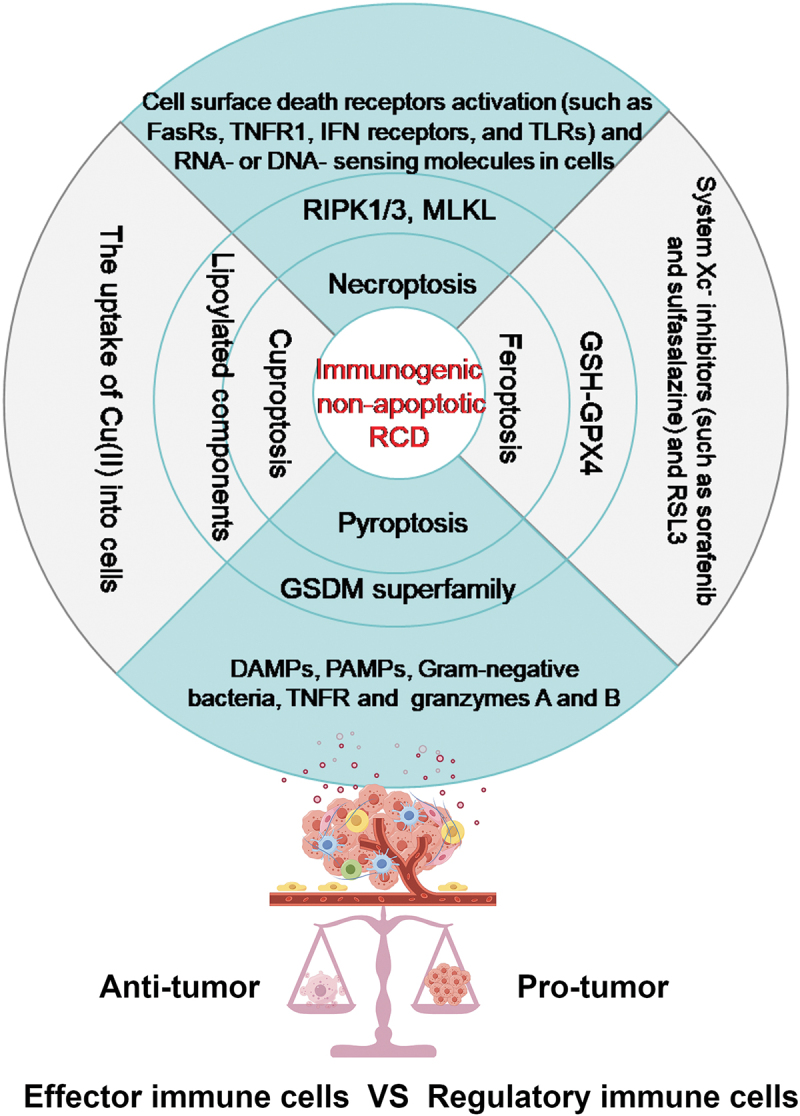
The core mechanism underlying four distinct forms of immunogenic non-apoptotic RCD. Within the context of various extracellular stresses and intracellular signaling pathways, cancer cells have the potential to undergo a specific type of cell death that is regulated by a specific set of genes and signaling molecules. These four types of RCD, namely necroptosis, pyroptosis, ferroptosis, and cuproptosis, represent typical immunological processes within the microenvironment(tme). These processes play a crucial role in balancing the TME by promoting the enrichment of either anti-tumor effector immune cells or regulatory immune cells, ultimately leading to either tumor regression or progression.
Immunogenic RCD plays a collaborative role in modulating the tumor microenvironment (TME).5,26,27 The four potentially novel mechanisms of immunogenic cell death, namely necroptosis, ferroptosis, pyroptosis, and cuproptosis, have been confirmed to exhibit reciprocal interaction between tumor cell death and the activation of antitumor immunity.3,28 Necroptosis, for instance, has been extensively studied concerning various stimuli, including the activation of death receptors (e.g., Fas and TNFRA), toll-like receptors (e.g., toll-like receptors 3 and 4), as well as RNA- and DNA-sensors (e.g., Z DNA-binding protein 1 [ZBP1], retinoic acid receptor responder 3 [RIG1], transmembrane protein 173 [TMEM173, also known as STING]).29 Mechanically, RIPK3-stimulated MLKL is necessary for membrane rupture formation in necroptosis;30 Pyroptosis is primarily induced by the cleavage of gasdermin D (GSDMD) by CASP1 and CASP11 in response to pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), or cytosolic lipopolysaccharide (LPS);31 Ferroptosis is typically initiated by the excessive accumulation of intracellular reactive oxygen species (ROS) following lipid peroxidation-induced destruction of cellular membranes in an iron-dependent manner.32 Enhanced susceptibility to ferroptosis is correlated with reduced expression of GPX4, which can be induced directly through the binding of compounds such as RSL3, or indirectly through the inhibition of the cystine/glutamate antiporter(system xc-);33 Cuproptosis is a recently discovered RCD that is linked to the immunogenicity of the tumor microenvironment. The underlying mechanism involves the transportation of excess Cu(II) from cells to mitochondria through ionophores. Within the mitochondria, the enzyme ferredoxin 1 (FDX1) reduces Cu(II) to Cu(I). The increased amount of Cu(I) directly binds to lipoylated components (like DLAT) of the tricarboxylic acid (TCA) cycle, leading to lipoylated proteins aggregation and destabilization of Fe – S cluster proteins, eventually, cell death.34
Detection of ICD
To distinguish the immunogenicity of certain variants of RCD from ICD, standardized experimental assays have been developed and guidelines for interpreting ICD have been established.10,35 Various cellular stressors have been linked to ICD in immunocompetent syngeneic hosts, some of which are commonly used in the therapy of cancer patients.36,37 Mechanistically, a crucial characteristic of molecules that induce ICD is their ability to render dying cells antigenicity and adjuvanticity through stress-responsive pathways, such as endoplasmic reticulum (ER) stress, and transcriptional/translational stress. The common inducers of ICD and the associated cellular stress related to antigenicity or adjuvanticity have been discussed, as shown in Figure 2.
Figure 2.
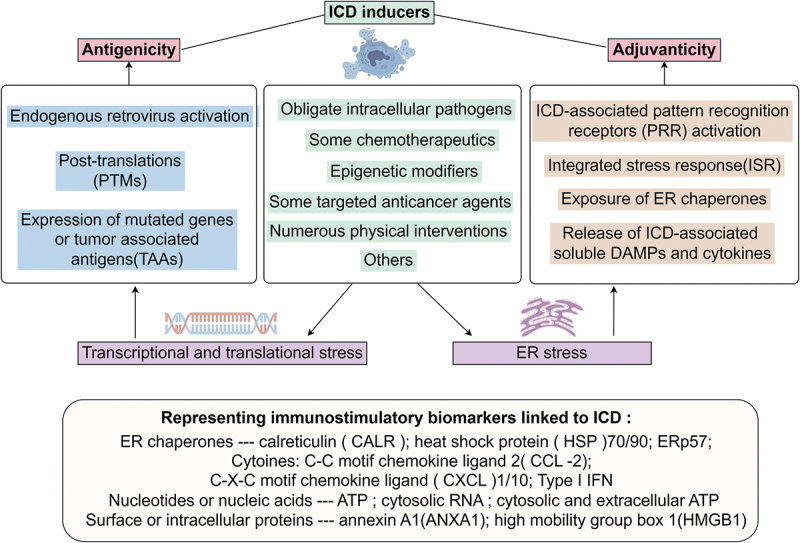
The core mechanisms of ICD involve the induction of transcriptional/translational stress or ER stress in dying cells by ICD inducers. These dying cells are then endowed with antigenicity or adjuvanticity through various approaches. Several immunostimulatory DAMPs and cytokines released during ICD have been identified as biomarkers of this process.
Antigenicity from ICD
Antigenicity is attributed to the expression and presentation of antigens that do not induce clonal deletion within the framework of central or peripheral tolerance in their basal state.38 Transcriptional and translational stress seem to be particularly effective in generating potential neoantigens.39 While there are at least three approaches to confer sources of ICD antigenicity, it is generally believed that the majority of ICD inducers have minimal impact on antigenicity. Firstly, it has been observed that latent endogenous retroviruses and/or retroviral genes can be activated in response to certain ICD stressors, leading to the production of potentially antigenic proteins.40 Secondly, the antigenic peptide repertoire can be enhanced through enzymatic or non-enzymatic post-translational modifications (PTMs) that modify the structure of proteins. These modifications include but are not limited to, phosphorylation, acetylation, glycosylation, citrullination, nitration/nitrosylation, glycation, oxidation, and ubiquitination.41 Lastly, the accumulation of mutations and the landscape of tumor neoantigens evolve in response to increased genetic stress, under the pressure of ICD stressors, such as chemotherapeutic or radiotherapeutic interventions.42,43
Adjuvanticity from ICD
Multiple mechanisms contribute to the adjuvanticity of ICD, which plays a significant role in the initiation of adaptive immunity. These mechanisms include: (1) ICD-associated pattern recognition receptors (PRRs) activation, encompass numerous Toll-like receptors (TLRs), cyclic GMP-AMP synthase (CGAS), RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), Z-DNA binding protein 1 (ZBP1), and heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1);44 (2)Integrated stress response(ISR) activation, which is a part of the ER stress response, ultimately stimulate the phosphorylation of eukaryotic translation initiation factor 2 subunit alpha(eIF2α) and the upregulation of activating transcription factor 4 (ATF4);45 (3)activation of autophagy, a cytoprotective mechanism related with ISR. However, the ultimate impact of autophagy on damage-associated molecular patterns (DAMPs) emission by ICD exhibits considerable context dependency. For example, autophagy limits the exposure of calreticulin(CALR) by cancer cells undergoing photodynamic therapy (PDT)-driven ICD, but optimizes ATP release in the course of chemotherapy-driven ICD;7,46 (4)release of immunostimulatory DAMPs and cytokines, most of them have been recognized as the biomarkers of ICD, which enable the recruitment of antigen processing cells (APCs) or their precursors to sites of ICD (eg, ATP), spatially guide the interaction between APCs and dying cells (eg, ANXA1), favor the phagocytosis of dying cells or their corpses (eg, CALR, ERp57, HSP70/90), promote the maturation and cross-presentation of APCs (eg, ATP, HMGB1, type I IFN and TFAM), or facilitate the recruitment of T cells (eg, CCL2, CXCL1 and CXCL10).8,47
Interpretation of ICD
It is important to note that not all inducers of ICD operate through the same molecular mechanisms, and the release of damage-associated molecular patterns (DAMPs) associated with ICD is not always sufficient for antigen-presenting cells (APCs) to initiate cytotoxic T lymphocyte (CTL)-dependent immune responses against dying cells.48,49 Therefore, it is necessary to conduct gold-standard vaccination and therapeutic assays in vivo to confirm genuine ICD inducers. In the vaccination assay, malignant cells that have undergone cell death in vitro due to a potential ICD inducer are utilized as a vaccine, either in their original form or loaded onto immature, syngeneic dendritic cells (DCs). The ability of mice to reject or control tumor growth reflects the level of immunogenicity.35,50 Specificity is confirmed by re-challenging tumor-free mice with another syngeneic cancer cell line at the conclusion of the experiment, which is anticipated to result in the development of palpable neoplastic lesions in 100% of the mice. In the therapeutic assay, grafted tumors that are either genetically-driven or chemically-induced are established in subcutaneous or orthotopic locations and subsequently treated with a putative inducer of ICD in both immunocompetent and immunodeficient mice. In this experimental configuration, genuine ICD inducers demonstrate optimal antineoplastic effects in immunocompetent mice, but not in immunodeficient mice.50,51 Importantly, the therapeutic assay is of significant importance in validating the outcomes of vaccination experiments. However, it is insufficient on its own to distinguish between the induction of ICD and immunostimulation unrelated to ICD.
Tumor subversion of ICD and corresponding restoring strategies
Malignant cells have devised various strategies to diminish the antigenicity and adjuvanticity of ICD, including direct inhibition of the essential components of the ICD-associated responsive apparatus. These mechanisms employed by cancerous cells enable them to evade the adaptive immune response triggered by ICD.52,53 Enhancing our comprehension of this process is expected to facilitate the clinical application of ICD.
Defects compromising the antigenicity and adjuvanticity in cancer
Numerous studies have documented the involvement of tumor subversion in the reduction of antigenicity, including loss of chromosome 6 and 15 (LOH), mutations in the beta-2-microglobulin (β2 M), and alterations in the IFN signaling pathway.54–56 These alterations significantly impact the synthesis of a functional major histocompatibility complex (MHC) class I exposure. Therefore, it is imperative to restore MHC class I defects in tumor cells to ensure efficient antigenicity of ICD.
Additionally, malignant cells can interfere with the release of ICD-associated damage-associated molecular patterns (DAMPs), such as Calreticulin (CALR), ATP, and annexin A1 (ANXA1), thereby subverting the adjuvanticity of ICD. For example, Certain malignant cells have the ability to manipulate CALR signaling through the internal retention of CALR upon interaction with stanniocalcin 1 (STC1) or by limiting CALR binding sites on the cell surface.57 Additionally, these malignant cells can evade the release of ATP associated with ICD by upregulating or promoting the upregulation of two ectonucleotidases, CD39 and CD73, which sequentially convert extracellular ATP into adenosine.58,59 Furthermore, studies have demonstrated that some malignant cells reduce the expression of ANXA1, thereby impacting the ability of cancer cells to expose CALR in response to ICD inducers.60
TME factors influencing ICD and corresponding enhancing strategies
In addition to the aforementioned factors originating from malignant cells, the microenvironment surrounding dying cancer cells significantly influences their capacity to induce adaptive immunity at the microenvironmental level, even when there is an adequate presence of antigens and adjuvants. The TME factors influencing ICD and corresponding enhancing strategies have been studied extensively, as shown in Figure 3.
Figure 3.
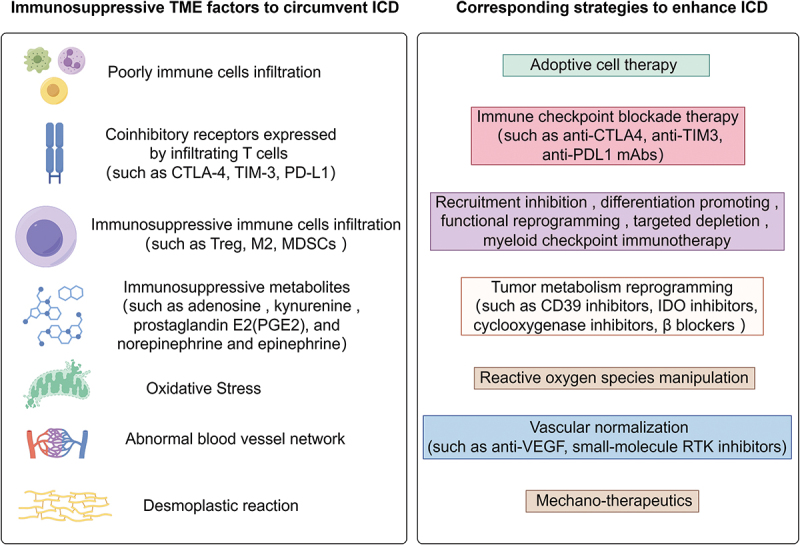
The TME factors influence the capacity of ICD to induce adaptive immunity at the microenvironmental level, along with corresponding strategies aimed at enhancing ICD for improved immunotherapy.
Firstly, The presence, activation, and costimulation of tumor-infiltrating lymphocytes (TILs), such as CD8+ T cells, CD4+ T cells, B cells, and innate lymphoid cells, are essential for a successful immune response against tumors and are correlated with favorable prognoses in various types of tumors.61,62 However, in tumors characterized as immune-desert or immune-excluded (“cold” tumors), tumor-infiltrating lymphocytes (TILs) are either absent or fail to efficiently penetrate the tumor, thereby impeding the induction of adaptive immunity through ICD. The utilization of adoptive cell therapy (ACT) involving TILs has been extensively studied in various solid tumors, revealing sustained responses by enhancing T-cell infiltration, even in patients resistant to immune checkpoint blockade (ICB).63,64 Nonetheless, in immune-inflamed tumors (“hot” tumors), the issue of T-cell exhaustion continues to pose a challenge to effective anti-cancer immune responses.65 Immune checkpoint blockade therapy targeting coinhibitory receptors expressed by infiltrating T cells, including CTLA-4, TIM-3, and PD-L1, has been shown to enhance the effectiveness of ICD.66,67
Secondly, immunosuppressive immune cells, such as myeloid-derived suppressor cells (MDSC), M2-like macrophages (M2), and regulatory T cells (Treg), play a crucial role in suppressing antitumor immune responses in cancer immunity.68,69 However, the efficacy of systemic cell depletion in promoting antitumor immunity is not always guaranteed, as these cells possess a wide range of functions and the tumor microenvironment is highly complex. Consequently, the utilization of various therapeutic approaches, currently being evaluated in clinical settings, encompasses diverse strategies such as targeting recruitment and differentiation, as well as engaging activating or inhibitory receptors (checkpoint receptors) to reprogram the functionality of immunosuppressive immune cells.70,71 Overall, these approaches are currently not considered independent strategies, but myeloid checkpoint therapy has demonstrated promising outcomes. Notably, the pharmaceutical inhibition of c-Rel, a myeloid checkpoint in MDSCs, has exhibited significant inhibition of cancer growth in mice.72
Thirdly, cancer cells possess the ability to suppress immune cells by generating immunosuppressive metabolic byproducts such as adenosine, kynurenine, prostaglandin E2 (PGE2), as well as norepinephrine and epinephrine.73,74 The synthesis and signaling pathways of these potent immunosuppressive metabolites exhibit inherent redundancy. This redundancy has posed a significant challenge in the development of effective pharmacological interventions against these metabolites. Consequently, the efficacy of inhibitors targeting only one metabolite is limited due to compensatory metabolic pathways. Currently, clinical trials are underway to evaluate the effectiveness of “pan-antagonists” for each subclass of the “metabolic immunosuppressive receptor,” including A2A/2 BAR, AhR, EP2/EP4, and β-adrenergic receptors. Additionally, broad inhibitors of kynurenine (IDO1/TDO or IDO1/IDO) and PGE2 (COX-1 and COX-2) are being tested.75,76
Fourthly, The intricate TME is characterized by a low pH, elevated redox status, and hypoxia, which have been linked to the suppression of immunotherapy.77 Reactive oxygen species (ROS) have been extensively investigated in the context of cancer and exhibit a dual nature. At lower to moderate concentrations, ROS serve as signal transducers, stimulating cell proliferation, migration, invasion, and angiogenesis. In contrast, elevated levels of reactive oxygen species (ROS) have the potential to induce cellular demise.78 Under conditions of oxidative stress, tumor-infiltrating regulatory T cells (Treg cells) undergo programmed cell death, known as apoptosis, resulting in a more potent immunosuppressive effect compared to viable Treg cells.79 Furthermore, ROS can impede the effectiveness of anti-tumor immune responses by activating endoplasmic reticulum (ER) stress-XBP1 signaling in dendritic cells (DCs).80 These collective findings suggest that heightened ROS levels represent a contributing mechanism underlying the resistance to immunotherapies, including the induction of anti-tumor immunity through ICD.
Fifthly, The tumor vasculature consists mainly of hypoxia-induced vessels that are distorted, malformed, and leaky, resulting in inefficiency. This abnormal vasculature has implications for the trafficking and accumulation of CD4+ and CD8+ cells within the tumor.81 Researchers have investigated vascular normalization therapy, including the use of anti-VEGF and small-molecule RTK inhibitors, to induce the development of high endothelial cells in the vasculature (HEVs).82,83 This may facilitate lymphocyte trafficking into the tumor and potentially enhance the effects of immunotherapy by promoting the formation of tertiary lymphoid structures.
Sixly, The desmoplastic reaction to a tumor refers to the proliferation of fibrous connective tissue surrounding tumor cells, which is closely linked to the infiltration of immune cells within the tumor. Several studies have shown that an immature desmoplastic reaction and the presence of myxoid stroma are associated with lower densities of tumor intraepithelial memory cytotoxic T cells and stromal M1-like macrophages.84,85 Additionally, the presence of a dense extracellular matrix (ECM) and fibrous stroma is also associated with the recruitment of immunosuppressive cells, such as Tregs, which hinder the effectiveness of antitumor immunity.86 The manipulation of mechanotransduction pathways in myofibroblasts has demonstrated the potential to mitigate organ fibrosis and reduce tumor burden in experimental models. The implementation of mechano-therapeutics, such as inducing a quiescent phenotype in myofibroblasts, promoting myofibroblast apoptosis, or inhibiting pro-fibrotic gene expression programs and TGF-β1 activation, could serve as innovative therapeutic interventions to enhance the efficacy of immunotherapy.87,88
ICD-based immunotherapy
In light of an enhanced comprehension of ICD within the context of cancer, ICD-based immunotherapy has emerged as a compelling option for integrating cancer immunotherapy combination regimens in clinical settings. These regimens encompass chemo-immunotherapy, radio-immunotherapy, photo-immunotherapy, and cancer vaccines. The process of ICD-based immunotherapeutic approaches in activating anti-tumor immunity is shown schematically in Figure 4.
Figure 4.
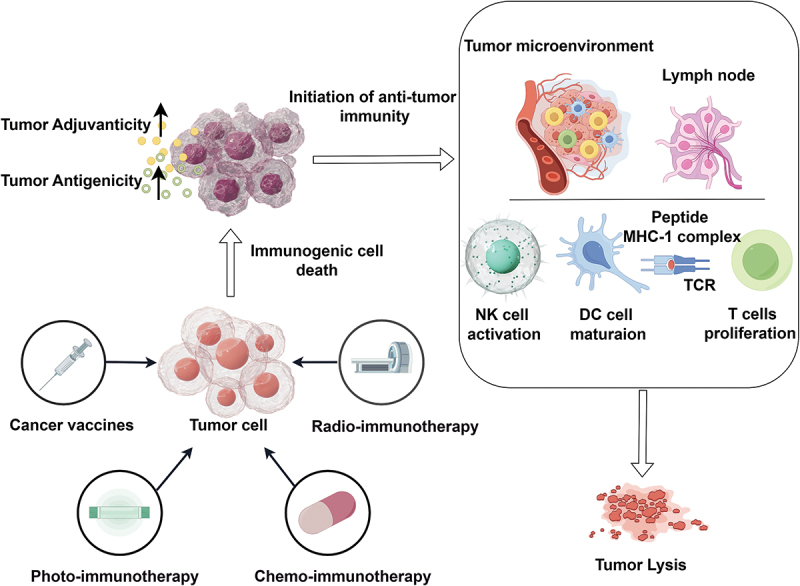
The process of icd-based immunotherapeutic approaches in activating anti-tumor immunity. Chemotherapy, radiotherapy, phototherapy, and cancer vaccine can induce the ICD of cancer by upregulating the antigenicity and adjuvanticity of cancer, then initiate the anti-tumor immunity, including NK cell activation, DC cell maturation, and T cell proliferation.
ICD-based chemo-immunotherapy
Several traditional chemotherapeutic drugs, including anthracyclines, taxanes, cyclophosphamide, bortezomib, crizotinib, and oxaliplatin, have been identified as genuine inducers of ICD. These drugs can trigger the immunogenic demise of tumor cells, leading to a CD8+T cell-mediated response against tumor antigens expressed by the dying cells.37,89 The combination of immune checkpoint inhibitors (ICIs) with ICD-inducing drugs has shown promising results in the treatment of various tumors, particularly in challenging-to-treat cancers, with minimal risks of overlapping toxicities among the individual drugs, although the optimal dose, timing, and sequence of chemo-immunotherapy combinations needed to be further explored.90,91
ICD-based radio-immunotherapy
Irradiated tumor cells experience ICD, which can stimulate a potent anti-tumor immune response. The integration of radiotherapy (RT) and immunotherapy is increasingly being utilized in routine clinical practice, despite limited high-quality evidence to inform clinical management.92 In addition to conventional photon RT (utilizing X-ray or gamma-ray beams), particle RT, such as proton RT, carbon-ion radiotherapy (CIRT), and boron neutron capture therapy (BNCT), also exerts a significant impact on tumor cells and various immune cells within the tumor microenvironment, resulting in the release of ICD biomarkers.93,94 However, the combination of particle RT with immunotherapy is still limited in clinical, because of costs and technique restrictions.
ICD-based photo-immunotherapy
Phototherapies employing suitable photoagents and light doses have been observed to elicit ICD within specific tumors, thereby generating tumor-associated antigens (TAAs) and damaged-associated molecular patterns (DAMPs) as potential sources.95 This phenomenon has the potential to instigate an inflammatory response. Notably, near-infrared (NIR) light has emerged as the predominant choice for phototherapy, owing to its ability to deeply penetrate biological tissues. This is often achieved through the administration of in situ or naturally occurring absorbance agents.96 Photosensitizers possess the ability to convert absorbed light energy into heat for photothermal effects, as observed in photothermal therapy (PTT), or into reactive oxygen species (ROS) for photochemical effects, as observed in photodynamic therapy (PDT).97 Notably, certain photosensitizers, including porphyrins, indocyanine green, methylene blue, and Rose Bengal, have been successfully employed in clinical settings for PDT. These photosensitizers exhibit robust optical absorption at therapeutic wavelengths, high photochemical conversion efficiency, and favorable biocompatibility. A combination of phototherapy and immunotherapy, particularly using immunostimulants, immune-targeting agents, and checkpoint inhibitors, can significantly induce antitumor immune responses.98,99
ICD-based cancer vaccine
The cancer vaccine has shown promise as an immunotherapy, but it continues to encounter obstacles, particularly in the identification of immunogenic neoepitopes on diverse cancer cells.39 The initiation of tumor-specific neoantigens through the process of ICD presents an opportunity for the development of endogenous cancer vaccines.100 A novel approach to in situ cancer vaccination involves utilizing the patient’s tumor antigens, which are produced by ICD inducers. The dendritic cells (DCs) recruited by ICD may also serve as a potential DC vaccine.101,102 Various strategies have been expanded upon to investigate the potential of neoantigen-based cancer vaccines, but a deeper investigation of their mechanisms and immune-related adverse events (irAE) need more attentions.103
Progress, obstacle, and future perspective in ICD-based nanoimmunotherapy in cancer
The efficacy and safety of traditional ICD inducers alone or combinational immunotherapy are constrained by various challenges.104 Firstly, the efficacy is hindered by inadequate targeting for solid tumors and an unfavorable tumor microenvironment for immunotherapeutics. Secondly, off-target adverse effects increase the likelihood of systemic toxicities and rates of immune-related adverse events (irAEs). To address these challenges, nanotechnology has emerged as an enhanced delivery technology for cancer immunotherapy utilizing ICD.14,105 The utilization of nanotechnology has the potential to enhance the concentration of immunotherapeutics within tumors, facilitating more precise targeting of desired tumor and immune cells, while also mitigating off-target adverse effects. Additionally, nanotechnology offers a distinctive approach to elicit enduring antitumor immune responses through sustained ICD mediation and concurrently remodeling the tolerogenic tumor immune microenvironment.106,107
ICD-based nanoimmunotherapy in cancer
Recent investigations have assessed the efficacy of novel inorganic and polymeric nanoparticles, including AuNPs, Metal-organic frameworks (MOFs), and micelles, as inducers of ICD.108 Nanotechnology presents a viable approach for inducing ICD and facilitating the targeted administration of ICD inducers, thereby enhancing the efficacy of conventional therapeutic strategies like chemotherapy, phototherapy, and radiotherapy.
In terms of chemotherapy, certain chemotherapeutic drugs that induce ICD have been incorporated into a nano-drug co-delivery system (NDCDS), which combines cytotoxicity and immunostimulatory properties.109 For instance, a prodrug of Cisplatin(IV) was conjugated to N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer (P-Cis) and coadministered with digoxin (Dig), which induces potent immunogenic cell death, leading to dendritic cell maturation and activation of CD8+ T cell responses.110 Poly(lactic-co-glycolic acid) nanoparticles (PLGA NPs) loaded with paclitaxel (PTX) and a Toll-like receptor 4 (TLR-4) agonist (SP-LPS) demonstrated enhanced in vivo antitumor activity and a higher proportion of activated immune cells in the TME than the Taxol-treated group.111
In terms of phototherapy, several inorganic nano-agents, including AuNPs, CuS NPs, GO, MoS2 nanosheets, and carbon nanotubes, possess inherent NIR light absorption capabilities.112,113 These nano-agents have been designed to deliver thermal energy and immunoadjuvants. For instance, Mao et al. conducted a study on near-infrared (NIR)-driven immunostimulants, where they combined upconversion nanoparticles with aggregation-induced emission luminogens (AIEgens).114 This combination resulted in the generation of high-dose reactive oxygen species (ROS) when exposed to high-power NIR irradiation, leading to enhanced immunogenic cell death and antigen release.
In terms of radiotherapy, the utilization of high-Z-element nanoparticles in radiotherapy has demonstrated the potential to improve the radiotherapeutic indices of localized tumors by minimizing radiation doses and adverse effects on healthy tissues. Additionally, these nanoparticles can stimulate the tumor microenvironment (TME) to elicit systemic antitumor immune responses, thereby augmenting abscopal effects.115,116 On the one hand, The utilization of inorganic nanoparticles (NPs) as radiosensitizers has been shown to enhance the absorption of ionizing radiation, including X-rays, photons, and gamma rays. On the other hand, Nanotechnology also offers the potential for nanovectorized ionizing radiation, which could effectively enhance the anti-tumor immune response associated with ICD following internal radiation.117 This approach can be tailored and designed to achieve predictable outcomes. For instance, Zhang et al. successfully designed a multifunctional nanoparticle (PIC) by employing a scalable and straightforward complexation method involving poly-l-lysine (PLL), CpG oligodeoxynucleotide (CpG), and iron oxide nanoparticles (ION). This nanoparticle formulation resulted in the activation of tumor-specific immunity and improved abscopal effects.118
Recently, Several intelligent nanoparticle-based platforms have been developed to induce potent ICD through various mechanisms of action, thereby enhancing antitumor immunity.119,120 For instance, nanoparticles capable of co-delivering ICD-inducing therapeutic drugs (such as OxP, DOX, and PTX) and photosensitizers (such as indocyanine green (ICG), Ce6, and pheophorbide A) have been designed to achieve a synergistic effect. Concurrently, the modulation of the immunosuppressive tumor immune microenvironment (TIME) through the use of agents such as indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors and anti-PD-L1 antibodies provides additional advantages by mitigating the immunosuppressive state and augmenting the effectiveness of cancer immunotherapy.121–123
Obstacles and future perspective of nanoimmunotherapy
In a similar vein, the effectiveness of nanoimmunotherapy is impeded by unfavorable tumor microenvironments characterized by limited tumor penetration due to elevated interstitial fluid pressure (IFP), dense extracellular matrix (ECM), transient tumor retention, and inadequate tumor cell uptake. To address these challenges, extensive endeavors have been undertaken to overcome these biological barriers. Primarily, the physiochemical attributes of nanomedicines play a significant role in tumor penetration, encompassing surface area, hydrodynamic diameter, shape, and surface zeta-potential.124 Moreover, the utilization of tumor-targeting RGD family peptides has demonstrated the ability to enhance the penetration of nanoparticles (NPs) into tumors. For instance, liposomes modified with nRGD and loaded with DOX exhibited superior tumoricidal effects in comparison to standard PEGylated liposomal DOX.125 Additionally, the degradation of the extracellular matrix (ECM) has also been achieved as a strategy. For instance, Cheng et al. developed PEG-PLGA NPs with surface-conjugated recombinant human hyaluronidase PH 20 (rHuPH20) to enhance tumor penetration. These NPs exhibited improved tumor penetration and more effective perfusion when compared to the physical mixture of free rHuPH20 and PLGA-PEG NPs, after loading with DOX.126
In summary, various nanoparticles, both established and newly discovered, have been assessed as carriers or modalities for inducing ICD, thereby enhancing the effectiveness of cancer immunotherapy while minimizing adverse effects on the entire system.127,128 Nevertheless, further investigation is required to determine the precise effects of each combined approach.129 It is worth noting that only a limited number of stimuli have been proven to genuinely induce ICD. Furthermore, the animal models used in current studies may not accurately represent real-world scenarios in human subjects. Consequently, numerous challenges must be thoroughly addressed prior to the widespread implementation of these approaches.
Conclusions
The initiation of adaptive immune responses that specifically target antigens found in tumors can be stimulated through the induction of immunological RCD in cancer cells. These cancer cells acquire antigenicity or adjuvanticity through stress-responsive pathways. However, there exist various mechanisms by which both the cancer cells themselves and the TME can hinder the initiation or execution of ICD. Therefore, the use of ICD sensitizers or enhancers becomes necessary in certain ICD-related immunotherapies to achieve optimal effectiveness. Apart from traditional strategies for ICD induction, the field of ICD-based nanoimmunotherapy is gaining increasing attention due to its unique characteristics.
Acknowledgments
We would like to thank all the staff who have taken part in this study.
Biographies
Guo Ai received his Ph.D. degree from Tongji Medical College affiliated to Huazhong University of Science and Technology in 2013. He is an associate chief physician in the Department of Pediatrics of Tongji Hospital, Wuhan. His research work is focused on the clinical immunity, including the tumor immunity.
Na Shen received his Ph.D. degree from Tongji Medical College affiliated to Huazhong University of Science and Technology. Now, She is an associate research fellow in the Department of Laboratory Medicine of Tongji Hospital, Wuhan. Her research work is focused on laboratory medicine, including bioinformatics analysis and clinical oncology.
Funding Statement
This project was supported by grants from the Hubei Provincial Natural Science Foundation of China [2024AFD052] and the Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province [Grant No. CXPJJH122005-015].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contributions
L. Dou, Y. Fang, G. Ai, and N. Shen contributed to the conception and design of this study.
L. Dou, Y. Fang, G. Ai, and N. Shen interpreted the data.
L. Dou and H.Y Yang prepared the first manuscript draft.
L. Dou, G. Ai drew the figures by Figdraw, and proofed the manuscript draft.
L. Dou, G. Ai, and N. Shen revised the draft critically, and all authors proofread the final version.
References
- 1.Lee E, Song CH, Bae SJ, Ha KT, Karki R.. Regulated cell death pathways and their roles in homeostasis, infection, inflammation, and tumorigenesis. Exp Mol Med. 2023;55(8):1632–12. doi: 10.1038/s12276-023-01069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasser A, Vaux DL. Cell death in the origin and treatment of cancer. Mol Cell. 2020;78(6):1045–1054. doi: 10.1016/j.molcel.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Hanggi K, Ruffell B. Cell death, therapeutics, and the immune response in cancer. Trends Cancer. 2023;9(5):381–396. doi: 10.1016/j.trecan.2023.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong X, Tang R, Xiao M, Xu J, Wang W, Zhang B, Liu J, Yu X, Shi S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol. 2022;15(1):174. doi: 10.1186/s13045-022-01392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu X, Chen L, Li Y, Hu Z, He F. Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: perspectives for immunotherapy of SCLC. Semin Cancer Biol. 2022;86(Pt 3):273–285. doi: 10.1016/j.semcancer.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Gao W, Wang Y, Zhou Y, Yu X, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196. doi: 10.1038/s41392-022-01046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloy N, Garcia P, Laumont CM, Pitt JM, Sistigu A, Stoll G, Yamazaki T, Bonneil E, Buque A, Humeau J, et al. Immunogenic stress and death of cancer cells: contribution of antigenicity vs adjuvanticity to immunosurveillance. Immunol Rev. 2017;280(1):165–174. doi: 10.1111/imr.12582. [DOI] [PubMed] [Google Scholar]
- 8.Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P, Zhao L, Spisek R, Kroemer G, Galluzzi L. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11(11):1013. doi: 10.1038/s41419-020-03221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birmpilis AI, Paschalis A, Mourkakis A, Christodoulou P, Kostopoulos IV, Antimissari E, Terzoudi G, Georgakilas AG, Armpilia C, Papageorgis P, et al. Immunogenic cell death, DAMPs and prothymosin α as a putative anticancer immune response biomarker. Cells. 2022;11(9):1415. doi: 10.3390/cells11091415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1):e000337. doi: 10.1136/jitc-2019-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Sun L, Peng X, Liu S, Zhu Z, Huang C. An immunogenic cell death-related signature predicts prognosis and immunotherapy response in stomach adenocarcinoma. Apoptosis. 2023;28(11–12):1564–1583. doi: 10.1007/s10495-023-01879-5. [DOI] [PubMed] [Google Scholar]
- 12.Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology. 2012;1(3):393–395. doi: 10.4161/onci.19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Zhu C, Chen X, Guan G, Zou C, Shen S, Wu J, Wang Y, Lin Z, Chen L, et al. Ferroptosis, as the most enriched programmed cell death process in glioma, induces immunosuppression and immunotherapy resistance. Neuro Oncol. 2022;24(7):1113–1125. doi: 10.1093/neuonc/noac033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58(3):670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Lai X, Fu S, Ren L, Cai H, Zhang H, Gu Z, Ma X, Luo K. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy efficiency. Adv Sci (Weinh). 2022;9(22):e2201734. doi: 10.1002/advs.202201734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding D, Jiang X. Advances in immunogenic cell death for cancer immunotherapy. Small Methods. 2023;7(5):e2300354. doi: 10.1002/smtd.202300354. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savitskaya MA, Zakharov II, Onishchenko GE. Apoptotic features in non-apoptotic processes. Biochem (Mosc). 2022;87(3):191–206. doi: 10.1134/S0006297922030014. [DOI] [PubMed] [Google Scholar]
- 20.Vandenabeele P, Vandecasteele K, Bachert C, Krysko O, Krysko DV. Immunogenic apoptotic cell death and anticancer immunity. Adv Exp Med Biol. 2016;930(133–49). doi: 10.1007/978-3-319-39406-0_6. [DOI] [PubMed] [Google Scholar]
- 21.Pan R, Ryan J, Pan D, Wucherpfennig KW, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185(9):1521–1538 e18. doi: 10.1016/j.cell.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gourisankar S, Krokhotin A, Ji W, Liu X, Chang CY, Kim SH, Li Z, Wenderski W, Simanauskaite JM, Yang H, et al. Rewiring cancer drivers to activate apoptosis. Nature. 2023;620(7973):417–425. doi: 10.1038/s41586-023-06348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razeghian E, Suksatan W, Sulaiman Rahman H, Bokov DO, Abdelbasset WK, Hassanzadeh A, Marofi F, Yazdanifar M, Jarahian M. Harnessing TRAIL-Induced apoptosis pathway for cancer immunotherapy and associated challenges. Front Immunol. 2021;12(699746). doi: 10.3389/fimmu.2021.699746. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Hadian K, Stockwell BR. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat Rev Drug Discov. 2023;22(9):723–742. doi: 10.1038/s41573-023-00749-8. [DOI] [PubMed] [Google Scholar]
- 25.Huang S, Zeng L. Flow cytometric analysis of regulated cell death. Methods Mol Biol. 2023;2712(165–178). doi: 10.1007/978-1-0716-3433-2_15. [DOI] [PubMed] [Google Scholar]
- 26.Cai J, Hu Y, Ye Z, Ye L, Gao L, Wang Y, Sun Q, Tong S, Yang J, Chen Q. Immunogenic cell death-related risk signature predicts prognosis and characterizes the tumour microenvironment in lower-grade glioma. Front Immunol. 2022;13(1011757). doi: 10.3389/fimmu.2022.1011757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J, Zhang W, Jiang T. Immunogenic cell death mediation patterns reveal novel paradigm for characterizing the immune microenvironment and immunotherapeutic responses in bladder cancer. Front Genet. 2022;13(1035484). doi: 10.3389/fgene.2022.1035484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan H, Liu P, Zhao L, Pan Y, Mao M, Kroemer G, Kepp O. Immunogenic cell stress and death in the treatment of cancer. Semin Cell Dev Biol. 2024;156(11):11–21. doi: 10.1016/j.semcdb.2023.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Gong Y, Fan Z, Luo G, Yang C, Huang Q, Fan K, Cheng H, Jin K, Ni Q, Yu X, et al. The role of necroptosis in cancer biology and therapy. Mol Cancer. 2019;18(1):100. doi: 10.1186/s12943-019-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan C, Huang M, Yang X, Hou J. MLKL: functions beyond serving as the executioner of Necroptosis. Theranostics. 2021;11(10):4759–4769. doi: 10.7150/thno.54072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan Y, Chen Q, Li J, Zeng Z, Xiong W, Li G, Yang X, Xiang B, Yi M, Yi M. Pyroptosis: a new paradigm of cell death for fighting against cancer. J Exp Clin Cancer Res. 2021;40(1):153. doi: 10.1186/s13046-021-01959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Wei W, Ma N, Qu Y, Liu Q. Molecular mechanisms of ferroptosis and its role in prostate cancer therapy. Crit Rev Oncol Hematol. 2022;176(103732):103732. doi: 10.1016/j.critrevonc.2022.103732. [DOI] [PubMed] [Google Scholar]
- 34.Xie J, Yang Y, Gao Y, He J. Cuproptosis: mechanisms and links with cancers. Mol Cancer. 2023;22(1):46. doi: 10.1186/s12943-023-01732-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsuno K, Han P, Edelson R, Hanlon D. Detection of immunogenic cell death in tumor vaccination mouse model. Methods Mol Biol. 2021;2255(171–186). doi: 10.1007/978-1-0716-1162-3_15. [DOI] [PubMed] [Google Scholar]
- 36.Amiri M, Molavi O, Sabetkam S, Jafari S, Montazersaheb S. Stimulators of immunogenic cell death for cancer therapy: focusing on natural compounds. Cancer Cell Int. 2023;23(1):200. doi: 10.1186/s12935-023-03058-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhai J, Gu X, Liu Y, Hu Y, Jiang Y, Zhang Z. Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: an update review. Front Pharmacol. 2023;14(1152934). doi: 10.3389/fphar.2023.1152934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zapata L, Caravagna G, Williams MJ, Lakatos E, AbdulJabbar K, Werner B, Chowell D, James C, Gourmet L, Milite S, et al. Immune selection determines tumor antigenicity and influences response to checkpoint inhibitors. Nat Genet. 2023;55(3):451–460. doi: 10.1038/s41588-023-01313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie N, Shen G, Gao W, Huang Z, Huang C, Fu L. Neoantigens: promising targets for cancer therapy. Signal Transduct Target Ther. 2023;8(1):9. doi: 10.1038/s41392-022-01270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi S, Tokita S, Moniwa K, Kitahara K, Iuchi H, Matsuo K, Kakizaki H, Kanaseki T, Torigoe T. Proteogenomic identification of an immunogenic antigen derived from human endogenous retrovirus in renal cell carcinoma. JCI Insight. 2023;8(16). doi: 10.1172/jci.insight.167712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kacen A, Javitt A, Kramer MP, Morgenstern D, Tsaban T, Shmueli MD, Teo GC, da Veiga Leprevost F, Barnea E, Yu F, et al. Post-translational modifications reshape the antigenic landscape of the MHC I immunopeptidome in tumors. Nat Biotechnol. 2023;41(2):239–251. doi: 10.1038/s41587-022-01464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lybaert L, Lefever S, Fant B, Smits E, De Geest B, Breckpot K, Dirix L, Feldman SA, van Criekinge W, Thielemans K, et al. Challenges in neoantigen-directed therapeutics. Cancer Cell. 2023;41(1):15–40. doi: 10.1016/j.ccell.2022.10.013. [DOI] [PubMed] [Google Scholar]
- 43.Andersen MH. Tumor microenvironment antigens. Semin Immunopathol. 2023;45(2):253–264. doi: 10.1007/s00281-022-00966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern recognition receptors and the host cell death molecular machinery. Front Immunol. 2018;9(2379). doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian X, Zhang S, Zhou L, Seyhan AA, Hernandez Borrero L, Zhang Y, El-Deiry WS. Targeting the integrated stress response in cancer therapy. Front Pharmacol. 2021;12(747837). doi: 10.3389/fphar.2021.747837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humeau J, Bezu L, Kepp O, Kroemer G. EIF2alpha phosphorylation: a hallmark of both autophagy and immunogenic cell death. Mol Cell Oncol. 2020;7(5):1776570. doi: 10.1080/23723556.2020.1776570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi K, Nikolos F, Lee YC, Jain A, Tsouko E, Gao H, Kasabyan A, Leung HE, Osipov A, Jung SY, et al. Tipping the immunostimulatory and inhibitory DAMP balance to harness immunogenic cell death. Nat Commun. 2020;11(1):6299. doi: 10.1038/s41467-020-19970-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitt JM, Kroemer G, Zitvogel L. Immunogenic and non-immunogenic cell death in the tumor microenvironment. Adv Exp Med Biol. 2017;1036(65–79). doi: 10.1007/978-3-319-67577-0_5. [DOI] [PubMed] [Google Scholar]
- 49.Garcia Garcia MR, Casares N, Martinez Perez LA, Juarez Curiel E, de Jesus Hernandez AA, Bogdanchikova N, Garibo D, Rodriguez-Hernandez AG, Pestryakov A, Castro Gamboa S, et al. Silver nanoparticles induce a non-immunogenic tumor cell death. J Immunotoxicol. 2023;20(1):2175078. doi: 10.1080/1547691X.2023.2175078. [DOI] [PubMed] [Google Scholar]
- 50.Humeau J, Levesque S, Kroemer G, Pol JG. Gold standard assessment of immunogenic cell death in oncological mouse models. Methods Mol Biol. 2019;1884(297–315). doi: 10.1007/978-1-4939-8885-3_21. [DOI] [PubMed] [Google Scholar]
- 51.Yamazaki T, Buque A, Rybstein M, Chen J, Sato A, Galluzzi L. Methods to detect immunogenic cell death in vivo. Methods Mol Biol. 2020;2055(433–452). doi: 10.1007/978-1-4939-9773-2_20. [DOI] [PubMed] [Google Scholar]
- 52.Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: indication, genotype, and immunotype. Immunity. 2023;56(10):2188–2205. doi: 10.1016/j.immuni.2023.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Hernandez Á-P, Juanes-Velasco P, Landeira-Vinuela A, Bareke H, Montalvillo E, Gongora R, Fuentes M. Restoring the immunity in the tumor microenvironment: insights into immunogenic cell death in onco-therapies. Cancers (Basel). 2021;13(11):2821. doi: 10.3390/cancers13112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: biological function, resistance and remedy. Cancer Lett. 2021;517(96):96–104. doi: 10.1016/j.canlet.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Garrido MA, Perea F, Vilchez JR, Rodriguez T, Anderson P, Garrido F, Ruiz-Cabello F, Aptsiauri N. Copy neutral LOH affecting the entire chromosome 6 is a frequent mechanism of HLA class I alterations in cancer. Cancers (Basel). 2021;13(20):5046. doi: 10.3390/cancers13205046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saleiro D, Platanias LC. Interferon signaling in cancer. Non-canonical pathways and control of intracellular immune checkpoints. Semin Immunol. 2019;43(101299):101299. doi: 10.1016/j.smim.2019.101299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kroemer G, Zitvogel L. Subversion of calreticulin exposure as a strategy of immune escape. Cancer Cell. 2021;39(4):449–451. doi: 10.1016/j.ccell.2021.01.014. [DOI] [PubMed] [Google Scholar]
- 58.Sun T, Li Y, Yang Y, Liu B, Cao Y, Yang W. Enhanced radiation-induced immunogenic cell death activates chimeric antigen receptor T cells by targeting CD39 against glioblastoma. Cell Death Dis. 2022;13(10):875. doi: 10.1038/s41419-022-05319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, Galluzzi L. ATP and cancer immunosurveillance. Embo J. 2021;40(13):e108130. doi: 10.15252/embj.2021108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baracco EE, Stoll G, Van Endert P, Zitvogel L, Vacchelli E, Kroemer G. Contribution of annexin A1 to anticancer immunosurveillance. Oncoimmunology. 2019;8(11):e1647760. doi: 10.1080/2162402X.2019.1647760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang J, Zheng J, Qiu J, Zhang M, Liu L, Wang Z, Zheng Q, Liu Y, Chen M, Li J, et al. Systemic immune-inflammatory index, tumor-infiltrating lymphocytes, and clinical outcomes in esophageal squamous cell carcinoma receiving concurrent chemoradiotherapy. J Immunol Res. 2023;2023(4275998):1–12. doi: 10.1155/2023/4275998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valenza C, Taurelli Salimbeni B, Santoro C, Trapani D, Antonarelli G, Curigliano G. Tumor infiltrating lymphocytes across breast cancer subtypes: current issues for biomarker assessment. Cancers (Basel). 2023;15(3):767. doi: 10.3390/cancers15030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Granhoj JS, Witness Praest Jensen A, Presti M, Met O, Svane IM, Donia M. Tumor-infiltrating lymphocytes for adoptive cell therapy: recent advances, challenges, and future directions. Expert Opin Biol Ther. 2022;22(5):627–641. doi: 10.1080/14712598.2022.2064711. [DOI] [PubMed] [Google Scholar]
- 64.Kristensen NP, Heeke C, Tvingsholm SA, Borch A, Draghi A, Crowther MD, Carri I, Munk KK, Holm JS, Bjerregaard AM, et al. Neoantigen-reactive CD8+ T cells affect clinical outcome of adoptive cell therapy with tumor-infiltrating lymphocytes in melanoma. J Clin Invest. 2022;132(2). doi: 10.1172/JCI150535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Indini A, Massi D, Pirro M, Roila F, Grossi F, Sahebkar A, Glodde N, Bald T, Mandala M. Targeting inflamed and non-inflamed melanomas: biological background and clinical challenges. Semin Cancer Biol. 2022;86(Pt 2):477–490. doi: 10.1016/j.semcancer.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Sun X, Zhao X, Yang C, Shi M, Zhang B, Hu H, Qiao M, Chen D, Zhao X. Combining immune checkpoint blockade with ATP-based immunogenic cell death amplifier for cancer chemo-immunotherapy. Acta Pharm Sin B. 2022;12(9):3694–3709. doi: 10.1016/j.apsb.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srivastava S, Furlan SN, Jaeger-Ruckstuhl CA, Sarvothama M, Berger C, Smythe KS, Garrison SM, Specht JM, Lee SM, Amezquita RA, et al. Immunogenic chemotherapy enhances recruitment of CAR-T cells to lung tumors and improves antitumor efficacy when combined with checkpoint blockade. Cancer Cell. 2021;39(2):193–208 e10. doi: 10.1016/j.ccell.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, Shu P, Li D, Wang Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6(1):362. doi: 10.1038/s41392-021-00670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sattiraju A, Kang S, Giotti B, Chen Z, Marallano VJ, Brusco C, Ramakrishnan A, Shen L, Tsankov AM, Hambardzumyan D, et al. Hypoxic niches attract and sequester tumor-associated macrophages and cytotoxic T cells and reprogram them for immunosuppression. Immunity. 2023;56(8):1825–1843 e6. doi: 10.1016/j.immuni.2023.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura K, Smyth MJ. Myeloid immunosuppression and immune checkpoints in the tumor microenvironment. Cell Mol Immunol. 2020;17(1):1–12. doi: 10.1038/s41423-019-0306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li T, Bou-Dargham MJ, Fultang N, Li X, Pear WS, Sun H, Chen YH. C-rel-dependent monocytes are potent immune suppressor cells in cancer. J Leukoc Biol. 2022;112(4):845–859. doi: 10.1002/JLB.1MA0422-518RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan M, Ding Y, Li Z, Wang X, Xu M. Metabolic manipulation of the tumour immune microenvironment. Immunology. 2022;165(3):290–300. doi: 10.1111/imm.13444. [DOI] [PubMed] [Google Scholar]
- 74.Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL, McCarthy PL, Abrams SI, Repasky EA. beta2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129(12):5537–5552. doi: 10.1172/JCI129502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jennings MR, Munn D, Blazeck J. Immunosuppressive metabolites in tumoral immune evasion: redundancies, clinical efforts, and pathways forward. J Immunother Cancer. 2021;9(10):e003013. doi: 10.1136/jitc-2021-003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watson MJ, Delgoffe GM. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J Clin Invest. 2022;132(2). doi: 10.1172/JCI148549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz-Gregorio A, Aranda-Rivera AK, Sciutto E, Fragoso G, Pedraza-Chaverri J. Redox state associated with antitumor and immunomodulatory peptides in cancer. Arch Biochem Biophys. 2022;730(109414):109414. doi: 10.1016/j.abb.2022.109414. [DOI] [PubMed] [Google Scholar]
- 78.Sarmiento-Salinas FL, Perez-Gonzalez A, Acosta-Casique A, Ix-Ballote A, Diaz A, Trevino S, Rosas-Murrieta NH, Millan-Perez-Pena L, Maycotte P. Reactive oxygen species: role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021;284(119942):119942. doi: 10.1016/j.lfs.2021.119942. [DOI] [PubMed] [Google Scholar]
- 79.Maj T, Wang S, Crespo J, Zhang H, Wei W, Vatan I, Zhao L, Shao L, Szeliga C, Lyssiotis W, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18(12):1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, Chopra S, Perales-Puchalt A, Song M, Zhang S, Bettigole SE, Gupta D, Holcomb K, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161(7):1527–1538. doi: 10.1016/j.cell.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mizukami Y, Sasajima J, Ashida T, Kohgo Y. Abnormal tumor vasculatures and bone marrow-derived pro-angiogenic cells in cancer. Int J Hematol. 2012;95(2):125–130. doi: 10.1007/s12185-012-1017-x. [DOI] [PubMed] [Google Scholar]
- 82.Choi Y, Jung K. Normalization of the tumor microenvironment by harnessing vascular and immune modulation to achieve enhanced cancer therapy. Exp Mol Med. 2023;55(11):2308–2319. doi: 10.1038/s12276-023-01114-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu P, Wang Y, Yuan D, Sun Y, Qin S, Li T. Vascular normalization: reshaping the tumor microenvironment and augmenting antitumor immunity for ovarian cancer. Front Immunol. 2023;14(1276694). doi: 10.3389/fimmu.2023.1276694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Akimoto N, Vayrynen JP, Zhao M, Ugai T, Fujiyoshi K, Borowsky J, Zhong R, Haruki K, Arima K, Lau MC, et al. Desmoplastic reaction, immune cell response, and prognosis in colorectal cancer. Front Immunol. 2022;13(840198). doi: 10.3389/fimmu.2022.840198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Noda Y, Ishida M, Ueno Y, Fujisawa T, Iwai H, Tsuta K. Novel pathological predictive factors for extranodal extension in oral squamous cell carcinoma: a retrospective cohort study based on tumor budding, desmoplastic reaction, tumor-infiltrating lymphocytes, and depth of invasion. BMC Cancer. 2022;22(1):402. doi: 10.1186/s12885-022-09393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lunardi S, Jamieson NB, Lim SY, Griffiths KL, Carvalho-Gaspar M, Al-Assar O, Yameen S, Carter RC, McKay CJ, Spoletini G, et al. IP-10/CXCL10 induction in human pancreatic cancer stroma influences lymphocytes recruitment and correlates with poor survival. Oncotarget. 2014;5(22):11064–11080. doi: 10.18632/oncotarget.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tschumperlin DJ, Lagares D. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacol Ther. 2020;212(107575):107575. doi: 10.1016/j.pharmthera.2020.107575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di X, Gao X, Peng L, Ai J, Jin X, Qi S, Li H, Wang K, Luo D. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Signal Transduct Target Ther. 2023;8(1):282. doi: 10.1038/s41392-023-01501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yerragopu AK, Vellapandian C. Chemoimmunotherapy with doxorubicin and caffeine combination enhanced ICD induction and T-cell infiltration in B16F10 melanoma tumors. J Biochem Mol Toxicol. 2023;37(5):e23327. doi: 10.1002/jbt.23327. [DOI] [PubMed] [Google Scholar]
- 90.Fang Y, Sun H, Xiao X, Tang M, Tian Z, Wei H, Sun R, Zheng X. Low-dose immunogenic chemotherapeutics promotes immune checkpoint blockade in microsatellite stability colon cancer. Front Immunol. 2022;13(1040256). doi: 10.3389/fimmu.2022.1040256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi MY, Liu HG, Chen XH, Tian Y, Chen ZN, Wang K. The application basis of immuno-checkpoint inhibitors combined with chemotherapy in cancer treatment. Front Immunol. 2022;13(1088886). doi: 10.3389/fimmu.2022.1088886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pointer KB, Pitroda SP, Weichselbaum RR. Radiotherapy and immunotherapy: open questions and future strategies. Trends Cancer. 2022;8(1):9–20. doi: 10.1016/j.trecan.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Vaes RDW, Hendriks LEL, Vooijs M, De Ruysscher D. Biomarkers of radiotherapy-induced immunogenic cell death. Cells. 2021;10(4):930. doi: 10.3390/cells10040930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu S, Wang Y, Tang J, Cao M. Radiotherapy induced immunogenic cell death by remodeling tumor immune microenvironment. Front Immunol. 2022;13(1074477). doi: 10.3389/fimmu.2022.1074477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9(1):e001926. doi: 10.1136/jitc-2020-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobayashi H, Choyke PL. Near-infrared photoimmunotherapy of cancer. Acc Chem Res. 2019;52(8):2332–2339. doi: 10.1021/acs.accounts.9b00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 98.Luo S, Luo X, Wang X, Li L, Liu H, Mo B, Gan H, Sun W, Wang L, Liang H, et al. Tailoring multifunctional small molecular photosensitizers to in vivo self-assemble with albumin to boost tumor-preferential accumulation. NIR Imag Photodyn Photothermal Immunother Small. 2022;18(27):e2201298. doi: 10.1002/smll.202201298. [DOI] [PubMed] [Google Scholar]
- 99.Zha M, Yang G, Li Y, Zhang C, Li B, Li K. Recent advances in AIEgen-based photodynamic therapy and immunotherapy. Adv Healthc Mater. 2021;10(24):e2101066. doi: 10.1002/adhm.202101066. [DOI] [PubMed] [Google Scholar]
- 100.Jin MZ, Wang XP. Immunogenic cell death-based cancer vaccines. Front Immunol. 2021;12(697964). doi: 10.3389/fimmu.2021.697964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lamberti MJ, Nigro A, Mentucci FM, Rumie Vittar NB, Casolaro V, Dal Col J. Dendritic cells and immunogenic cancer cell death: a combination for improving antitumor immunity. Pharmaceutics. 2020;12(3):256. doi: 10.3390/pharmaceutics12030256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Redkin TS, Sleptsova EE, Turubanova VD, Saviuk MO, Lermontova SA, Klapshina LG, Peskova NN, Balalaeva IV, Krysko O, Mishchenko TA, et al. Dendritic cells pulsed with tumor lysates induced by Tetracyanotetra(aryl)porphyrazines-based photodynamic therapy effectively trigger anti-tumor immunity in an orthotopic mouse glioma model. Pharmaceutics. 2023;15(10):2430. doi: 10.3390/pharmaceutics15102430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee KW, Yam JWP, Mao X. Dendritic cell vaccines: a shift from conventional approach to new generations. Cells. 2023;12(17):2147. doi: 10.3390/cells12172147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanatkar SA, Heidari A, Rezaei N. Cancer immunotherapy: diverse approaches and obstacles. Curr Pharm Des. 2022;28(29):2387–2403. doi: 10.2174/1381612828666220728160519. [DOI] [PubMed] [Google Scholar]
- 105.Li J, Zhao M, Sun M, Wu S, Zhang H, Dai Y, Wang D. Multifunctional nanoparticles boost cancer immunotherapy based on modulating the immunosuppressive tumor microenvironment. ACS Appl Mater Interface. 2020;12(45):50734–50747. doi: 10.1021/acsami.0c14909. [DOI] [PubMed] [Google Scholar]
- 106.Dou L, Meng X, Yang H, Dong H. Advances in technology and applications of nanoimmunotherapy for cancer. Biomark Res. 2021;9(1):63. doi: 10.1186/s40364-021-00321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheng R, Santos HA. Smart nanoparticle-based platforms for regulating tumor microenvironment and cancer immunotherapy. Adv Healthc Mater. 2023;12(8):e2202063. doi: 10.1002/adhm.202202063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qi J, Jin F, Xu X, Du Y. Combination cancer immunotherapy of nanoparticle-based immunogenic cell death inducers and immune checkpoint inhibitors. Int J Nanomed. 2021;16(1435):1435–1456. doi: 10.2147/IJN.S285999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang M, Zeng J, Zhao L, Zhang M, Ma J, Guan X, Zhang W. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo-immunotherapy. Nanoscale. 2021;13(41):17218–17235. doi: 10.1039/d1nr05512g. [DOI] [PubMed] [Google Scholar]
- 110.Xiang Y, Chen L, Li L, Huang Y. Restoration and enhancement of immunogenic cell death of cisplatin by coadministration with digoxin and conjugation to HPMA copolymer. ACS Appl Mater Interface. 2020;12(1):1606–1616. doi: 10.1021/acsami.9b19323. [DOI] [PubMed] [Google Scholar]
- 111.Ma P, Huang J, Liu J, Zhu Y, Chen J, Chen J, Lei L, Guan Z, Ban J, Lu Z. Nanoformulation of paclitaxel: exploring the Cyclodextrin/RGD nano delivery carrier to slow down paclitaxel release, enhance accumulation in vivo. J Cancer. 2023;14(5):759–769. doi: 10.7150/jca.82410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jia J, Wu X, Long G, Yu J, He W, Zhang H, Wang D, Ye Z, Tian J. Revolutionizing cancer treatment: nanotechnology-enabled photodynamic therapy and immunotherapy with advanced photosensitizers. Front Immunol. 2023;14(1219785). doi: 10.3389/fimmu.2023.1219785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Guo Z, Zhu AT, Fang RH, Zhang L. Recent developments in Nanoparticle-based photo-immunotherapy for cancer treatment. Small Methods. 2023;7(5):e2300252. doi: 10.1002/smtd.202300252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mao D, Hu F, Yi Z, Kenry SX, Yan Z, Luo S, Wu Z, Wang W, Kong D, Kong D, et al. Aiegen-coupled upconversion nanoparticles eradicate solid tumors through dual-mode ROS activation. Sci Adv. 2020;6(26):eabb2712. doi: 10.1126/sciadv.abb2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhen W, Weichselbaum RR, Lin W. Nanoparticle-mediated radiotherapy remodels the tumor microenvironment to enhance antitumor efficacy. Adv Mater. 2023;35(21):e2206370. doi: 10.1002/adma.202206370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu M, Yang M, Zhang J, Yin Y, Fan X, Zhang Y, Qin S, Zhang H, Yu F. Immunogenic cell death induction by ionizing radiation. Front Immunol. 2021;12(705361). doi: 10.3389/fimmu.2021.705361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vanpouille-Box C, Hindre F. Nanovectorized radiotherapy: a new strategy to induce anti-tumor immunity. Front Oncol. 2012;2(136). doi: 10.3389/fonc.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Y, Rahman MM, Clark PA, Sriramaneni RN, Havighurst T, Kerr CP, Zhu M, Jones J, Wang X, Kim K, et al. In situ vaccination following intratumoral injection of IL2 and poly-l-lysine/iron Oxide/CpG Nanoparticles to a radiated tumor site. ACS Nano. 2023;17(11):10236–10251. doi: 10.1021/acsnano.3c00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hao Y, Li H, Zhao H, Liu Y, Ge X, Sun X, Chen H, Yang A, Zou J, Li X, et al. An intelligent Nanovehicle armed with multifunctional navigation for precise delivery of toll-like receptor 7/8 agonist and immunogenic cell death amplifiers to eliminate solid tumors and trigger durable antitumor immunity. Adv Healthc Mater. 2022;11(12):e2102739. doi: 10.1002/adhm.202102739. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y, Chang R, Xing R, Yan X. Bioactive peptide nanodrugs based on supramolecular assembly for boosting immunogenic cell death-induced cancer immunotherapy. Small Methods. 2023;7(5):e2201708. doi: 10.1002/smtd.202201708. [DOI] [PubMed] [Google Scholar]
- 121.Song J, Cheng M, Xie Y, Li K, Zang X. Efficient tumor synergistic chemoimmunotherapy by self-augmented ROS-responsive immunomodulatory polymeric nanodrug. J Nanobiotechnol. 2023;21(1):93. doi: 10.1186/s12951-023-01842-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bian Y, Liu B, Ding B, Wang M, Yuan M, Ma P, Lin J. Tumor microenvironment-activated nanocomposite for self-amplifying Chemodynamic/Starvation therapy enhanced IDO-Blockade tumor immunotherapy. Adv Sci (Weinh). 2023;10(34):e2303580. doi: 10.1002/advs.202303580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li L, Cai H, Ren Z, Ma H, Zhu X, Gong H, Zhang Q, Gu K, Luo Z, Luo K. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of anti-PD-L1 antibody. Bioact Mater. 2023;21(299–312):299–312. doi: 10.1016/j.bioactmat.2022.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lu L, Kang S, Sun Z, Guo C, Li T, Zhang J, Luo B, Liu X, Liu B. Multifunctional nanoparticles in precise cancer treatment: considerations in design and functionalization of nanocarriers. Curr Top Med Chem. 2020;20(27):2427–2441. doi: 10.2174/1568026620666200825170030. [DOI] [PubMed] [Google Scholar]
- 125.Ren Y, Yuan B, Hou S, Sui Y, Yang T, Lv M, Zhou Y, Yu H, Li S, Peng H, et al. Delivery of RGD-modified liposome as a targeted colorectal carcinoma therapy and its autophagy mechanism. J Drug Target. 2021;29(8):863–874. doi: 10.1080/1061186X.2021.1882469. [DOI] [PubMed] [Google Scholar]
- 126.Cheng D, Ji Y, Wang B, Wang Y, Tang Y, Fu Y, Xu Y, Qian X, Zhu W. Dual-responsive nanohybrid based on degradable silica-coated gold nanorods for triple-combination therapy for breast cancer. Acta Biomater. 2021;128(435):435–446. doi: 10.1016/j.actbio.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 127.Kiaie SH, Salehi-Shadkami H, Sanaei MJ, Azizi M, Shokrollahi Barough M, Nasr MS, Sheibani M. Nano-immunotherapy: overcoming delivery challenge of immune checkpoint therapy. J Nanobiotechnol. 2023;21(1):339. doi: 10.1186/s12951-023-02083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen BQ, Zhao Y, Zhang Y, Pan YJ, Xia HY, Kankala RK, Wang SB, Liu G, Chen AZ. Immune-regulating camouflaged nanoplatforms: a promising strategy to improve cancer nano-immunotherapy. Bioact Mater. 2023;21(1):1–19. doi: 10.1016/j.bioactmat.2022.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang Z, Zhang X, Luo Y, Wang Y, Zhou S. Kcl nanoparticles as potential inducer of immunogenic cell death for cancer immunotherapy. ACS Appl Bio Mater. 2023;6(6):2404–2414. doi: 10.1021/acsabm.3c00219. [DOI] [PubMed] [Google Scholar]


