Abstract
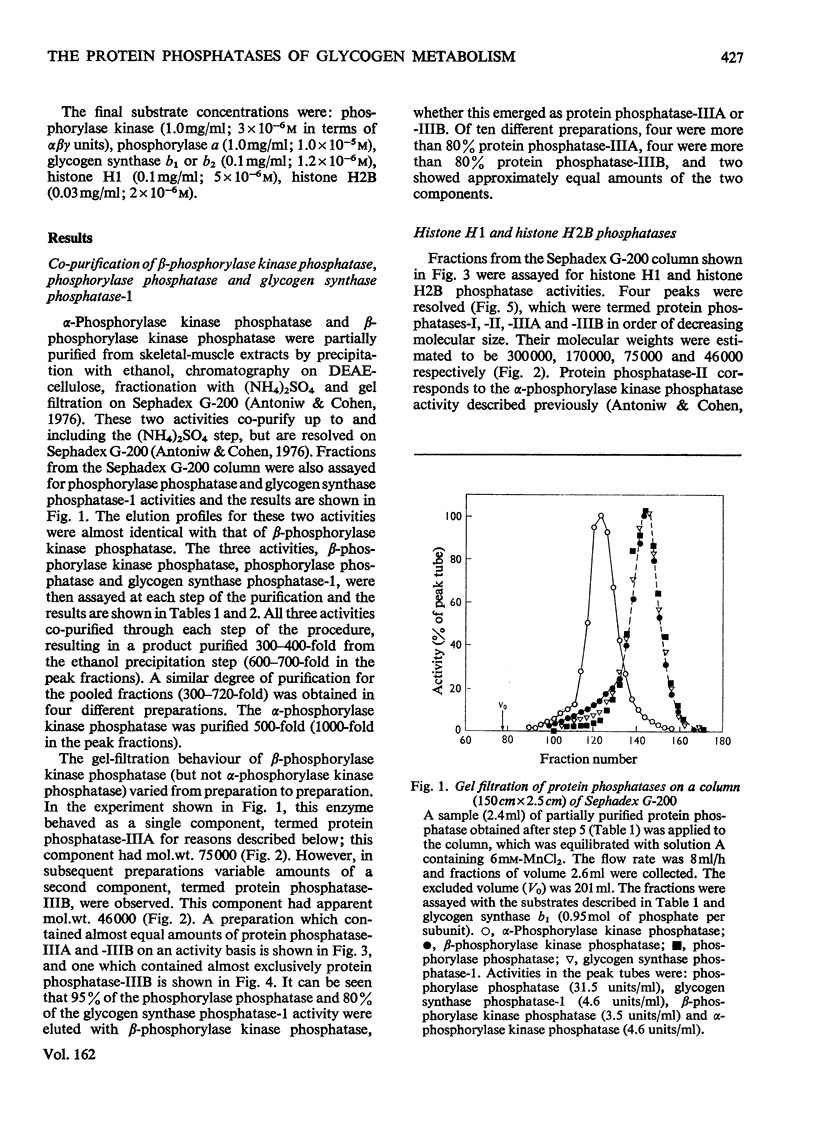
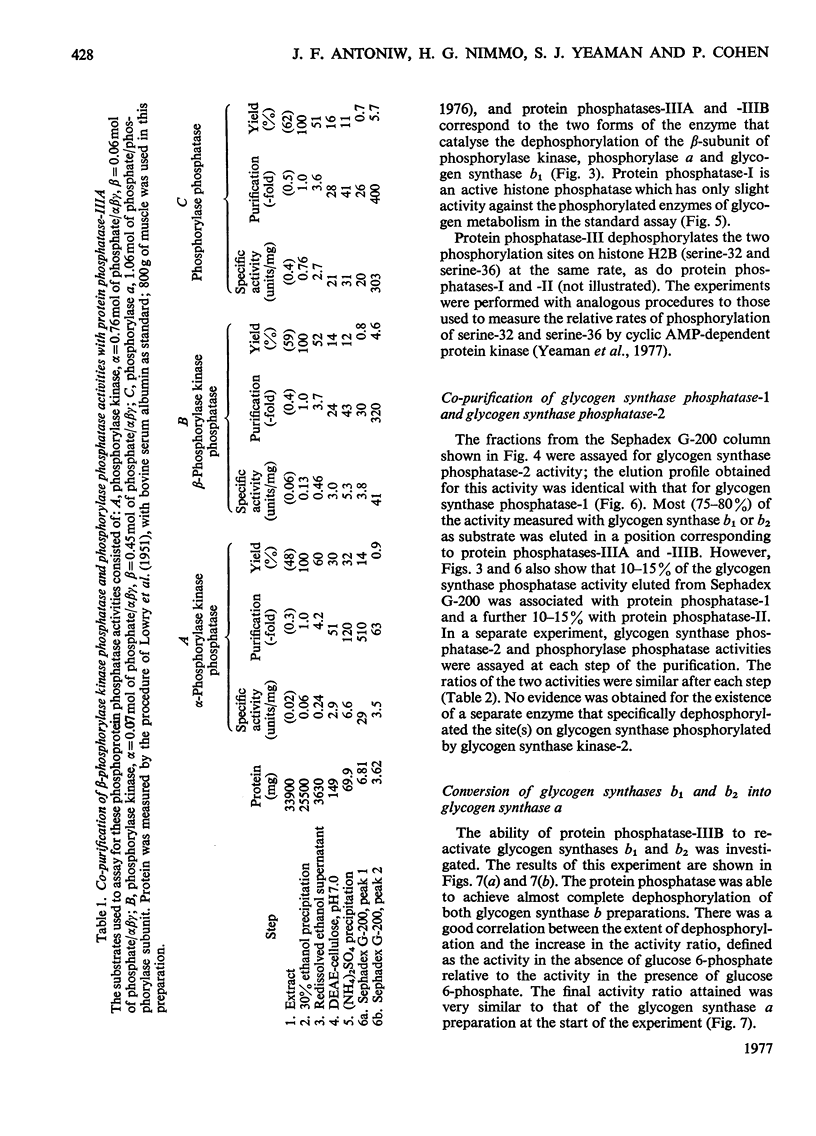
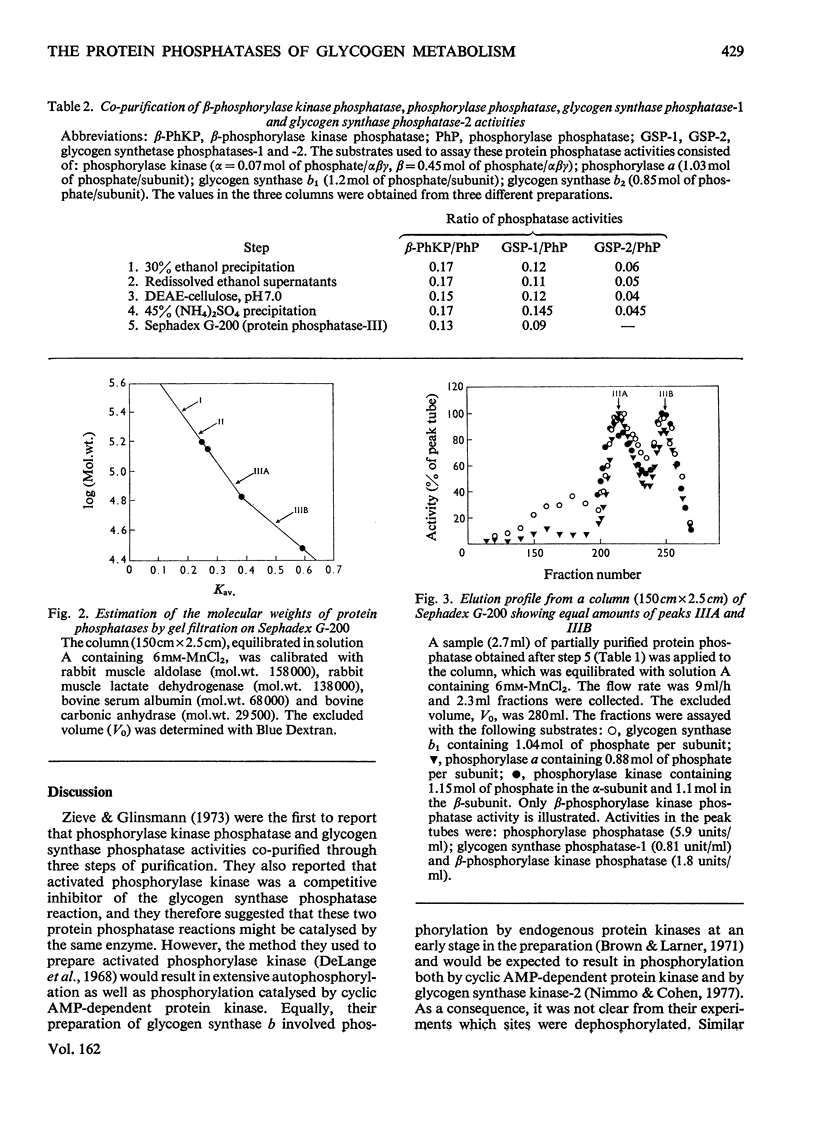
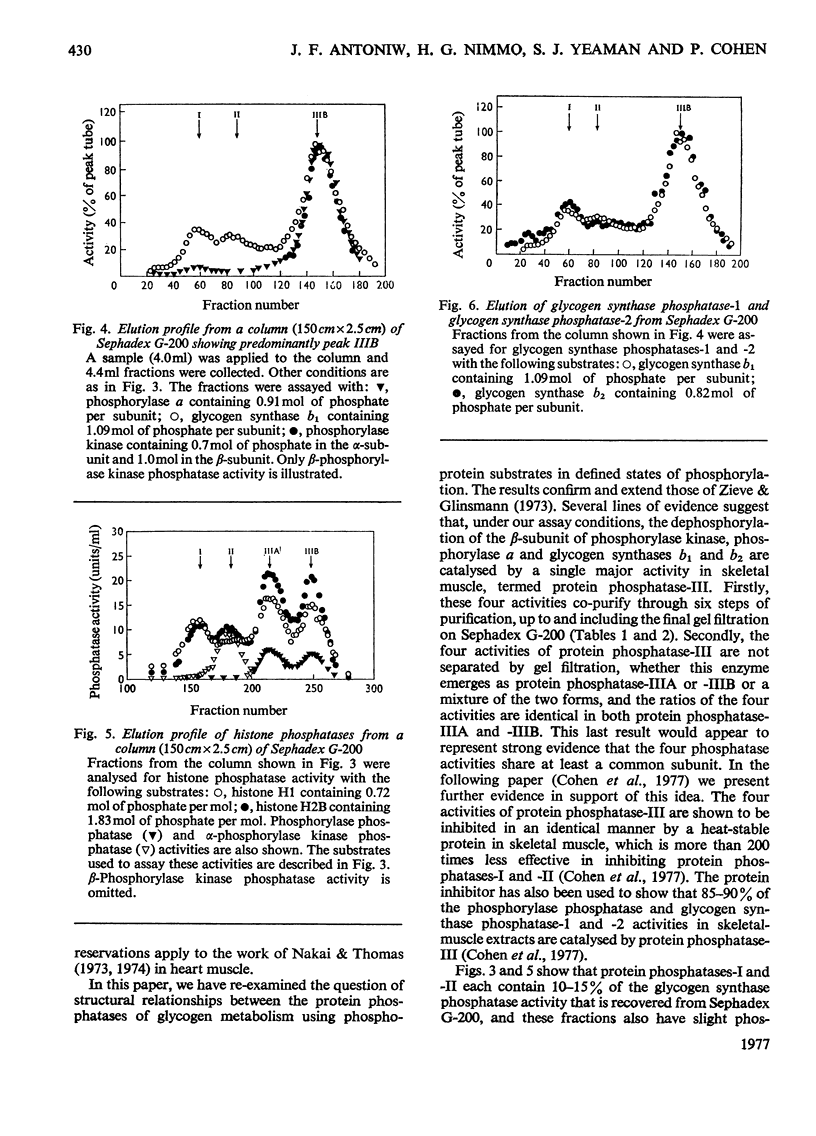
Muscle extracts were subjected to fractionation with ethanol, chromatography on DEAE-cellulose, precipitation with (NH4)2SO4 and gel filtration on Sephadex G-200. These fractions were assayed for protein phosphatase activities by using the following seven phosphoprotein substrates: phosphorylase a, glycogen synthase b1, glycogen synthase b2, phosphorylase kinase (phosphorylated in either the alpha-subunit or the beta-subunit), histone H1 and histone H2B. Three protein phosphatases with distinctive specificities were resolved by the final gel-filtration step and were termed I, II and III. Protein phosphatase-I, apparent mol.wt. 300000, was an active histone phosphatase, but it accounted for only 10-15% of the glycogen synthase phosphatase-1 and glycogen synthase phosphatase-2 activities and 2-3% of the phosphorylase kinase phosphatase and phosphorylase phosphatase activity recovered from the Sephadex G-200 column. Protein phosphatase-II, apparent mol.wt. 170000, possessed histone phosphatase activity similar to that of protein phosphatase-I. It possessed more than 95% of the activity towards the alpha-subunit of phosphorylase kinase that was recovered from Sephadex G-200. It accounted for 10-15% of the glycogen synthase phosphatase-1 and glycogen synthase phosphatase-2 activity, but less than 5% of the activity against the beta-subunit of phosphorylase kinase and 1-2% of the phosphorylase phosphatase activity recovered from Sephadex G-200. Protein phosphatase-III was the most active histone phosphatase. It possessed 95% of the phosphorylase phosphatase and beta-phosphorylase kinase phosphatase activities, and 75% of the glycogen synthase phosphatase-1 and glycogen synthase phosphatase-2 activities recovered from Sephadex G-200. It accounted for less than 5% of the alpha-phosphorylase kinase phosphatase activity. Protein phosphatase-III was sometimes eluted from Sephadex-G-200 as a species of apparent mol.wt. 75000(termed IIIA), sometimes as a species of mol.wt. 46000(termed IIIB) and sometimes as a mixture of both components. The substrate specificities of protein phosphatases-IIA and -IIB were identical. These findings, taken with the observation that phosphorylase phosphatase, beta-phosphorylase kinase phosphatase, glycogen synthase phosphatase-1 and glycogen synthase phosphatase-2 activities co-purified up to the Sephadex G-200 step, suggest that a single protein phosphatase (protein phosphatase-III) catalyses each of the dephosphorylation reactions that inhibit glycogenolysis or stimulate glycogen synthesis. This contention is further supported by results presented in the following paper [Cohen, P., Nimmo, G.A. & Antoniw, J.F. (1977) Biochem. J. 1628 435-444] which describes a heat-stable protein that is a specific inhibitor of protein phosphatase-III.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniw J. F., Cohen P. Separation of two phosphorylase kinase phosphatase activities in rabbit skeletal muscle. Biochem Soc Trans. 1975;3(1):83–84. doi: 10.1042/bst0030083. [DOI] [PubMed] [Google Scholar]
- Antoniw J. F., Cohen P. Separation of two phosphorylase kinase phosphatases from rabbit skeletal muscle. Eur J Biochem. 1976 Sep;68(1):45–54. doi: 10.1111/j.1432-1033.1976.tb10763.x. [DOI] [PubMed] [Google Scholar]
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972 Oct 25;247(20):6637–6642. [PubMed] [Google Scholar]
- Brandt H., Capulong Z. L., Lee E. Y. Purification and properties of rabbit liver phosphorylase phosphatase. J Biol Chem. 1975 Oct 25;250(20):8038–8044. [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- Brown N. E., Larner J. Molecular characteristics of the totally dependent and independent forms of glycogen synthase of rabbit skeletal muscle. I. Preparation and characteristics of the totally glucose 6-phosphate dependent form. Biochim Biophys Acta. 1971 Jul 21;242(1):69–80. doi: 10.1016/0005-2744(71)90088-x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Antoniw J. F., Nimmo H. G., Yeaman S. J. Protein phosphorylation and hormone action. Ciba Found Symp. 1976;41:281–295. doi: 10.1002/9780470720233.ch15. [DOI] [PubMed] [Google Scholar]
- Cohen P., Antoniw J. F. The control of phosphorylase kinase phosphatase by "second site phosphorylation"; a new form of enzyme regulation. FEBS Lett. 1973 Aug 1;34(1):43–47. doi: 10.1016/0014-5793(73)80699-4. [DOI] [PubMed] [Google Scholar]
- Cohen P., Duewer T., Fischer E. H. Phosphorylase from dogfish skeletal muscle. Purification and a comparison of its physical properties to those of rabbit muscle phosphorylase. Biochemistry. 1971 Jul 6;10(14):2683–2694. doi: 10.1021/bi00790a005. [DOI] [PubMed] [Google Scholar]
- Cohen P., Nimmo G. A., Antoniw J. F. Specificity of a protein phosphatase inhibitor from rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):435–444. doi: 10.1042/bj1620435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Nimmo G. A., Antoniw J. F. Specificity of a protein phosphatase inhibitor from rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):435–444. doi: 10.1042/bj1620435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Watson D. C., Dixon G. H. The hormonal control of activity of skeletal muscle phosphorylase kinase. Amino-acid sequences at the two sites of action of adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1975 Feb 3;51(1):79–92. doi: 10.1111/j.1432-1033.1975.tb03909.x. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Kemp R. G., Riley W. D., Cooper R. A., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by adenosine triphosphate and adenosine 3',5'-monophosphate. J Biol Chem. 1968 May 10;243(9):2200–2208. [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Histones: structure and function. Annu Rev Biochem. 1971;40:279–314. doi: 10.1146/annurev.bi.40.070171.001431. [DOI] [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Hashimoto E., Takeda M., Nishizuka Y. Phosphorylated sites of calf thymus histone H2B by adenosine 3',5'-monophosphate-dependent protein kinase from silkworm. Biochem Biophys Res Commun. 1975 Sep 16;66(2):547–555. doi: 10.1016/0006-291x(75)90545-8. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Glinsmann W. H. Inactivation of rabbit muscle phosphorylase phosphatase by cyclic AMP-dependent kinas. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3004–3008. doi: 10.1073/pnas.72.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandelwal R. L., Vandenheede J. R., Krebs E. G. Purification, properties, and substrate specificities of phosphoprotein phosphatase(s) from rabbit liver. J Biol Chem. 1976 Aug 25;251(16):4850–4858. [PubMed] [Google Scholar]
- Killilea S. D., Brandt H., Lee E. Y., Whelan W. J. Evidence for the coordinate control of activity of liver glycogen synthase and phosphorylase by a single protein phosphatase. J Biol Chem. 1976 Apr 25;251(8):2363–2368. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Cyclic AMP and histone phosphorylation. Ann N Y Acad Sci. 1971 Dec 30;185:166–180. doi: 10.1111/j.1749-6632.1971.tb45246.x. [DOI] [PubMed] [Google Scholar]
- Larner J., Villar-Palasi C., Goldberg N. D., Bishop J. S., Huijing F., Wenger J. I., Sasko H., Brown N. B. Hormonal and non-hormonal control of glycogen synthesis-control of transferase phosphatase and transferase I kinase. Adv Enzyme Regul. 1968;6:409–423. doi: 10.1016/0065-2571(68)90025-3. [DOI] [PubMed] [Google Scholar]
- Nakai C., Thomas J. A. Properties of a phosphoprotein phosphatase from bovine heart with activity on glycogen synthase, phosphorylase, and histone. J Biol Chem. 1974 Oct 25;249(20):6459–6467. [PubMed] [Google Scholar]
- Nakai C., Thomas J. A. Substrate specificity of glycogen synthase phosphatase from bovine heart: action on phosphorylase a and histone. Biochem Biophys Res Commun. 1973 May 15;52(2):530–536. doi: 10.1016/0006-291x(73)90744-4. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Glycogen synthetase kinase 2 (GSK 2); the identification of a new protein kinase in skeletal muscle. FEBS Lett. 1974 Oct 1;47(1):162–166. doi: 10.1016/0014-5793(74)80450-3. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The phosphorylation of rabbit skeletal muscle glycogen synthase by glycogen synthase kinase-2 and adenosine-3':5'-monophosphate-dependent protein kinase. Eur J Biochem. 1976 Sep;68(1):31–44. doi: 10.1111/j.1432-1033.1976.tb10762.x. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Proud C. G., Cohen P. The purification and properties of rabbit skeletal muscle glycogen synthase. Eur J Biochem. 1976 Sep;68(1):21–30. doi: 10.1111/j.1432-1033.1976.tb10761.x. [DOI] [PubMed] [Google Scholar]
- Riley W. D., DeLange R. J., Bratvold G. E., Krebs E. G. Reversal of phosphorylase kinase activation. J Biol Chem. 1968 May 10;243(9):2209–2215. [PubMed] [Google Scholar]
- Soderling T. R., Hickenbottom J. P., Reimann E. M., Hunkeler F. L., Walsh D. A., Krebs E. G. Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1970 Dec 10;245(23):6317–6328. [PubMed] [Google Scholar]
- Titani K., Cohen P., Walsh K. A., Neurath H. Amino-terminal sequence of rabbit muscle phosphorylase. FEBS Lett. 1975 Jul 15;55(1):120–123. doi: 10.1016/0014-5793(75)80974-4. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Stull J. T., Huang T. S., Krebs E. G. A study on the autoactivation of rabbit muscle phosphorylase kinase. J Biol Chem. 1976 Aug 10;251(15):4521–4527. [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P. The specificity of adenosine 3':5'-cyclic monophosphate-dependent protein kinase. Biochem Soc Trans. 1976;4(6):1027–1030. doi: 10.1042/bst0041027. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Cohen P., Watson D. C., Dixon G. H. The substrate specificity of adenosine 3':5'-cyclic monophosphate-dependent protein kinase of rabbit skeletal muscle. Biochem J. 1977 Feb 15;162(2):411–421. doi: 10.1042/bj1620411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve F. J., Glinsmann W. H. Activation of glycogen synthetase and inactivation of phosphorylase kinase by the same phosphoprotein phosphatase. Biochem Biophys Res Commun. 1973 Feb 5;50(3):872–878. doi: 10.1016/0006-291x(73)91326-0. [DOI] [PubMed] [Google Scholar]


