Abstract
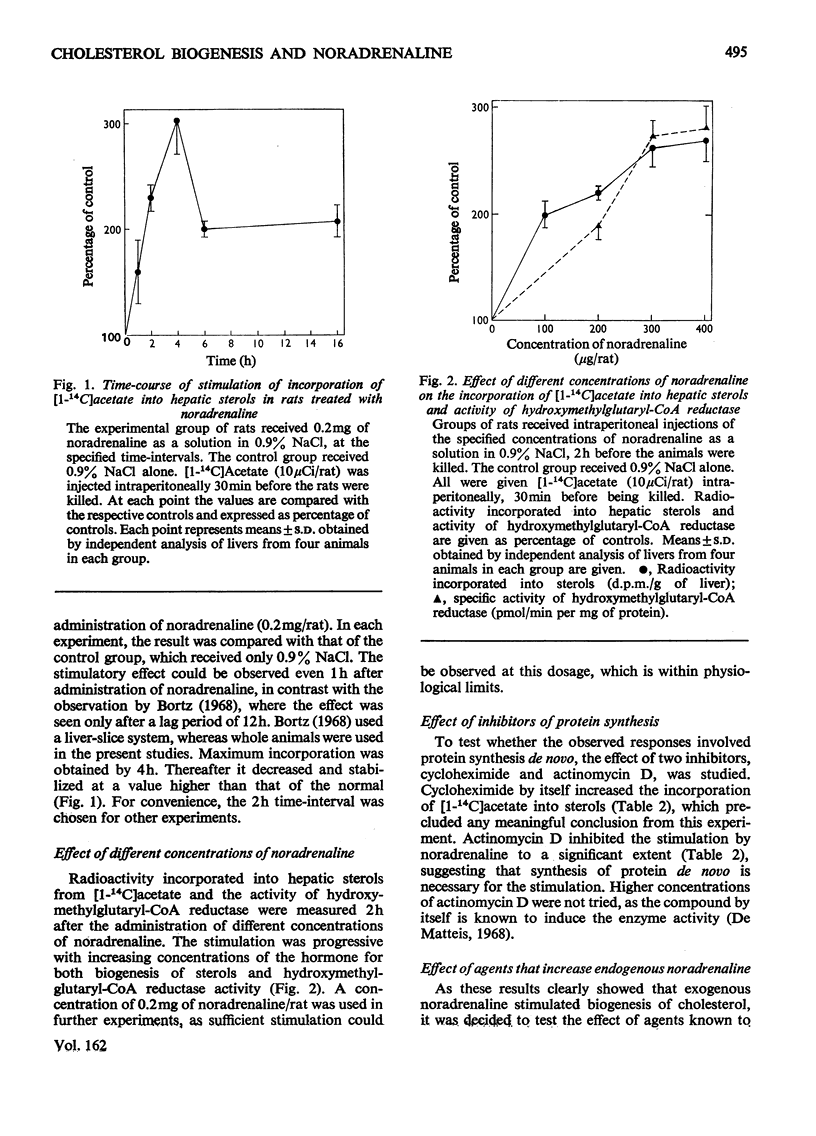
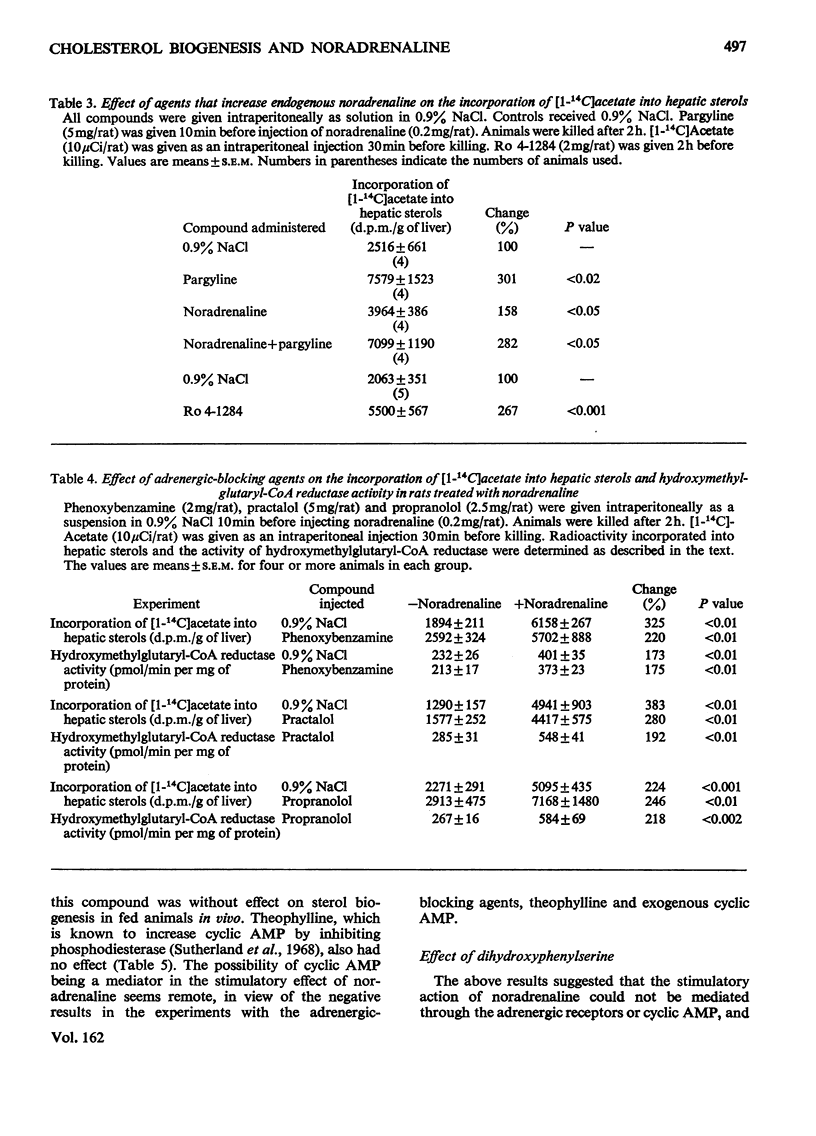
1. Administration of noradrenaline increased the incorporation of [1-14C]acetate into hepatic sterols and the activity of liver microsomal 3-hydroxy-3-methylglutaryl-CoA reductase. 2. The stimulation was observed at short time-intervals with a maximum at 4h and was progressive with increasing concentrations of noradrenaline. 3. Protein synthesis de novo was a necessary factor for the effect. 4. The stimulatory effect was not mediated through the adrenergic receptors, but appears to involve a direct action of the hormone within the hepatocyte.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bortz W. M. Noradrenalin-induced increase in hepatic cholesterol synthesis and its blockade by puromycin. Biochim Biophys Acta. 1968 May 1;152(3):619–626. doi: 10.1016/0005-2760(68)90102-1. [DOI] [PubMed] [Google Scholar]
- Bortz W. M. On the control of cholesterol synthesis. Metabolism. 1973 Dec;22(12):1507–1524. doi: 10.1016/0026-0495(73)90019-x. [DOI] [PubMed] [Google Scholar]
- Bricker L. A., Levey G. S. Evidence for regulatin of cholesterol and fatty acid synthesis in liver by cyclic adenosine 3',5'-monophosphate. J Biol Chem. 1972 Aug 10;247(15):4914–4915. [PubMed] [Google Scholar]
- Dairman W., Christenson J. G., Udenfriend S. Changes in tyrosine hydroxylase and dopa decarboxylase induced by pharmacological agents. Pharmacol Rev. 1972 Jun;24(2):269–289. [PubMed] [Google Scholar]
- De Matteis F. Stimulation of liver cholesterol synthesis by actinomycin D. Biochem J. 1968 Oct;109(5):775–785. doi: 10.1042/bj1090775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A. Effect of adrenalectomy and hypophysectomy on the circadian rhythm of -hydroxy- -methylglutaryl coenzyme A reductase activity in rat liver. J Biol Chem. 1973 Apr 25;248(8):2912–2917. [PubMed] [Google Scholar]
- Edwards P. A. The influence of catecholamines and cyclic AMP on 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and lipid biosynthesis in isolated rat hepatocytes. Arch Biochem Biophys. 1975 Sep;170(1):188–203. doi: 10.1016/0003-9861(75)90110-1. [DOI] [PubMed] [Google Scholar]
- Hellerman L., Erwin V. G. Mitochondrial monoamine oxidase. II. Action of various inhibitors for the bovine kidney enzyme. Catalytic mechanism. J Biol Chem. 1968 Oct 25;243(20):5234–5243. [PubMed] [Google Scholar]
- Hornbrook K. R. Adrenergic receptors for metabolic responses in the liver. Fed Proc. 1970 Jul-Aug;29(4):1381–1385. [PubMed] [Google Scholar]
- Huber J., Guder W., Latzin S., Hamprecht B. The influence of insulin and glucagon on hydroxymethylglutaryl coenzyme A reductase activity in rat liver. Hoppe Seylers Z Physiol Chem. 1973 Jul;354(7):795–798. [PubMed] [Google Scholar]
- Louw A. I., Bekersky I., Mosbach E. H. Improved synthesis of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). J Lipid Res. 1969 Nov;10(6):683–686. [PubMed] [Google Scholar]
- Nepokroeff C. M., Lakshmanan M. R., Ness G. C., Dugan R. E., Porter J. W. Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys. 1974 Feb;160(2):387–396. doi: 10.1016/0003-9861(74)90412-3. [DOI] [PubMed] [Google Scholar]
- Ness G. C., Dugan R. E., Lakshmanan M. R., Nepokroeff C. M., Porter J. W. Stimulation of hepatic beta-hydroxy-beta-methylglutaryl coenzyme A reductase activity in hypophysectomized rats by L-triiodothyronine. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3839–3842. doi: 10.1073/pnas.70.12.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. S., George R., Ramasarma T. Stimulation of hepatic biogenesis of sterols on administration of adenosine compounds. Biochem J. 1976 Mar 15;154(3):639–645. doi: 10.1042/bj1540639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh Y., Ui M. Stimulation of glycogenolysis and gluconeogenesis by epinephrine independent of its beta-adrenergic function in perfused rat liver. Biochem Pharmacol. 1976 Apr 1;25(7):841–845. doi: 10.1016/0006-2952(76)90156-8. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Nordstrom J. L., Mitschelen J. J., Rodwell V. W., Schimke R. T. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta. 1974 Dec 29;370(2):369–377. doi: 10.1016/0005-2744(74)90098-9. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Rodwell V. W. Regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and cholesterol synthesis. J Biol Chem. 1971 May 25;246(10):3210–3216. [PubMed] [Google Scholar]


