Abstract
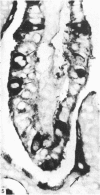
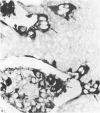
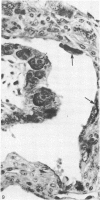
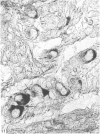
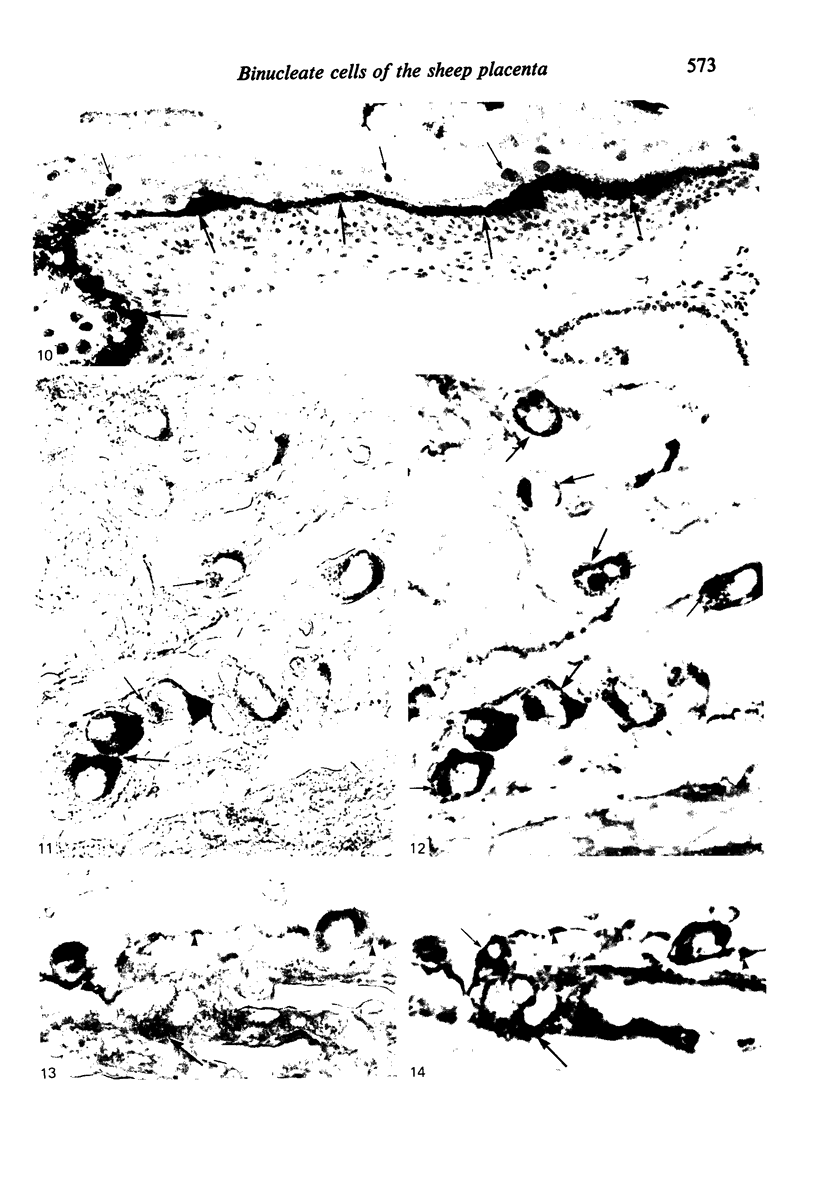
The distribution of cells recognised by the monoclonal antibody SBU-3 raised against trophoblast microvilli during development of the sheep placenta was investigated using the indirect immunoperoxidase technique. At 21 days of gestation, the placental antigen recognised by the monoclonal antibody SBU-3 was observed in the binucleate cells in the trophoblast located in close apposition to the caruncular epithelium. From 30-100 days there was a dramatic increase in the number of SBU-3-positive cells in the placentomal trophoblast and the syncytial layer in the placentome. An insignificant number of SBU-3-positive cells was observed in the interplacentomal trophoblast. By 120-145 days, the syncytial layer became less intensely stained, but strongly SBU-3-positive binucleate cells were still present in the placentomal trophoblast. It is concluded that the antigen recognised by the monoclonal antibody SBU-3 is a secretory product of the binucleate cells of the trophoblast, whose function is at present unknown. The findings in this study are consistent with the theory that the syncytium is formed by fusion of migrating fetal binucleate cells.
Full text
PDF



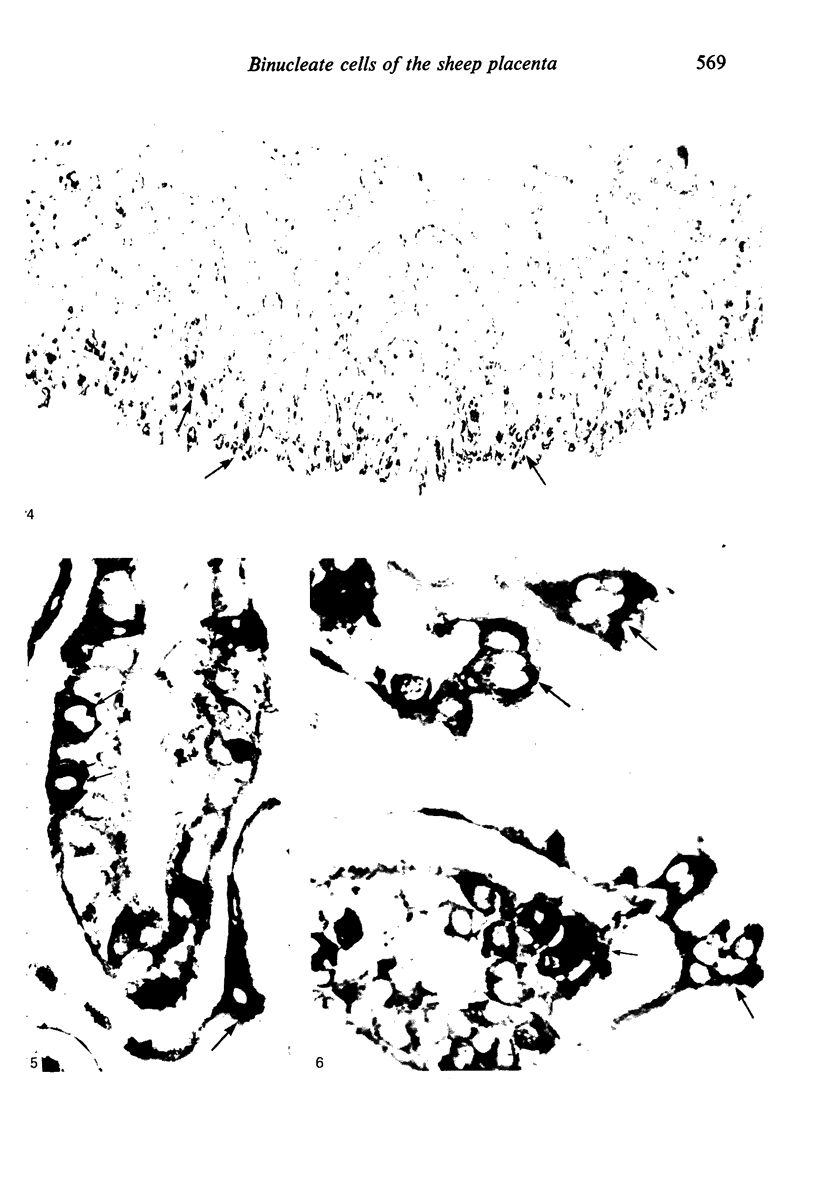

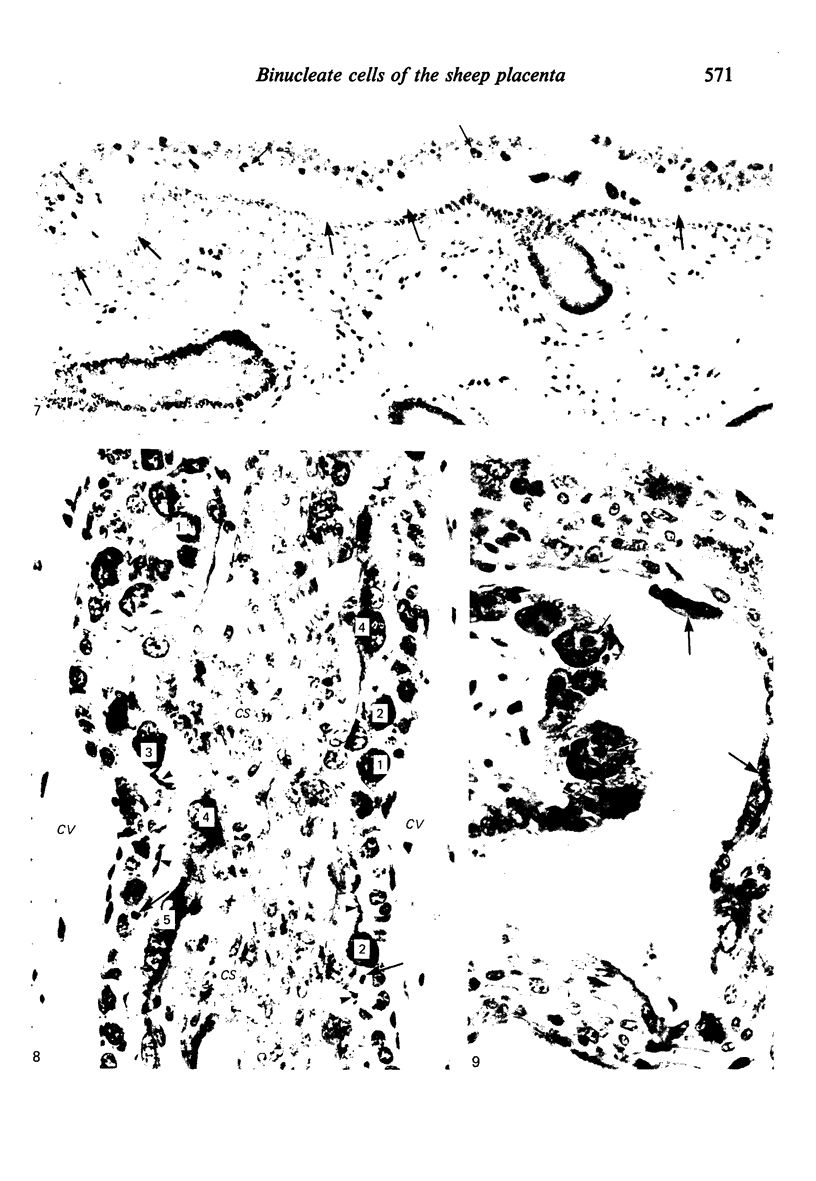




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BJOERKMAN N. FINE STRUCTURE OF THE OVINE PLACENTOME. J Anat. 1965 Apr;99:283–297. [PMC free article] [PubMed] [Google Scholar]
- Boshier D. P. A histological and histochemical examination of implantation and early placentome formation in sheep. J Reprod Fertil. 1969 Jun;19(1):51–61. doi: 10.1530/jrf.0.0190051. [DOI] [PubMed] [Google Scholar]
- Boshier D. P., Holloway H. The sheep trophoblast and placental function: an ultrastructural study. J Anat. 1977 Nov;124(Pt 2):287–298. [PMC free article] [PubMed] [Google Scholar]
- Bryden M. M., Evans H. E., Binns W. Embryology of the sheep. I. Extraembryonic membranes and the development of body form. J Morphol. 1972 Oct;138(2):169–185. doi: 10.1002/jmor.1051380204. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. Multinucleate giant cells. J Pathol. 1978 Nov;126(3):125–148. doi: 10.1002/path.1711260302. [DOI] [PubMed] [Google Scholar]
- Chan J. S., Robertson H. A., Friesen H. G. The purification and characterization of ovine placental lactogen. Endocrinology. 1976 Jan;98(1):65–76. doi: 10.1210/endo-98-1-65. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Martal J., Djiane J., Dubois M. P. Immunofluorescent localization of ovine placental lactogen. Cell Tissue Res. 1977 Nov 23;184(4):427–433. doi: 10.1007/BF00220966. [DOI] [PubMed] [Google Scholar]
- Reddy S., Watkins W. B. Immunofluorescence localization of ovine placental lactogen. J Reprod Fertil. 1978 Jan;52(1):173–174. doi: 10.1530/jrf.0.0520173. [DOI] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G. Preparation and characterization of human syncytiotrophoblast plasma membrane. Med Biol. 1978 Oct;56(5):272–276. [PubMed] [Google Scholar]
- Steven D. H., Bass F., Jansen C. J., Krane E. J., Mallon K., Samuel C. A., Thomas A. L., Nathanielsz P. W. Ultrastructural changes in the placenta of the ewe after fetal pituitary stalk section. Q J Exp Physiol Cogn Med Sci. 1978 Jul;63(3):221–229. doi: 10.1113/expphysiol.1978.sp002437. [DOI] [PubMed] [Google Scholar]
- WIMSATT W. A. Observations on the morphogenesis, cytochemistry, and significance of the binocleate giant cells of the placenta of ruminants. Am J Anat. 1951 Sep;89(2):233–281. doi: 10.1002/aja.1000890204. [DOI] [PubMed] [Google Scholar]
- Watkins W. B., Reddy S. Ovine placental lactogen in the cotyledonary and intercotyledonary placenta of the ewe. J Reprod Fertil. 1980 Mar;58(2):411–414. doi: 10.1530/jrf.0.0580411. [DOI] [PubMed] [Google Scholar]
- Wooding F. B., Chambers S. G., Perry J. S., George M., Heap R. B. Migration of binucleate cells in the sheep placenta during normal pregnancy. Anat Embryol (Berl) 1980;158(3):361–370. doi: 10.1007/BF00301823. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. Electron microscopic localization of binucleate cells in the sheep placenta using phosphotungstic acid. Biol Reprod. 1980 Mar;22(2):357–365. doi: 10.1093/biolreprod/22.2.357. [DOI] [PubMed] [Google Scholar]
- Wooding F. B., Flint A. P., Heap R. B., Hobbs T. Autoradiographic evidence for migration and fusion of cells in the sheep placenta: resolution of a problem in placental classification. Cell Biol Int Rep. 1981 Aug;5(8):821–827. doi: 10.1016/0309-1651(81)90254-x. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. Localization of ovine placental lactogen in sheep placentomes by electron microscope immunocytochemistry. J Reprod Fertil. 1981 May;62(1):15–19. doi: 10.1530/jrf.0.0620015. [DOI] [PubMed] [Google Scholar]