Abstract
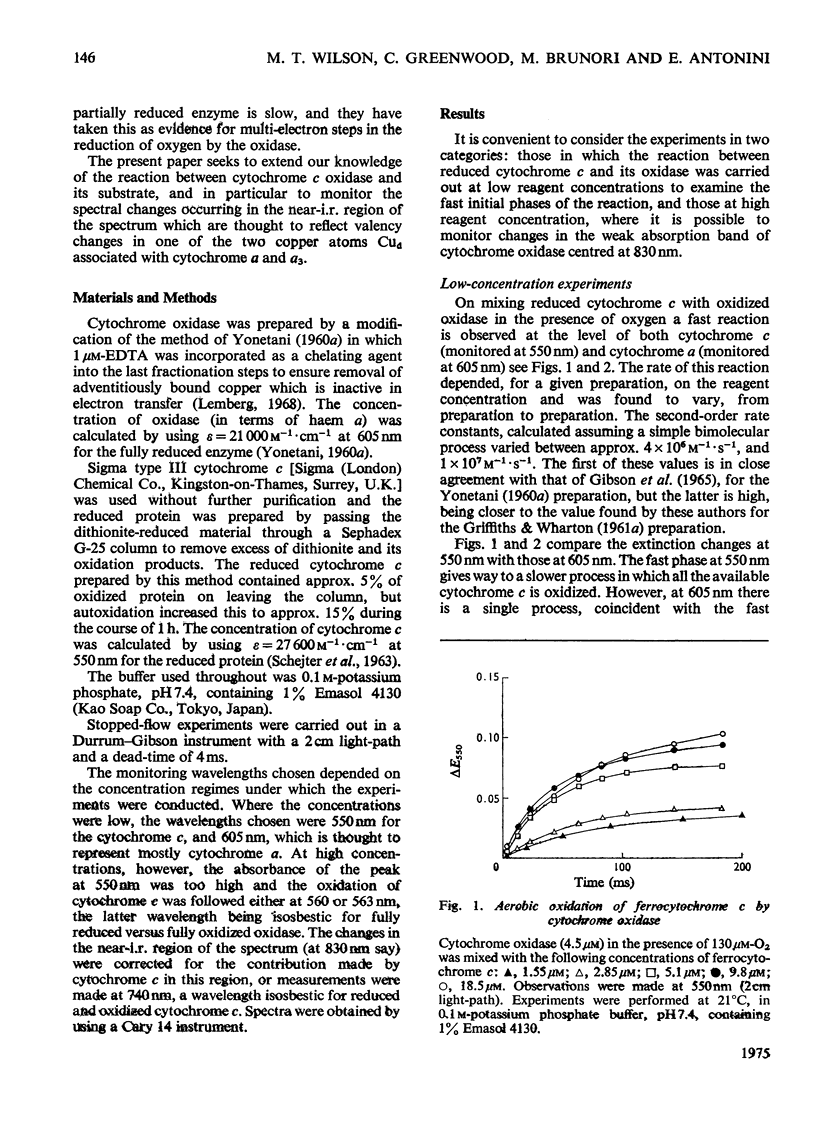
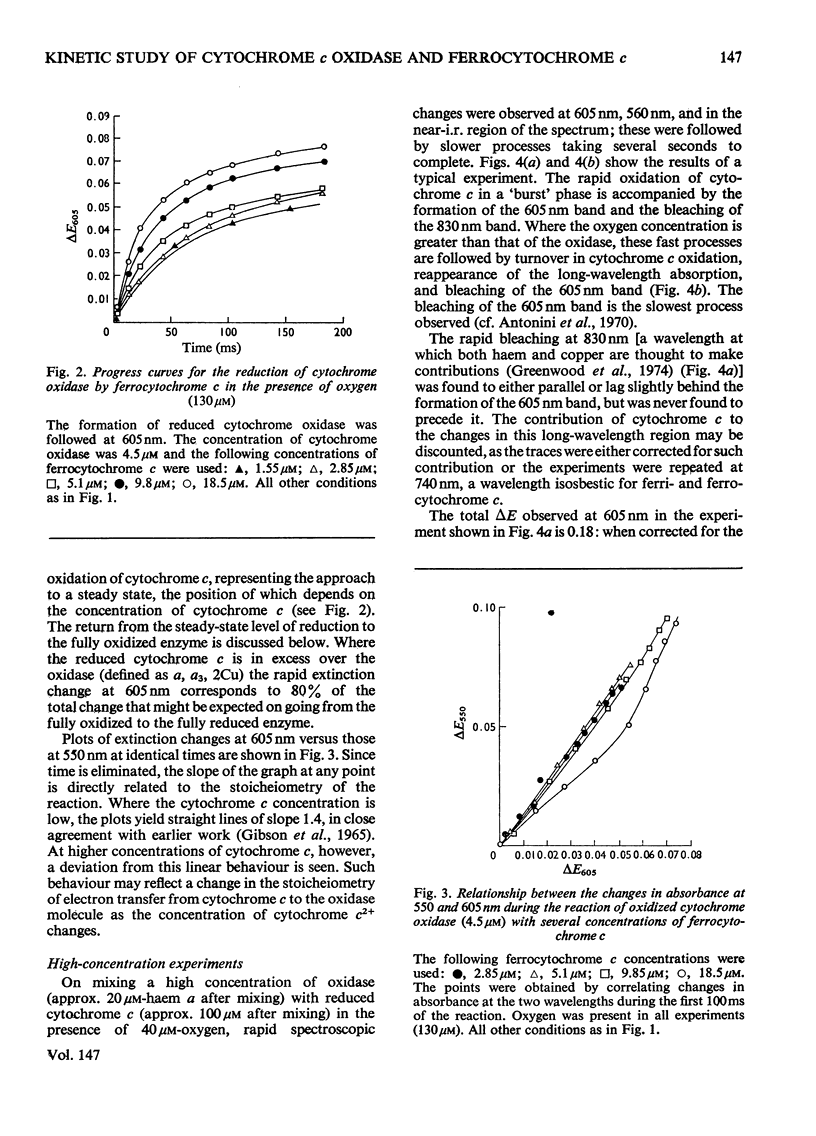
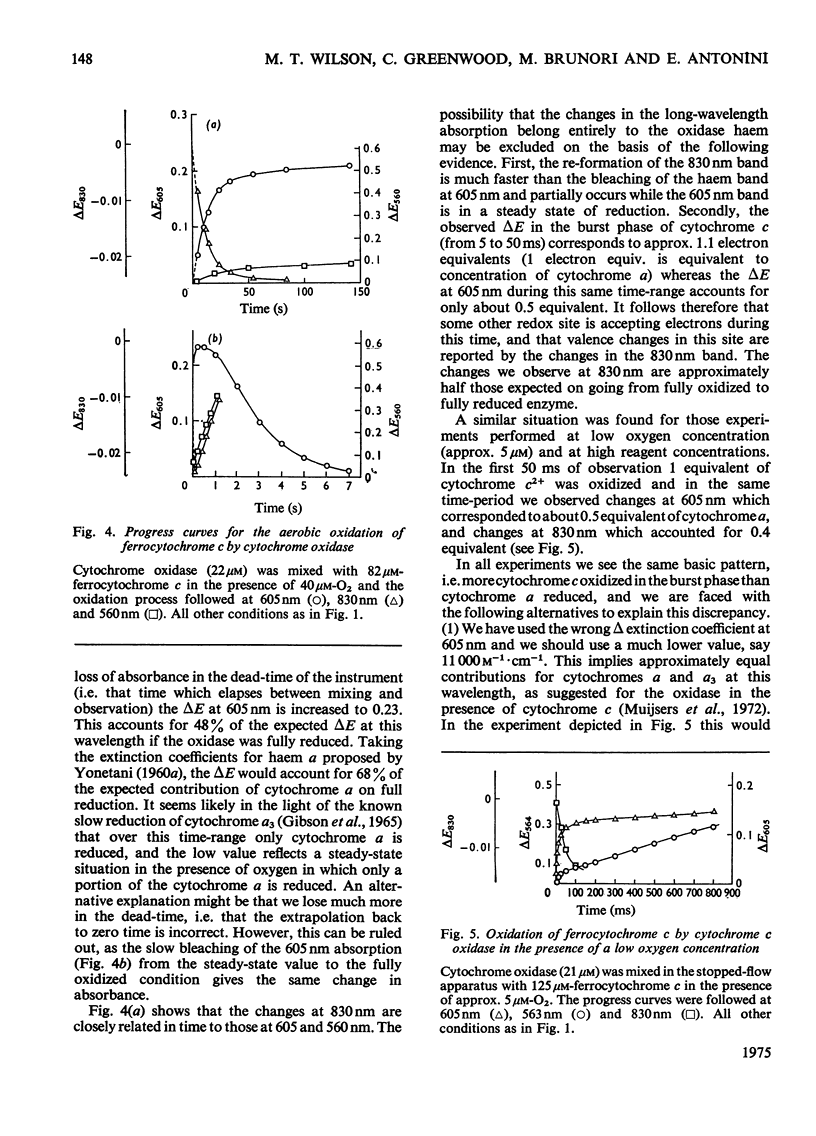
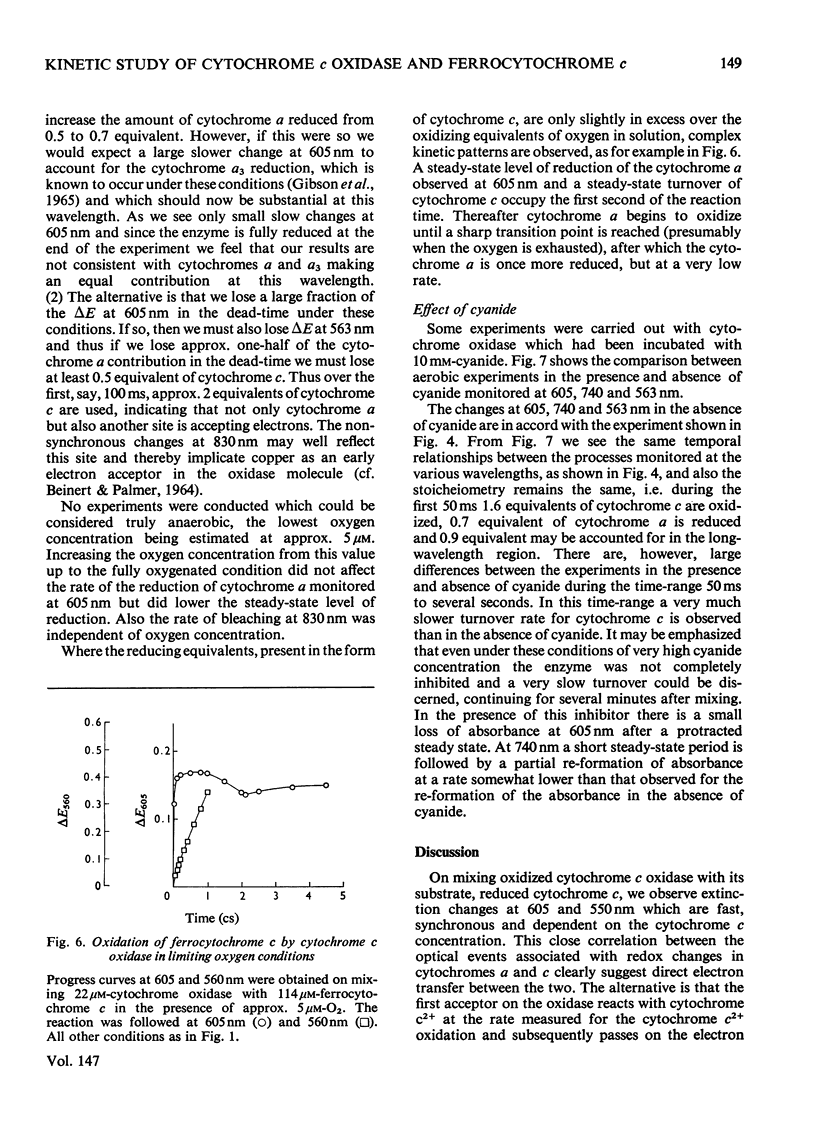
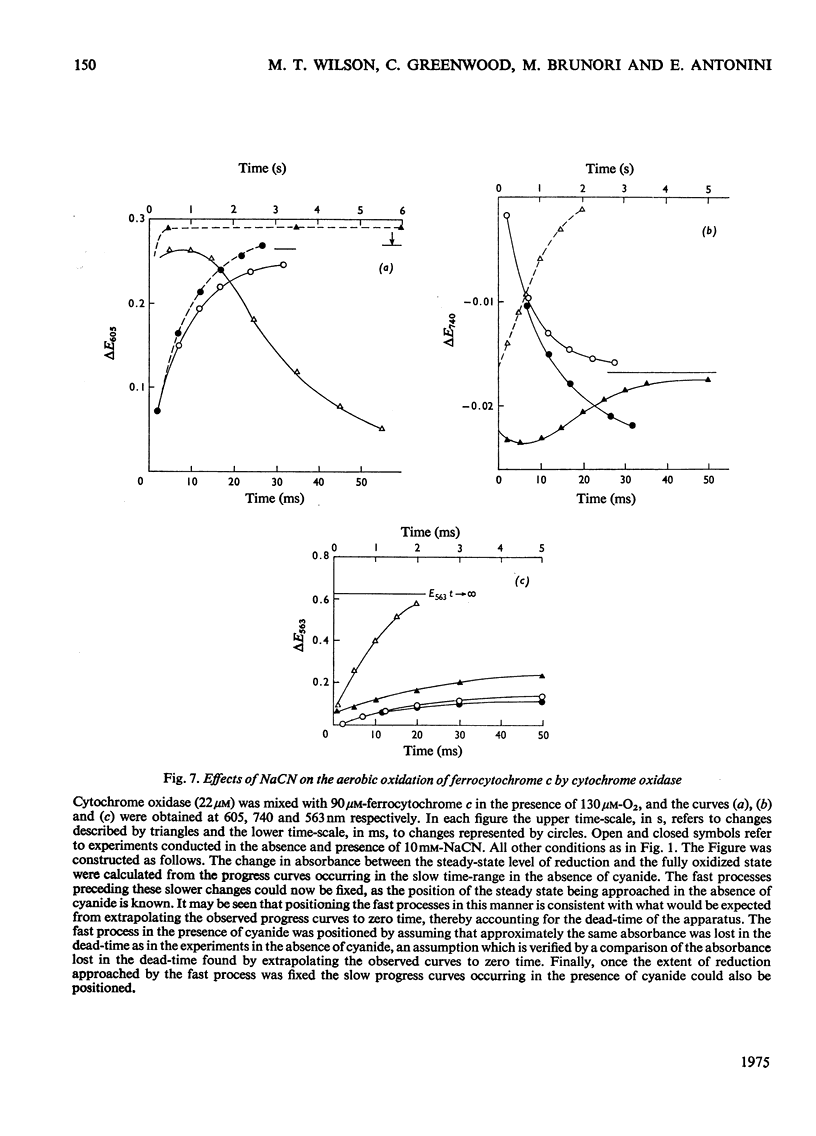
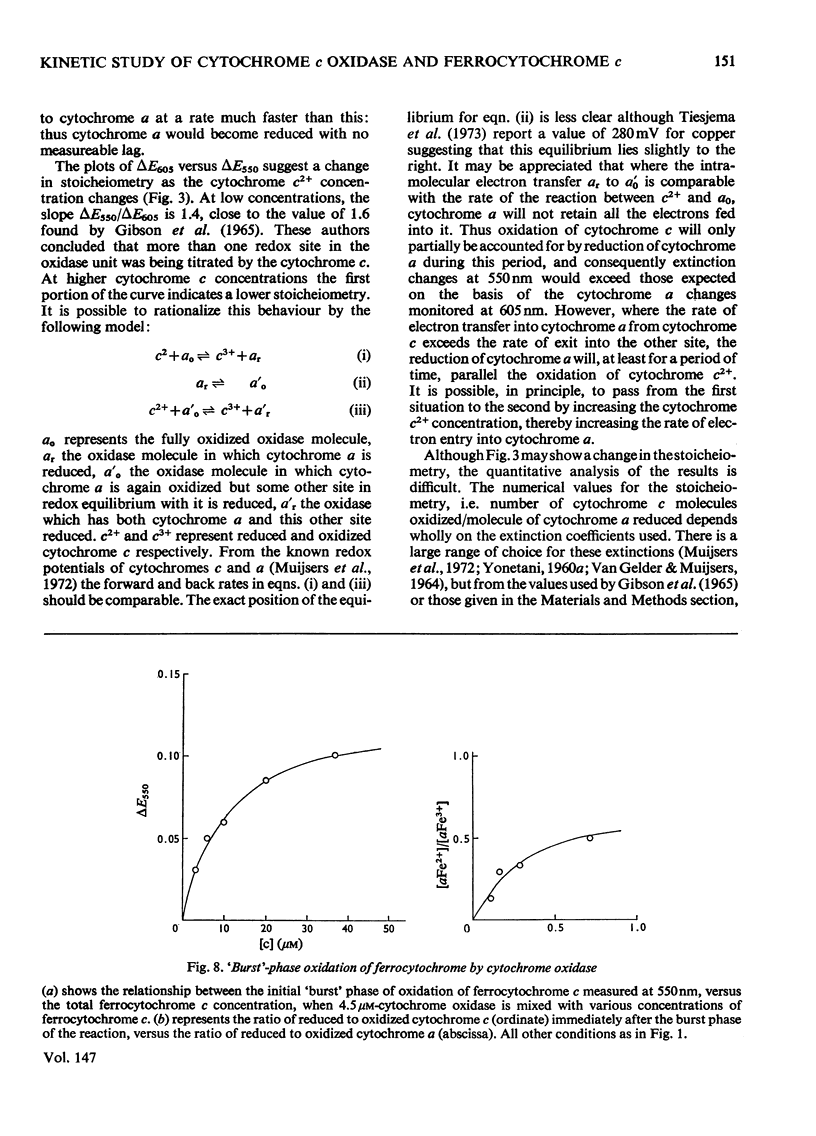
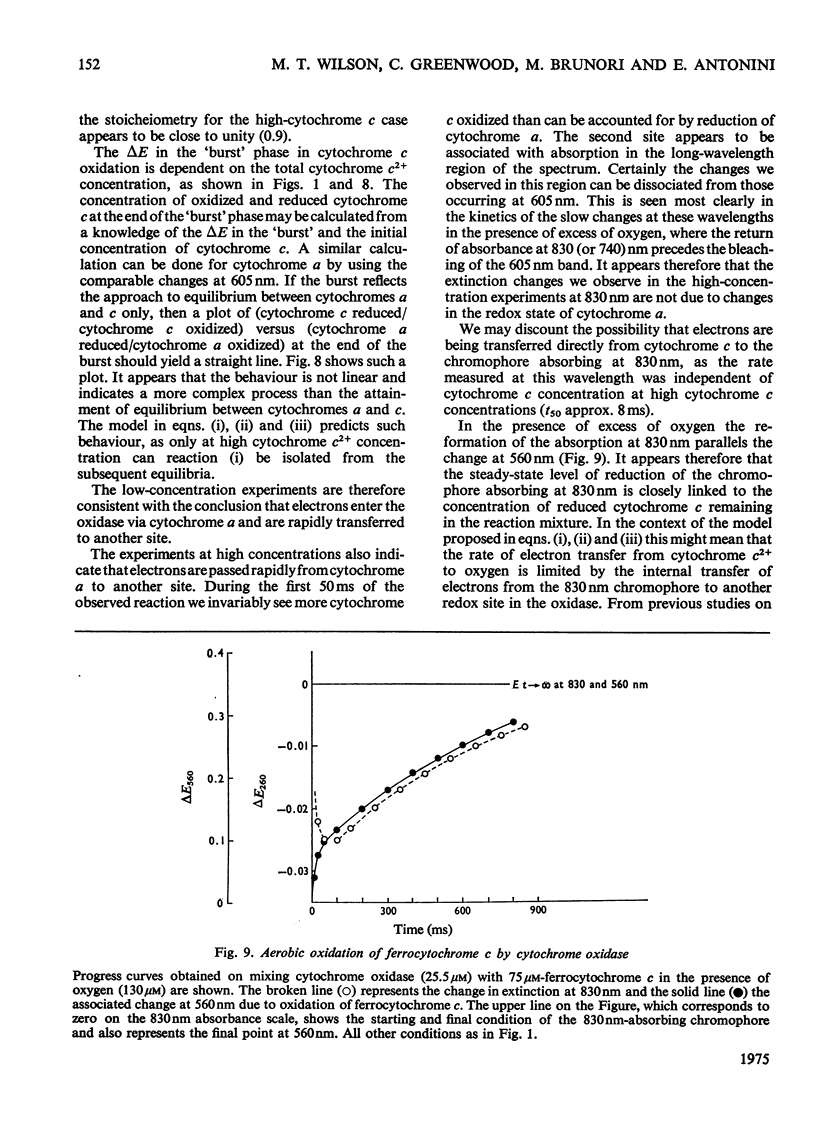
In stopped-flow experiments in which oxidized cytochrome c oxidase was mixed with ferrocytochrome c in the presence of a range of oxygen concentrations and in the absence and presence of cyanide, a fast phase, reflecting a rapid approach to an equilibrium, was observed. Within this phase, one or two molecules of ferrocytochrome were oxidized per haem group of cytochrome a, depending on the concentration of ferrocytochrome c used. The reasons for this are discussed in terms of a mechanism in which all electrons enter through cytochrome a, which, in turn, is in rapid equilibrium with a second site, identified with 'visible' copper (830 nm-absorbing) Cud (Beinert et al., 1971). The value of the bimolecular rate constant for the reaction between cytochromes c2+ and a3+ was between 10(6) and 10(7) M(-1)-S(-1); some variability from preparation to preparation was observed. At high ferrocytochrome c concentrations, the initial reaction of cytochrome c2+ with cytochrome a3+ could be isolated from the reaction involving the 'visible' copper and the stoicheiometry was found to approach one molecule of cytochrome c2+ oxidized for each molecule of cytochrome a3+ reduced. At low ferrocytochrome c concentrations, however, both sites (i.e. cytochrome a and Cud) were reduced simultaneously and the stoicheiometry of the initial reaction was closer to two molecules of cytochrome c2+ oxidized per molecule of cytochrome a reduced. The bleaching of the 830 nm band lagged behind or was simultaneous with the formation of the 605 nm band and does not depend on the cytochrome c concentration, whereas the extinction at the steady-state does. The time-course of the return of the 830 nm-absorbing species is much faster than the bleaching of the 605 nm-absorbing component, and parallels that of the turnover phase of cytochrome c2+ oxidation. Additions of cyanide to the oxidase preparations had no effect on the observed stoicheiometry or kinetics of the reduction of cytochrome a and 'visible' copper, but inhibited electron transfer to the other two sites, cytochrome a3 and the undetectable copper, Cuu.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andréasson L. -E., Malmström B. G., Strömberg C., Vänngård T. The reaction of ferrocytochrome c with cytochrome oxidase: A new look. FEBS Lett. 1972 Dec 15;28(3):297–301. doi: 10.1016/0014-5793(72)80735-x. [DOI] [PubMed] [Google Scholar]
- Antonini E., Brunori M., Greenwood C., Malmström B. G. Catalytic mechanism of cytochrome oxidase. Nature. 1970 Dec 5;228(5275):936–937. doi: 10.1038/228936a0. [DOI] [PubMed] [Google Scholar]
- Antonini E., Brunori M., Greenwood C., Malmström B. G., Rotilio G. C. The interaction of cyanide with cytochrome oxidase. Eur J Biochem. 1971 Nov 11;23(2):396–400. doi: 10.1111/j.1432-1033.1971.tb01633.x. [DOI] [PubMed] [Google Scholar]
- BEINERT H., PALMER G. OXIDATION-REDUCTION OF THE COPPER COMPONENT OF CYTOCHROME OXIDASE. KINETIC STUDIES WITH A RAPID FREEZING TECHNIQUE. J Biol Chem. 1964 Apr;239:1221–1227. [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. THE REACTION OF CYTOCHROME OXIDASE WITH CYTOCHROME C. J Biol Chem. 1965 Feb;240:888–894. [PubMed] [Google Scholar]
- GRIFFITHS D. E., WHARTON D. C. Studies of the electron transport system. XXXV. Purification and properties of cytochrome oxidase. J Biol Chem. 1961 Jun;236:1850–1856. [PubMed] [Google Scholar]
- GRIFFITHS D. E., WHARTON D. C. Studies of the electron transport system. XXXVI. Properties of copper in cytochroma oxidase. J Biol Chem. 1961 Jun;236:1857–1862. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell C. R., Hansen R. E., Beinert H. Electron carriers of cytochrome oxidase detectable by electron paramagnetic resonance and their relationship to those traditionally recognized in this enzyme. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2477–2481. doi: 10.1073/pnas.70.9.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg M. R. Cytochrome oxidase. Physiol Rev. 1969 Jan;49(1):48–121. doi: 10.1152/physrev.1969.49.1.48. [DOI] [PubMed] [Google Scholar]
- MINNAERT K. The kinetics of cytochrome c oxidase. I. The system: cytochrome c-cytochrome oxidase-oxygen. Biochim Biophys Acta. 1961 Jun 10;50:23–34. doi: 10.1016/0006-3002(61)91055-1. [DOI] [PubMed] [Google Scholar]
- MINNAERT K. The kinetics of cytochrome c oxidase. II. The system: reducing agent-cytochrome c-cytochrome oxidase-oxygen. Biochim Biophys Acta. 1961 Nov 25;54:26–41. doi: 10.1016/0006-3002(61)90934-9. [DOI] [PubMed] [Google Scholar]
- Muijsers A. O., Tiesjema R. H., Henderson R. W., Van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VII. The effect of cytochrome c on the oxidation-reduction potential of isolated cytochrome aa 3 . Biochim Biophys Acta. 1972 Apr 20;267(1):216–221. doi: 10.1016/0005-2728(72)90154-5. [DOI] [PubMed] [Google Scholar]
- SCHEJTER A., GLAUSER S. C., GEORGE P., MARGOLIASH E. SPECTRA OF CYTOCHROME C MONOMER AND POLYMERS. Biochim Biophys Acta. 1963 Aug 6;73:641–643. doi: 10.1016/0006-3002(63)90334-2. [DOI] [PubMed] [Google Scholar]
- SMITH L. Reactions of cytochromes a and a3. I. Studies of oxidation and reduction of the pigments in a purified preparation. J Biol Chem. 1955 Aug;215(2):833–846. [PubMed] [Google Scholar]
- Tiesjema R. H., Muijsers A. O., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. X. Spectral and potentiometric properties of the hemes and coppers. Biochim Biophys Acta. 1973 Apr 27;305(1):19–28. doi: 10.1016/0005-2728(73)90227-2. [DOI] [PubMed] [Google Scholar]
- WHARTON D. C., TZAGOLOFF A. STUDIES ON THE ELECTRON TRANSFER SYSTEM. LVII. THE NEAR INFRARED ABSORPTION BAND OF CYTOCHROME OXIDASE. J Biol Chem. 1964 Jun;239:2036–2041. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. II. Steady state properties. J Biol Chem. 1960 Nov;235:3138–3143. [PubMed] [Google Scholar]


