Abstract
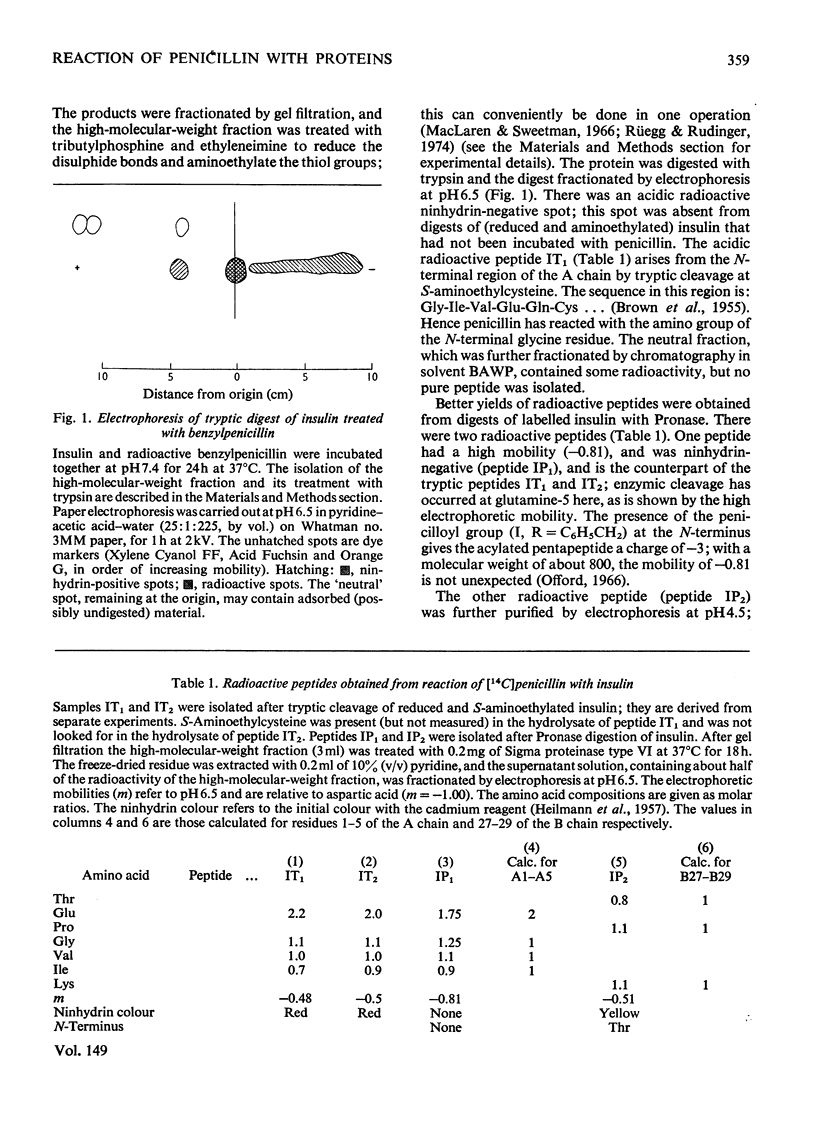
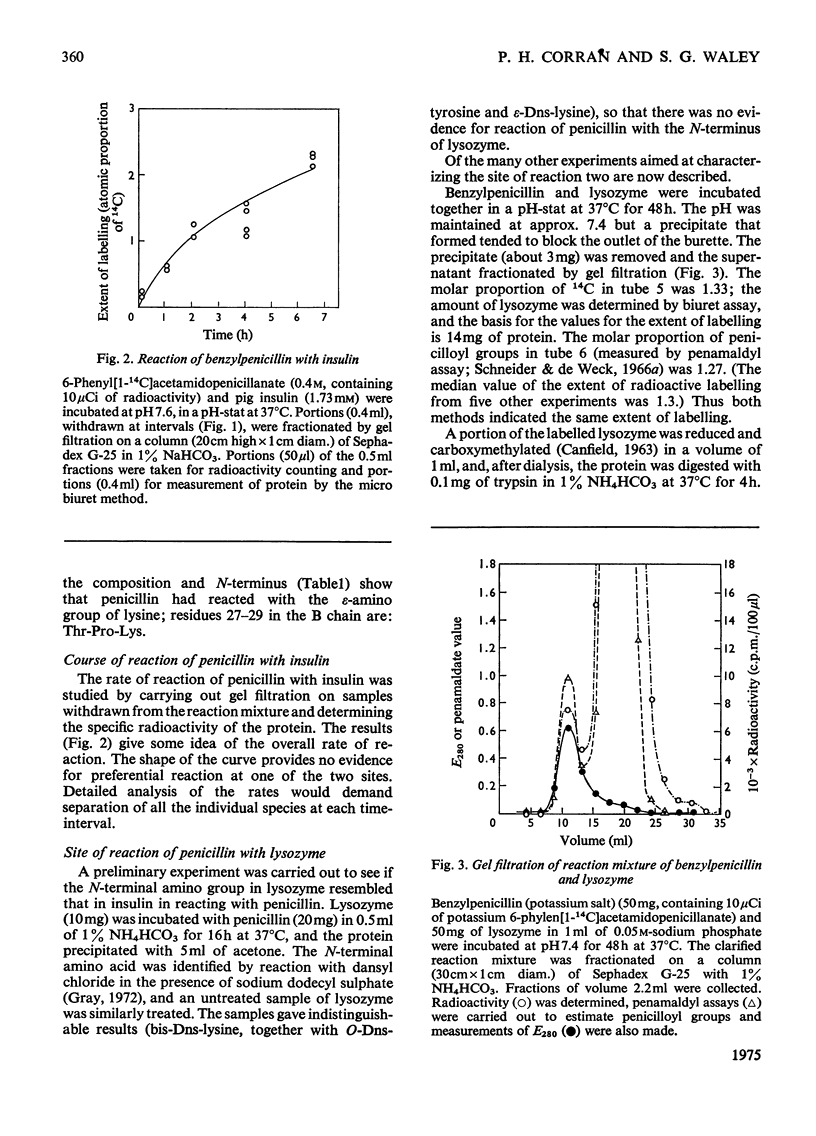
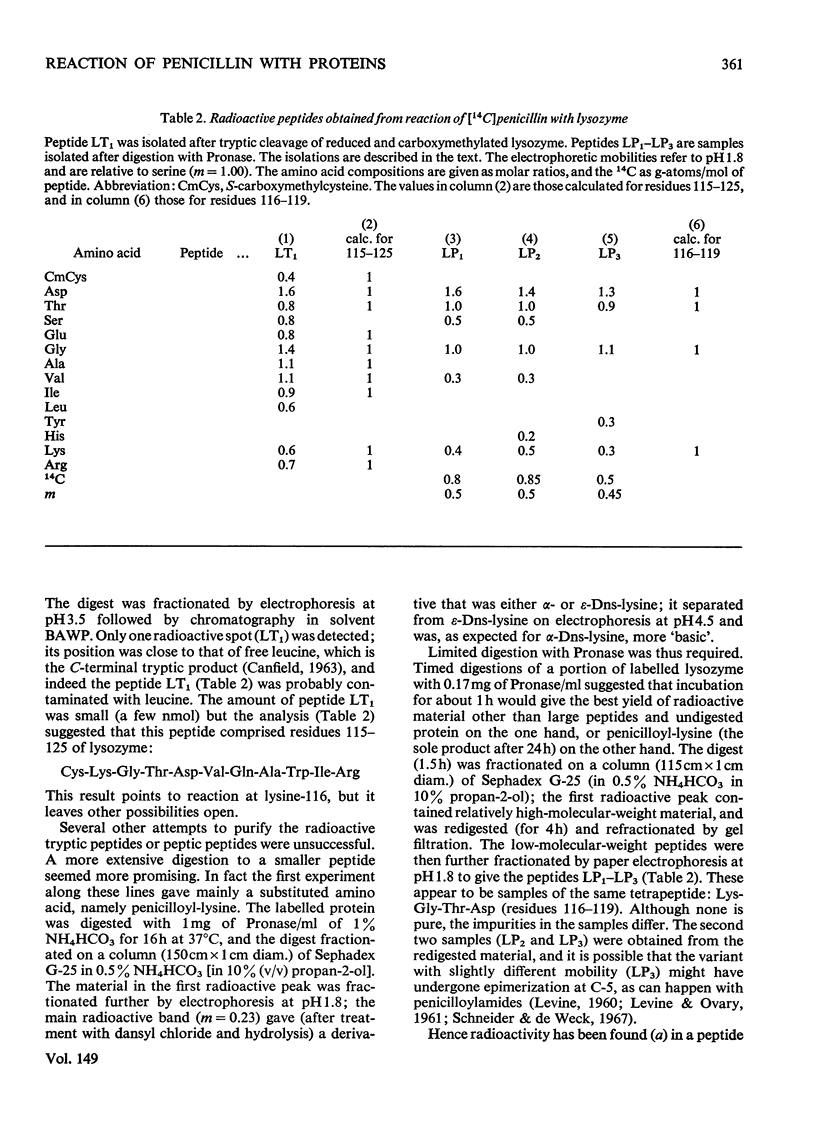
The mode of reaction of benzylpenicillin with two proteins was studied, with particular reference to the allergenicity of penicillin. These reactions, with pig insulin, and with hen's-egg-white lysozyme, were carried out in neutral solution at 37 degrees C. High concentrations of penicillin are needed to label the proteins, owing to concurrent hydrolysis of penicillin. Evidence has been obtained that the penicillin-reactive sites on the insulin molecule are the alpha-amino group at the N-terminus of the A chain and the epsilon-amino group of the lysine residue; whereas a site of reaction with lysozyme appears to be the epsilon-amino group of lysine-116.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN H., SANGER F., KITAI R. The structure of pig and sheep insulins. Biochem J. 1955 Aug;60(4):556–565. doi: 10.1042/bj0600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor F. R., Dewdney J. M., Gazzard D. Penicillin allergy: the formation of the penicilloyl determinant. Nature. 1965 Apr 24;206(982):362–364. doi: 10.1038/206362a0. [DOI] [PubMed] [Google Scholar]
- Brandenburg D., Gattner H. G., Wollmer A. Darstellung und Eigenschaften von Acetylderivaten des Rinderinsulins. Hoppe Seylers Z Physiol Chem. 1972 Apr;353(4):599–617. [PubMed] [Google Scholar]
- Browne C. A., Waley S. G. Studies of triose phosphate isomerase by hydrogen exchange. Biochem J. 1974 Sep;141(3):753–760. doi: 10.1042/bj1410753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANFIELD R. E. PEPTIDES DERIVED FROM TRYPTIC DIGESTION OF EGG WHITE LYSOZYME. J Biol Chem. 1963 Aug;238:2691–2697. [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The tryptic peptides of rabbit muscle triose phosphate isomerase. Biochem J. 1974 Apr;139(1):1–10. doi: 10.1042/bj1390001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Abraham E. P. Specificities of haemagglutinating antibodies evoked by members of the cephalosporin C family and benzylpenicillin. Biochem J. 1971 Jun;123(2):183–190. doi: 10.1042/bj1230183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idsoe O., Guthe T., Willcox R. R., de Weck A. L. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38(2):159–188. [PMC free article] [PubMed] [Google Scholar]
- Johnson L. N. An interaction between lysozyme and penicillin. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):439–440. doi: 10.1098/rspb.1967.0042. [DOI] [PubMed] [Google Scholar]
- LEVINE B. B. Degradation of benzylpenicillin at pH 7.5 to D-benzylpenicilloic acid. Nature. 1960 Sep 10;187:939–940. doi: 10.1038/187939a0. [DOI] [PubMed] [Google Scholar]
- LEVINE B. B. N(ALPHA-D-PENICILLOYL) AMINES AS UNIVALENT HAPTEN INHIBITORS OF ANTIBODYDEPENDENT ALLERGIC REACTIONS TO PENICILLIN. J Med Pharm Chem. 1962 Sep;91:1025–1034. doi: 10.1021/jm01240a016. [DOI] [PubMed] [Google Scholar]
- LEVINE B. B., OVARY Z. Studies on the mechanism of the formation of the penicillin antigen. III. The N-(D-alpha-benzylpenicilloyl) group as an antigenic determinant responsible for hypersensitivity to penicillin G. J Exp Med. 1961 Dec 1;114:875–904. doi: 10.1084/jem.114.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. G., Shall S. The acetylation of insulin. Biochem J. 1971 Mar;121(5):737–745. doi: 10.1042/bj1210737a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. C., Waley S. G. Amino acid sequences around the cysteine residues of rabbit muscle triose phosphate isomerase. Biochem J. 1971 Apr;122(2):209–218. doi: 10.1042/bj1220209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K., Piszkiewicz D., Smith E. L. Amino acd sequence of chicken liver glutamate dehydrogenase. J Biol Chem. 1973 May 10;248(9):3093–3107. [PubMed] [Google Scholar]
- NAKKEN K. F., ELDJARN L., PIHL A. The mechanism of inactivation of penicillin by cysteine and other mercaptoamines. Biochem Pharmacol. 1960 May;3:89–100. doi: 10.1016/0006-2952(60)90025-3. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Jao L., Dahlquist F. W., Borders C. L., Jr, Racs J., Groff T., Raftery M. A. The nature of amino acid side chains which are critical for the activity of lysozyme. Biochemistry. 1969 Feb;8(2):700–712. doi: 10.1021/bi00830a036. [DOI] [PubMed] [Google Scholar]
- Phillips D. C. The three-dimensional structure of an enzyme molecule. Sci Am. 1966 Nov;215(5):78–90. doi: 10.1038/scientificamerican1166-78. [DOI] [PubMed] [Google Scholar]
- SLUYTERMAN L. A. Electrophoretic behaviour in filter paper and molecular weight of insulin. Biochim Biophys Acta. 1955 Jun;17(2):169–176. doi: 10.1016/0006-3002(55)90347-4. [DOI] [PubMed] [Google Scholar]
- Schneider C. H., de Weck A. L. Chemische Aspekte der Penicillin-Allergie: Die direkte Penicilloylierung von epsilon-Aminogruppen durch Penicilline bei pH 7,4. Helv Chim Acta. 1966 Jul 11;49(5):1695–1706. doi: 10.1002/hlca.19660490532. [DOI] [PubMed] [Google Scholar]
- Schneider C. H., de Weck A. L. Studies on the direct neutral penicilloylation of functional groups occurring on proteins. Biochim Biophys Acta. 1968 Sep 10;168(1):27–35. doi: 10.1016/0005-2795(68)90230-4. [DOI] [PubMed] [Google Scholar]
- Schneider C. H., de Weck A. L. Unterscheidung der Penicilloinsäuren von funktionellen Derivaten ihrer alpha-Carboxylgruppe mittels Penamaldat-Stabilitätsbestimmung. Helv Chim Acta. 1966 Jul 11;49(5):1689–1694. doi: 10.1002/hlca.19660490531. [DOI] [PubMed] [Google Scholar]
- Shrake A., Rupley J. A. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J Mol Biol. 1973 Sep 15;79(2):351–371. doi: 10.1016/0022-2836(73)90011-9. [DOI] [PubMed] [Google Scholar]
- Smith H., Marshall A. C. Polymers formed by some beta-lactam antibiotics. Nature. 1971 Jul 2;232(5305):45–46. doi: 10.1038/232045a0. [DOI] [PubMed] [Google Scholar]
- Stewart G. T. Allergy to penicillin and related antibiotics: antigenic and immunochemical mechanism. Annu Rev Pharmacol. 1973;13:309–324. doi: 10.1146/annurev.pa.13.040173.001521. [DOI] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weck A. L., Schneider C. H., Gutersohn J. The role of penicilloylated protein impurities, penicillin polymers and dimers in penicillin allergy. Int Arch Allergy Appl Immunol. 1968;33(6):535–567. doi: 10.1159/000230070. [DOI] [PubMed] [Google Scholar]


