Abstract
Background
Protein energy wasting (PEW) is prevalent in adult maintenance hemodialysis (MHD) patients. Concurrently, cardiovascular diseases (CVD) remain a leading cause of mortality in MHD patients. However, the relationship between PEW and CVD in MHD patients remains unclear.
Methods
We conducted a retrospective cohort study at Shanghai East Hospital. According to the inclusion and exclusion criteria, a total of 210 adult MHD patients were finally enrolled. Patients were categorized into two groups based on PEW diagnostic criteria, including 122 patients (58.1%) with PEW and 88 patients (41.9%) without PEW. We further analyzed the incidence of major adverse cardiovascular events (MACE) and all-cause mortality in one year, along with their risk factors.
Results
MACE incidence was significantly higher in the PEW group compared with the non-PEW group (p = 0.015). Multivariate Cox regression showed PEW, CVD, high N-terminal pro-B-type natriuretic peptide (NT-proBNP) and low Kt/V urea were the risk factors of MACE. Age ≥ 65 years and high NT-proBNP were the risk factors of all-cause death. Among patients aged ≥ 65 years, PEW was associated with a higher risk of all-cause death (p = 0.043). Total cholesterol < 3.4 mmol/L, albumin < 38 g/L and prealbumin < 280 mg/L were the thresholds for MACE incidence in MHD patients with PEW.
Conclusion
Adult MHD patients with PEW had an increased risk of MACE and all-cause mortality. Strategies aimed at optimizing total cholesterol, albumin, and prealbumin levels may improve cardiovascular outcomes in adult MHD patients with PEW.
Keywords: Protein energy wasting, major adverse cardiovascular events, hemodialysis, mortality, risk factors
1. Introduction
The prevalence of chronic kidney diseases (CKD) has a significant impact on global health due to its direct contribution to global mortality and an important risk factor for cardiovascular diseases (CVD) [1]. Individuals afflicted with CKD, in particular those on maintenance hemodialysis (MHD), have an elevated risk for protein energy wasting (PEW) [2,3]. PEW is a state of decreased protein and energy reserves in the body, clinically manifested as a syndrome characterized by inadequate dietary nutrient and calorie intake, low body mass index (BMI), low serum albuminemia, a microinflammatory state, and progressive skeletal muscle wasting [2,4,5]. This syndrome has been shown to significantly impact the quality of life and increase the risk of morbidity and mortality with a global prevalence ranging from 28%-54% [6–8].
Furthermore, CVD has been confirmed as the leading cause of mortality among dialysis patients [9,10]. Research indicates that there is an additive interaction between these factors, meaning that when PEW and CVD coexist, the risk of death is significantly higher than expected [11]. The mechanism by which PEW contributes to CVD likely involves the combined effects of inflammation and malnutrition [12,13]. Inflammation alters vascular endothelium and plasma protein composition, promoting vascular injury [14,15]. Malnutrition depletes muscle proteins and decreases serum albumin concentrations, impairing repair mechanisms [12]. Additionally, malnutrition may contribute to the progression of heart failure by inducing morphological and functional deterioration of the myocardium [16].
Nevertheless, the evidence indicating that PEW promotes the occurrence of cardiovascular events is limited. The relationship between PEW and CVD remains unclear. We previously found that N-terminal pro-B-type natriuretic peptide (NT-proBNP) was an independent predictor for PEW [17]. NT-proBNP has gained recognition as a valuable biomarker, offering diagnostic and prognostic utility of heart failure in CVD patients [18,19]. The objective of this retrospective study was to elucidate the association between PEW and MACE, and to assess the factors associated with all-cause mortality in MHD patients.
2. Materials and methods
2.1. Study population
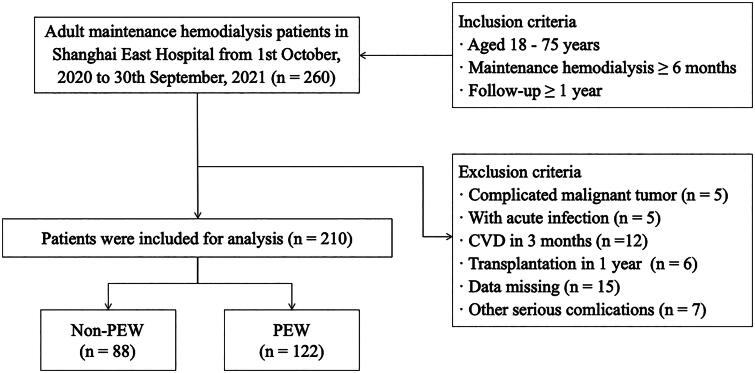
This was a single-center retrospective cohort study involving adult patients undergoing maintenance hemodialysis (MHD) in Shanghai East Hospital between October 1, 2020 and September 30, 2021. The inclusion criteria of patients were: age ≤ 75 years; more than 6 months with hemodialysis duration; more than 1 year follow-up. Patients were excluded based on the following criteria: complicated malignant tumor; acute infection; transplantation within 1 year; CVD within 3 months; missing data and other conditions (pregnancy; thyroid dysfunction; corticosteroid or immunosuppressive medication; operations; trauma; patients enrolled in other clinical studies; poor compliance). A total of 210 eligible patients were ultimately included in the study. According to the diagnostic criteria of the PEW concept introduced by the International Society for the Study of Renal Medicine (ISRNM) in 2008 [20], patients were separated into two groups: PEW group and non-PEW group. The layout of the research program is shown in the schematic diagram in Figure 1.
Figure 1.
Flowchart of the study.
260 adult maintenance hemodialysis patients were screened in the study, and 50 patients were excluded according to the exclusion criteria. 210 patients were finally enrolled, consisted of 122 patients with PEW and 88 patients with non-PEW.
PEW: protein energy wasting.
This study was conducted according to the guidelines of the Helsinki Declaration. The study protocol was approved by the Human Research Ethics Committee of Shanghai East Hospital (ChiCTR2000038127).
2.2. Data collection
Clinical and laboratory data were obtained from medical records and consisted of the following main elements: age, gender, education level, dialysis duration, height, weight, primary renal disease, comorbidities (hypertension, diabetes, hyperlipidemia, stroke, and CVD), medicates (antiplatelet, angiotensin converting enzyme inhibitors/angiotensin receptor blocker, β-blockers, statins), systolic blood pressure, diastolic blood pressure. A standardized questionnaire was used to obtain mid-arm muscle circumference (MAMC) and daily protein intake (DPI). MAMC was calculated using the following formula: MAMC = arm circumference (mm) − 3.14 ∗ triceps skin-fold thickness (mm) [21]. DPI was estimated using a 3-day dietary questionnaire to record the dietary intake of each patient for three consecutive days (including two working days and one weekend) [22]. BMI was calculated by dividing the dry weight of dialysis patients by their heightˆ2.
Venous blood samples were obtained 12 h after an overnight fast. Biochemistry data including kidney function, liver function, blood lipid profile, complete blood count, vitamin D, NT-proBNP, inflammation markers and urea clearance index (Kt/V urea) were collected. All central laboratory data testing methods were harmonized.
2.3. The diagnostic criteria for PEW
The diagnostic criteria for PEW are as follows [20]: At least three of the following four categories must fulfill the diagnostic requirements for PEW associated with kidney disease, and each criterion should be documented at least three times, preferably at 2-4 week intervals: (1) biochemistry: serum albumin < 38 g/L, serum prealbumin < 300 mg/L, or serum total cholesterol (TC) < 1 g/L; (2) body mass: BMI < 23 kg/m2, unintentional 5% weight loss over 3 months or 10% weight loss over 6 months, and total body fat percentage < 10%; (3) muscle mass: reduced 5% muscle mass over 3 months or 10% over 6 months, reduction of MAMC area over 10% about 50th percentile of reference population, and creatinine appearance; and (4) DPI: unintentional low DPI < 0.80 g/kg per day for at least 2 months for dialysis patients or DPI < 0.60 g/kg per day for patients with CKD stages 2–5 [20].
2.4. Definitions and end points
The primary endpoint was defined as first onset of MACE during follow-up period, which included myocardial infarction (ICD-10: I21-I22), heart failure (ICD-10: I50.0-I50.9), or cardiovascular mortality (ICD-10: I00-I99). The secondary endpoint was all-cause mortality. The time frame for the survival analysis was defined as the period from the date of inclusion to one year.
2.5. Statistical analysis
Descriptive statistics are reported as frequencies and proportions for categorical variables and as medians (IQR) or means (SD) for continuous variables. The independent sample t-test, Mann–Whitney U test, or test was used to compare differences between PEW and non-PEW participants. The risk factors of MACE and all-cause death were identified using univariate and multivariate Cox proportional-hazards models. Variables of biochemical parameters were Ln-transformed to approximate normality for analysis. The endpoints were defined as the first onset of MACE and all-cause mortality during follow-up period. The percentage of patients with MACE was presented in percentage-packed histograms. Cumulative rate of MACE incidence and all-cause mortality between two groups were calculated and analyzed using the Kaplan-Meier method. The associations between continuous variables and MACE incidence in the PEW group were evaluated with restricted cubic spline (RCS) diagram based on Cox proportional hazards. All probabilities were two-tailed, and the significance level was set at 0.05.
Statistical analyses were performed using SPSS (version 23.0), and RStudio (version 2021.09.1 + 372). The histograms and the Kaplan-Meier Curves were performed by Graph Prism 8.0 and the RCS curves were performed by RStudio.
3. Results
3.1. Patients
A total of 260 MHD patients were screened. According to the inclusion and exclusion criteria, 210 patients entered the final analysis (Figure 1). Patients were classified into two groups based on the ISRNM diagnostic criteria of PEW, including 122 patients (58.1%) in the PEW group and 88 patients (41.9%) in the non-PEW group. The baseline demographic and clinical characteristics of the two groups patients were shown in Table 1. The two groups had similar ages, gender distribution, education levels, dialysis duration and blood pressure (p > 0.05). The number of patients with hyperlipidemia and smokers was higher in the non-PEW group than in the PEW group (p < 0.05). The prevalence of CVD was similar between the two groups (p > 0.05). In terms of the biochemical parameters, albumin, prealbumin, blood urea nitrogen, serum creatinine, serum uric acid and high-density lipoprotein cholesterol were lower in the PEW group than in the non-PEW group (p < 0.05). Additionnally, the NT-proBNP level in the PEW group was higher compare to the non-PEW group (p < 0.05). There was no significant difference in medication between the two groups (p > 0.05). The categories of CVD among the patients at baseline were shown in Table 2.
Table 1.
Baseline characteristics between the PEW and the non-PEW groups.
| Variables | PEW (n = 122) | Non-PEW (n = 88) | Reference value | t/x2/Z | P value |
|---|---|---|---|---|---|
| Age, years | 65 (58, 70) | 65 (56, 71) | – | −0.032 | 0.974 |
| Male, n (%) | 72 (59.02) | 58 (65.91) | – | 1.030 | 0.310 |
| Education level, n (%) | – | 5.457 | 0.141 | ||
| Primary school | 20 (16.39) | 22 (25.00) | |||
| Junior school | 43 (35.25) | 22 (25.00) | |||
| High school | 32 (26.23) | 18 (25.00) | |||
| College and above | 27 (22.13) | 26 (29.55) | |||
| Hypertension, n (%) | 108 (90.00) | 79 (90.80) | – | 0.037 | 0.847 |
| Diabetes, n (%) | 77 (64.71) | 50 (57.47) | – | 1.113 | 0.292 |
| Hyperlipidemia, n (%) | 61 (50.41) | 67 (77.01) | – | 15.128 | <0.001 |
| CVD, n (%) | 42 (35.00) | 26 (29.55) | – | 0.686 | 0.407 |
| Stroke, n (%) | 15 (12.50) | 10 (11.49) | – | 0.048 | 0.827 |
| Smoker, n (%) | 20 (19.23) | 28 (35.44) | – | 6.099 | 0.014 |
| Dialysis duration (months) | 40 (17, 73) | 37 (24, 59) | – | −0.292 | 0.771 |
| Frequency of monthly HDF | 4 (4, 5) | 4 (4, 5) | – | −0.155 | 0.877 |
| SBP (mmHg) | 149.98 ± 26.02 | 151.22 ± 21.13 | – | −0.307 | 0.759 |
| DBP (mmHg) | 80.38 ± 13.04 | 82.08 ± 14.21 | – | −0.795 | 0.428 |
| BMI (kg/m2) | 21.44 ± 2.77 | 24.59 ± 3.09 | – | −7.725 | <0.001 |
| Serum albumin (g/L) | 38.07 ± 4.02 | 40.87 ± 5.03 | 40 - 55 | −4.477 | <0.001 |
| Serum prealbumin (mg/L) | 285.23 ± 83.09 | 337.98 ± 71.95 | 180-350 | −4.779 | <0.001 |
| ALT (U/L) | 11.69 ± 8.00 | 12.35 ± 6.97 | 7-40 | −0.618 | 0.537 |
| AST (U/L) | 12.82 ± 6.29 | 13.90 ± 7.43 | 13-35 | −1.131 | 0.259 |
| Hemoglobin (g/L) | 109.00 (100.00, 116.00) | 110.00 (100.00, 120.00) | 115-150 | −0.795 | 0.426 |
| CRP (mg/L) | 2.10 (1.60, 6.32) | 2.00 (1.60, 5.62) | 0-6 | −0.498 | 0.618 |
| BUN (mmol/L) | 23.47 ± 6.05 | 25.36 ± 6.22 | 2.6-7.5 | −2.191 | 0.030 |
| Scr (μmol/L) | 889.54 ± 227.66 | 981.90 ± 247.73 | 41-73 | −2.795 | 0.006 |
| SUA (μmol/L) | 416.07 ± 98.81 | 447.05 ± 98.05 | 143-339 | −2.249 | 0.026 |
| TG (mmol/L) | 1.41 (0.96, 2.34) | 2.20 (1.27, 3.03) | < 1.7 | −3.544 | <0.001 |
| TC (mmol/L) | 3.45 (2.64, 4.18) | 3.58 (2.97, 4.33) | < 5.2 | −1.951 | 0.051 |
| HDL-c (mmol/L) | 1.02 (0.84, 1.16) | 0.89 (0.77, 1.05) | > 1.04 | −2.451 | 0.014 |
| LDL-c (mmol/L) | 1.90 (1.22, 2.59) | 1.93 (1.52, 2.47) | < 3.4 | −1.217 | 0.224 |
| Plasma calcium (mmol/L) | 2.36 ± 0.19 | 2.36 ± 0.18 | 2.11-2.52 | 0.042 | 0.967 |
| Plasma magnesium (mmol/L) | 1.16 ± 0.70 | 1.09 ± 0.15 | 0.75-1.02 | 0.857 | 0.392 |
| Plasma phosphorous (mmol/L) | 1.70 ± 0.50 | 1.81 ± 0.52 | 0.85-1.51 | −1.602 | 0.111 |
| Serum iron (μmol/L) | 9.90 (8.18, 14.10) | 11.55 (8.45, 14.08 | 7.8-32.2 | −1.131 | 0.258 |
| iPTH (pg/mL) | 264.50 (150.75, 434.75) | 271.00 (195.25, 433.25) | 15-65 | −0.734 | 0.463 |
| FBG (mmol/L) | 7.36 (6.18, 9.66) | 7.05 (6.31, 10.09) | 3.9-6.1 | −0.370 | 0.712 |
| Vitamin D (ng/mL) | 13.09 (10.00, 18.25) | 15.00 (11.79, 21.57) | ≥ 20 | −1.758 | 0.079 |
| NT-proBNP (ng/L) | 6,339.50 (2315.25, 19993.25) | 3,679.50 (1795.75, 10584.50) | 0-125 | −2.233 | 0.026 |
| Kt/V urea | 1.39 (1.27, 1.62) | 1.31 (1.20, 1.57) | – | −1.933 | 0.053 |
| Medicate, n (%) | – | ||||
| Antiplatelet drugs | 42 (34.43) | 31 (35.23) | 0.014 | 0.904 | |
| ACEI / ARB | 44 (36.07) | 30 (34.09) | 0.087 | 0.768 | |
| β-blockers | 35 (28.69) | 34 (38.64) | 2.293 | 0.130 | |
| Statins | 48 (39.34) | 34 (38.64) | 0.011 | 0.917 |
Mean ± SD presented for variables according with normal distribution while median (IQR) presented for variables with abnormal distribution.
ACEI / ARB: angiotension converting enzyme inhibitors / angiotensin receptor blocker; ALT: alanine aminotransferas; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CRP: C-reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; FBG: fasting blood glucose; HDL-c: high density lipoprotein cholesterol; Kt/V urea: urea clearance index; LDL-c: low density lipoprotein cholesterol; NT-proBNP: N-terminal pro-B-Type Natriuretic Peptide; iPTH: intact Parathyroid Hormone; SBP: systolic blood pressure; Scr: serum creatinine; SUA: serum uric acid; HD: hemodialysis; HDF: hemodiafiltration; TC: total cholesterol; TG: triglycerides.
Note: p < 0.05 was considered as statistically significant.
Table 2.
The categories of CVD in basline.
| CVD categories | PEW (n = 122) | Non-PEW (n = 88) | x 2 | P value |
|---|---|---|---|---|
| Coronary atherosclerotic heart disease, n (%) | 33 (27.05) | 17 (19.32) | 1.684 | 0.194 |
| Hypertensive heart disease, n (%) | 11 (9.02) | 7 (7.95) | 0.074 | 0.786 |
| Atrial fibrillation, n (%) | 7 (5.74) | 3 (3.41) | 0.611 | 0.434 |
| Other severe arrhythmias, n (%) | 0 (0.00) | 2 (2.27) | 2.799 | 0.094 |
| Valvular heart disease, n (%) | 1 (0.82) | 1 (1.14) | 0.054 | 0.816 |
CVD: cardiovascular disease; PEW: protein energy wasting.
Note: p < 0.05 was considered as statistically significant.
3.2. Percentage of patients with MACE in two groups
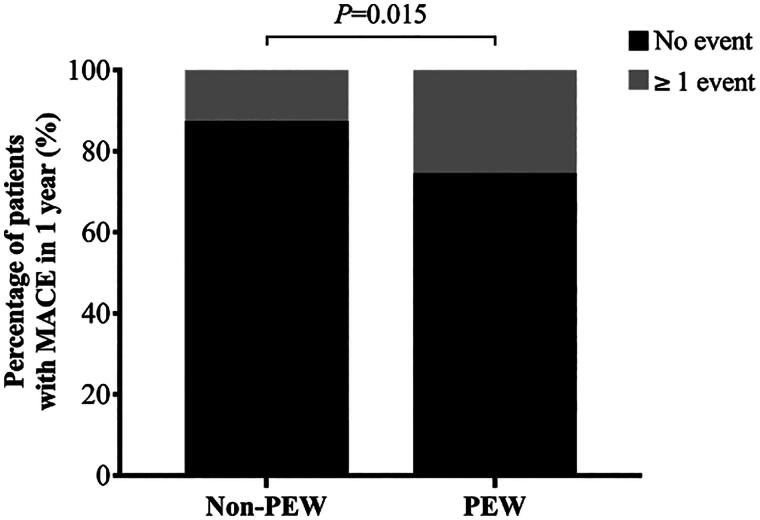
A total of 61 cardiovascular events occurred in both groups, with 43 events in the PEW group and 18 in the non-PEW group. The percentage of patients with MACE was compared between the PEW group and the non-PEW group over a one-year period, as shown in Figure 2. In the non-PEW group, a total of 11 patients (12.5%) experienced more than one MACE during the year. In contrast, in the PEW group, 31 patients (25.4%) experienced more than one MACE. It can be reasonably inferred that the percentage of patients with MACE was higher in the PEW population undergoing MHD (p = 0.015).
Figure 2.
The percentage of MHD patients with MACE between PEW and non-PEW.
Histogram showed the percentage of MHD patients with more than 1 MACE in 1 year between the two groups.
MACE: major adverse cardiovascular event; MHD: maintenance hemodialysis; PEW: protein energy wasting. P < 0.05 was considered to be statistically significant.
3.3. The risk factors for MACE incidence in MHD
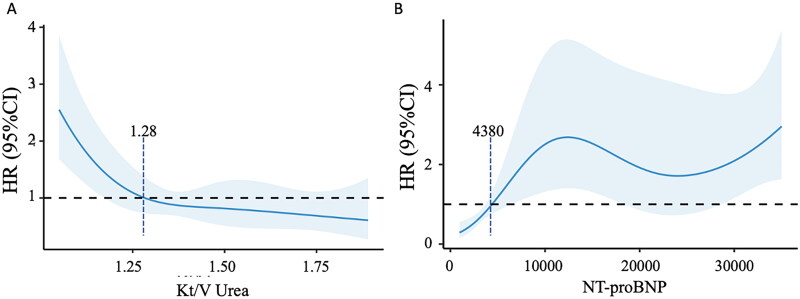
To identify potential risk factors for MACE incidence in MHD patients, we conducted a Cox regression analysis as shown in Table 3. The endpoint was defined as the first onset of MACE during the follow-up period. We included all variables with a p-value less than 0.100 into the multivariate Cox regression model. In the multivariate model, PEW was an independent risk factor for MACE incidence (HR 2.13, 95% CI 1.06 − 4.28, p = 0.034). In addition, CVD (HR 2.15, 95% CI 1.03 − 4.50, p = 0.042), high NT-proBNP (HR 1.38, 95% CI 1.05 − 1.82, p = 0.023), and low Kt/V urea (HR 0.64, 95% CI 0.44 − 0.93, p = 0.02) were also independent factors for the occurrence of MACE. These results suggested that PEW, CVD, higher NT-proBNP and lower Kt/V urea were independent risk factors for MACE incidence in MHD patients. The optimal thresholds of Kt/V urea and NT-proBNP were evaluated by RCS curves as 1.28 and 4380 ng/mL, respectively, as shown in Figure 3.
Table 3.
The risk factors of MACE based on univariate and multivariate Cox regression analysis.
| MACE |
||||
|---|---|---|---|---|
| univariate analysis |
multivariate analysis |
|||
| variables | HR (95%CI) | P | HR (95%CI) | P |
| Gender (male) | 0.55 (0.28-1.90) | 0.088 | 0.94 (0.45-1.98) | 0.871 |
| Age (per year) | 1.03 (0.99-1.06) | 0.134 | ||
| PEW | 2.17 (1.09-4.32) | 0.027 | 2.13 (1.06-4.28) | 0.034 |
| Dialysis duration (months) | 1.00 (0.99-1.01) | 0.757 | ||
| Frequency of monthly HDF | 0.93 (0.77-1.11) | 0.410 | ||
| Education level | 0.537 | |||
| Primary school | ref | |||
| Junior school | 1.22 (0.48-3.11) | 0.672 | ||
| High school | 0.96 (0.35-2.65) | 0.939 | ||
| College and above | 1.80 (0.73-4.47) | 0.203 | ||
| Hypertension | 1.45 (0.45-4.70) | 0.553 | ||
| Diabetes | 1.26 (0.67-2.40) | 0.476 | ||
| Hyperlipidemia | 0.93 (0.50-1.73) | 0.821 | ||
| Stroke | 1.24 (0.52-2.93) | 0.633 | ||
| CVD | 4.08 (2.19-7.60) | <0.001 | 2.15 (1.03-4.50) | 0.042 |
| Smoker | 1.14 (0.58-2.23) | 0.702 | ||
| SBP (per 10 mmHg) | 1.00 (0.99-1.01) | 0.829 | ||
| DBP (per10 mmHg) | 0.99 (0.97-1.02) | 0.609 | ||
| ALT (U/L)a | 1.12 (0.86-1.46) | 0.392 | ||
| AST (U/L)a | 1.06 (0.80-1.40) | 0.680 | ||
| BUN (mmol/L)a | 0.91 (0.67-1.24) | 0.562 | ||
| Scr (μmol/L)a | 0.79 (0.58-1.07) | 0.124 | ||
| SUA (μmol/L)a | 0.83 (0.63-1.10) | 0.197 | ||
| HDL-c (mmol/L)a | 0.83 (0.59-1.16) | 0.270 | ||
| LDL-c (mmol/L)a | 0.59 (0.42-0.84) | 0.003 | 0.79 (0.55-1.25) | 0.217 |
| Plasma calcium (mmol/L)a | 0.81 (0.59-1.09) | 0.163 | ||
| Plasma magnesium (mmol/L)a | 0.49 (0.16-1.47) | 0.202 | ||
| Plasma phosphorous(mmol/L)a | 1.12 (0.87-1.52) | 0.460 | ||
| Serum iron (μmol/L)a | 1.22 (0.91-1.62) | 0.183 | ||
| iPTH (pg/mL)a | 1.15 (0.84-1.57) | 0.381 | ||
| FBG (mmol/L)a | 1.09 (0.83-1.42) | 0.542 | ||
| Vitamin D (ng/mL)a | 0.97 (0.71-1.34) | 0.857 | ||
| NT-proBNP (ng/L)a | 1.68 (1.30-2.17) | <0.001 | 1.38 (1.05-1.82) | 0.023 |
| Hemoglobin (g/L)a | 0.86 (0.64-1.15) | 0.307 | ||
| CRP (mg/L)a | 1.18 (0.96-1.45) | 0.112 | ||
| Kt/V urea a | 0.54 (0.37-0.79) | 0.001 | 0.64 (0.44-0.93) | 0.020 |
| Antiplatelet drugs | 0.35 (0.19-0.64) | 0.001 | 1.56 (0.70-3.47) | 0.277 |
| ACEI / ARB | 0.64 (0.35-1.16) | 0.141 | ||
| β-blockers | 0.98 (0.51-1.86) | 0.944 | ||
| Statins | 0.39 (0.21-0.72) | 0.003 | 1.23 (0.38-2.62) | 0.594 |
aAll laboratory indicators transformed using formula LN(X) to approach a normal distribution.
ACEI / ARB: angiotension converting enzyme inhibitors / angiotensin receptor blocker; ALT: alanine aminotransferas; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CI: confidence interval; CRP: C-reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; FBG: fasting blood glucose; HDF: hemodialysis filtration; HDL-c: high density lipoprotein cholesterol; Kt/V urea: urea clearance index; LDL-c: low density lipoprotein cholesterol; NT-proBNP: N-terminal pro-B-Type Natriuretic Peptide; OR: odds ratio; iPTH: intact Parathyroid Hormone; SBP: systolic blood pressure; Scr: serum creatinine; SUA: serum uric acid.
Note: p < 0.05 was considered as statistically significant. Bold was for p < 0.05 in multivariate Cox regression analysis.
Figure 3.
Association between MACE incidence and Kt/V urea and NT-proBNP with RCS curves in MHD patients.
(A) The relationship of Kt/V urea and MACE incidence based on Cox proportional hazard models. The vertical dashed line indicates the cutoff value of 1.28 mmol/L. (B) The relationship of NT-proBNP and MACE incidence based on Cox proportional hazard models. The vertical dashed line indicates the cutoff value of 4380 ng/mL.
HR: hazard ration; Kt/V urea: urea clearance index; MACE: major adverse cardiovascular event; MHD: maintenance hemodialysis; NT-proBNP: N-terminal pro-B-Type Natriuretic Peptide; RCS: restricted cubic spline.
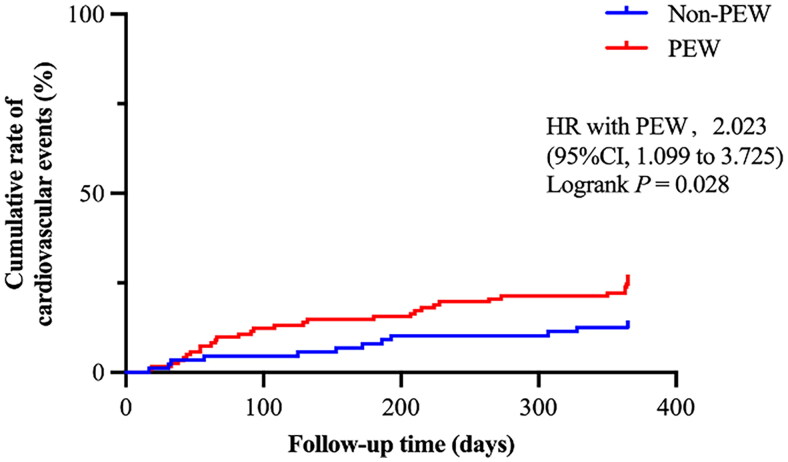
Further Kaplan-Meier curve was conducted and showed that patients with PEW had a higher cumulative rate of MACE incidence in one year than those without PEW (HR with PEW 2.023, 95% CI 1.099 − 3.725, Logrank p = 0.028), as shown in Figure 4. Patients in the PEW group were more likely to experience MACE.
Figure 4.
Kaplan-Meier curve for the MACE in MHD patients with PEW and with non-PEW.
Kaplan-Meier curve was shown for the proportion of cumulative MACE incidence during the follow-up period in MHD patients with PEW compared with patients with non-PEW.
MACE: major adverse cardiovascular event; MHD: maintenance hemodialysis; PEW: protein energy wasting; 95% CI: 95% confidence interval; HR: hazard ratio. P < 0.05 was considered to be statistically significant.
3.4. The risk factors for all-cause mortality in MHD patients
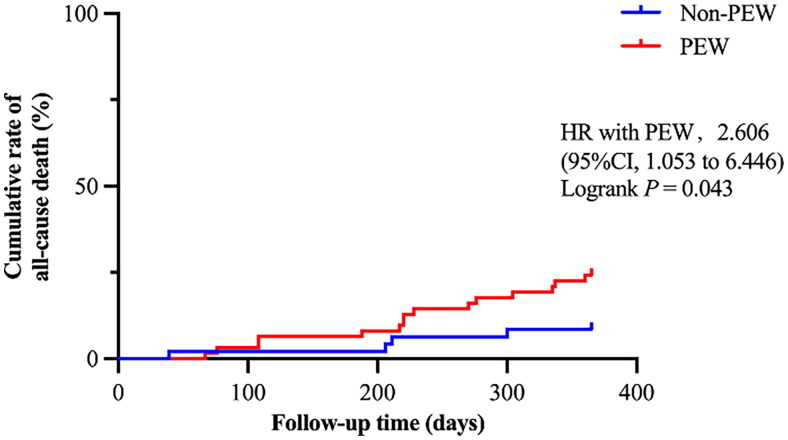
In addition, during the one-year follow-up period, a total of 26 (12.38%) deaths occurred, with 9 attributed to cardiovascular causes. In the PEW group, there were 19 (15.57%) deaths, while in the non-PEW group, there were 7 (7.95%) deaths. To identify the risk factors for all-cause mortality in MHD patients, we conducted a Cox regression analysis as shown in Table 4. We included all variables with a p-value less than 0.100 into the multivariate Cox regression model (including age, diastolic blood pressure, serum uric acid, iron, vitamin D, NT-proBNP, hemoglobin, C-reactive protein and β-blockers). The results showed that age ≥ 65 years (HR 4.7, 95% CI 1.27 − 17.36, p = 0.020) and high NT-proBNP (HR 2.33, 95% CI 1.47 − 3.68, p < 0.001) were independent risk factors for all-cause death. Consequently, we conducted a further Kaplan-Meier survival analysis in patients aged ≥ 65 years (as presented in Figure 5). A total of 108 (51.43%) patients aged 65 ≥ years were enrolled, of which 61 were with PEW and 47 were without PEW. A total of 19 deaths occurred, 15 in the PEW group and 4 in the non-PEW group. The result showed that there was a higher cumulative rate of all-cause death in the PEW group than in the non-PEW group (HR 2.606, 95% CI 1.053 − 6.446, p = 0.043). Accordingly, the all-cause mortality for PEW patients aged 65 and older was 2.606 times higher than that of non-PEW patients.
Table 4.
The risk factors of all-cause death based on univariate and multivariate Cox regression analysis.
| All-cause death |
||||
|---|---|---|---|---|
| univariate analysis |
multivariate analysis |
|||
| variables | HR (95%CI) | P | HR (95%CI) | P |
| Sex (male) | 0.71 (0.31-1.63) | 0.419 | ||
| Age (per year) | 1.04 (1.00-1.09) | 0.078 | ||
| Age < 65 years old | ref | |||
| ≥ 65 years old | 2.66 (1.12-6.34) | 0.027 | 4.70 (1.27-17.36) | 0.020 |
| PEW | 2.04 (0.86-4.86) | 0.106 | ||
| Dialysis duration (months) | 1.00 (1.00-1.01) | 0.515 | ||
| Frequency of monthly HDF | 0.87 (0.70-1.08) | 0.215 | ||
| Hypertension | 0.53 (0.19-1.55) | 0.251 | ||
| Diabetes | 0.71 (0.33-1.53) | 0.382 | ||
| Hyperlipidemia | 0.72 (0.33-1.55) | 0.393 | ||
| Stroke | 1.83 (0.69-4.85) | 0.225 | ||
| CVD | 1.86 (0.86-4.02) | 0.115 | ||
| Smoker | 1.15 (0.48-2.78) | 0.753 | ||
| SBP (per 10 mmHg) | 1.00 (0.98-1.01) | 0.581 | ||
| DBP (per10 mmHg) | 0.97 (0.94-1.00) | 0.045 | 0.96 (0.91-1.00) | 0.062 |
| ALT (U/L)a | 0.66 (0.38-1.15) | 0.142 | ||
| AST (U/L)a | 0.75 (0.47-1.22) | 0.247 | ||
| BUN (mmol/L)a | 0.91 (0.61-1.36) | 0.636 | ||
| Scr (μmol/L)a | 0.74 (0.51-1.09) | 0.131 | ||
| SUA (μmol/L)a | 1.00 (0.99-1.00) | 0.074 | 0.88 (0.56-1.39) | 0.580 |
| HDL-c (mmol/L)a | 0.84 (0.55-1.28) | 0.410 | ||
| LDL-c (mmol/L)a | 0.74 (0.49-1.12) | 0.158 | ||
| Plasma calcium (mmol/L)a | 0.85 (0.58-1.25) | 0.410 | ||
| Plasma magnesium (mmol/L)a | 0.47 (0.11-1.97) | 0.303 | ||
| Plasma phosphorous (mmol/L)a | 1.09 (0.74-1.61) | 0.664 | ||
| Serum iron (μmol/L)a | 0.54 (0.32-0.92) | 0.023 | 0.65 (0.32-1.32) | 0.230 |
| iPTH (pg/mL)a | 1.26 (0.90-1.78) | 0.180 | ||
| FBG (mmol/L)a | 1.01 (0.69-1.48) | 0.952 | ||
| Vitamin D (ng/mL)a | 0.61 (0.37-1.02) | 0.058 | 0.50 (0.22-1.10) | 0.084 |
| NT-proBNP (ng/L)a | 1.71 (1.20-2.45) | 0.003 | 2.33 (1.47-3.68) | <0.001 |
| Hemoglobin (g/L)a | 0.74 (0.52-1.05) | 0.091 | 1.27 (0.70-2.31) | 0.431 |
| CRP (mg/L)a | 1.27 (1.02-1.59) | 0.030 | 0.83 (0.58-1.20) | 0.321 |
| Kt/V urea a | 0.74 (0.49-1.11) | 0.141 | ||
| Antiplatelet drugs | 1.18 (0.54-2.60) | 0.681 | ||
| ACEI / ARB | 0.95 (0.42-2.13) | 0.899 | ||
| β-blockers | 0.26 (0.08-0.85) | 0.026 | 0.23 (0.05-1.13) | 0.070 |
| Statins | 1.16 (0.53-2.53) | 0.708 | ||
aAll laboratory indicators transformed using formula LN(X) to approach a normal distribution.
ACEI / ARB: angiotension converting enzyme inhibitors / angiotensin receptor blocker; ALT: alanine aminotransferas; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CI: confidence interval; CRP: C-reactive protein; CVD: cardiovascular disease; DBP: diastolic blood pressure; FBG: fasting blood glucose; HDF: hemodialysis filtration; HDL-c: high density lipoprotein cholesterol; Kt/V urea: urea clearance index; LDL-c: low density lipoprotein cholesterol; NT-proBNP: N-terminal pro-B-Type Natriuretic Peptide; OR: odds ratio; iPTH: intact Parathyroid Hormone; SBP: systolic blood pressure; Scr: serum creatinine; SUA: serum uric acid.
Note: p < 0.05 was considered as statistically significant. Bold was for p < 0.05 in multivariate Cox regression analysis.
Figure 5.
Kaplan-Meier curve for the all-cause death in MHD patients aged ≥ 65 with PEW and with non-PEW.
Kaplan-Meier curve was shown for the proportion of cumulative all-cause death during the follow-up period in hemodialysis patients aged 65 years or older with PEW compared with patients without PEW.
MHD: maintenance hemodialysis; PEW: protein energy wasting; 95% CI: 95% confidence interval; HR: hazard ratio. P < 0.05 was considered to be statistically significant.
3.5. Thresholds for the occurrence of MACE
To further clarify the risk factors for MACE in patients with PEW, we performed a univariable Cox analysis as shown in Table 5. Given that the study population consisted of individuals with PEW, diagnostic criteria for PEW, including BMI, MAMC, DPI, TC, prealbumin, and albumin, were also considered in the univariate analysis. The results indicated that CVD (HR 4.34, 95% CI 2.07 − 9.06, p < 0.001), low prealbumin (HR 0.67, 95% CI 0.46 − 0.98, p = 0.038), low TC (HR 0.57, 95% CI 0.37 − 0.85, p = 0.006), high NT-proBNP (HR 1.56, 95% CI 1.14 − 2.14, p = 0.005), and the use of antiplatelet (HR 2.73, 95% CI 1.34 − 5.55, p = 0.005) and statins (HR 2.81, 95% CI 1.36 − 5.79, p = 0.005) were associated with an increased risk of MACE.
Table 5.
The risk factors of MACE based on univariate Cox regression analysis in the PEW population.
| Univariate cox |
||
|---|---|---|
| Variable | HR (95%CI) | P |
| Age, years | 1.04 (1.00-1.09) | 0.062 |
| CVD | 4.34 (2.07-9.06) | <0.001 |
| BMI (kg/m2) | 1.08 (0.96-1.22) | 0.209 |
| Serum albumin (g/L)a | 0.75 (0.54-1.05) | 0.094 |
| Serum prealbumin (mg/L)a | 0.67 (0.46-0.98) | 0.038 |
| DPI (g/kg/d)a | 0.81 (0.56-1.18) | 0.274 |
| MAMC (cm)a | 1.21 (0.85-1.72) | 0.296 |
| TC (mmol/L)a | 0.57 (0.37-0.85) | 0.006 |
| NT-proBNP (ng/L)a | 1.56 (1.14-2.14) | 0.005 |
| Antiplatelet drugs | 2.73 (1.34-5.55) | 0.005 |
| Statins | 2.81 (1.36-5.79) | 0.005 |
aAll laboratory indicators transformed using formula LN(X) to approach a normal distribution.
BMI: body mass index; CVD: cardiovascular disease; DPI: dietary protein intake; MACE: major adverse cardiovascular event; MAMC: mid-arm muscle circumference; NT-proBNP: N-terminal pro-B-Type Natriuretic Peptide; PEW: protein energy wasting; TC: total cholesterol.
Univariate cox was done in key indicators of PEW.
Note: p < 0.05 was considered as statistically significant.
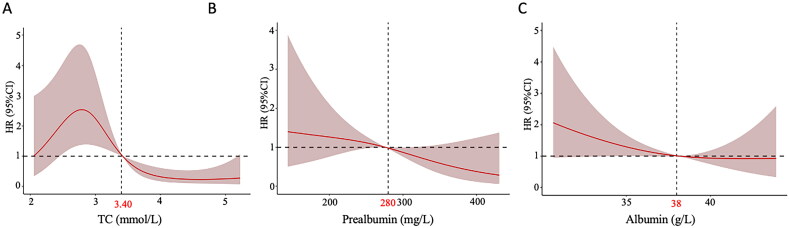
We aimed to identify key cutoff values for increased risk of MACE in our cohort to guide future intervention trials. And we plotted RCS curves to clarify the relationship between MACE and biochemical indices of PEW diagnostic factors, including TC, albumin, and prealbumin. Additionally, we determined the optimal thresholds for these indices to mitigate the risk of MACE, as displayed in Figure 6. The RCS curve showed that the incidence of MACE increased when TC was less than 3.4 mmol/L, and it reached its peak when at 2.8 mmol/L (as shown in Figure 6A). The incidence of MACE gradually increased with decreasing prealbumin and albumin concentration, reaching critical thresholds at 280 mg/L and 38 g/L (as shown in Figure 6B and Figure 6C).
Figure 6.
Association between MACE incidence and albumin, prealbumin and TC with RCS curves in MHD patients with PEW.
(A) The relationship of TC and MACE incidence based on Cox proportional hazard models. The vertical dashed line indicates the cutoff value of 3.40mmol/L. (B) The relationship of prealbumin and MACE incidence based on Cox proportional hazard models. The vertical dashed line indicates the cutoff value of 280mg/L. (C) The relationship of albumin and MACE incidence based on Cox proportional hazard models. The vertical dashed line indicates the cutoff value of 38g/L.
The solid red lines were adjusted hazard ratios, with grey red area showing 95% confidence intervals derived from RCS regressions with four knots. Reference lines for no association are indicated by the solid bold lines at a hazard ratio of 1.0.
HR: hazard ration; MHD: maintenance hemodialysis; MACE: major adverse cardiovascular event; PEW: protein energy wasting; RCS: restricted cubic spline; TC: total cholesterol.
4. Discussion
The aim of this study was to clarify the correlation between PEW and cardiovascular outcomes by investigating MACE incidence and all-cause mortality in patients receiving MHD over one year. In this retrospective study, we found that the incidence rate of MACE in the PEW population was 25.4%, significantly higher than the incidence rate of 12.5% in the non-PEW population. In addition, PEW was an independent risk factor for MACE in MHD patients, with the risk of MACE in MHD patients with PEW being 2.023 times higher than in patients without PEW. Regarding all-cause mortality, we stratified hemodialysis patients by age and found that MHD patients aged ≥ 65 years with PEW had a significantly higher all-cause mortality rate compared to patients without PEW. Therefore, for MHD patients, we need to strengthen the detection of PEW and follow up regularly to reduce the risk of PEW. Furthermore, we found that TC, prealbumin, CVD and NT-proBNP were risk factors for MACE in MHD patients with PEW. To identify targets for preventing MACE and improving prognosis, we explored the threshold of biochemistry markers in the cohort that can be modulated in PEW and lead to the occurrence of MACE. The results showed that the risk of MACE was significantly elevated in individuals with TC levels below 3.4 mmol/L, albumin levels below 38 g/L, and prealbumin levels below 280 mg/L. This may suggest that for MHD patients with PEW, aggressive intervention to maintain optimal TC, albumin, and prealbumin levels can help reduce the occurrence of MACE and even improve prognosis.
PEW is a common complication observed in MHD patients, and it is associated with increased mortality. It arises from a hypercatabolic state, uremic toxins, malnutrition, and inflammation [20,23]. It has been hypothesized that PEW could intensify preexisting inflammatory pathways and expedite the progression of atherosclerosis, consequently elevating the risk of cardiovascular events. This hypothesis suggests that PEW, beyond its association with malnutrition, may also play a role in the development of CVD [24]. Previous studies in CVD have shown that NT-proBNP is a risk factor for MACE [25,26]. This is similar to our findings. However, in our research, even after adjusting for confounding factors, we discovered that PEW remains an independent risk factor for MACE in hemodialysis patients. This finding supports the theory that PEW contributes to the accelerated development of CVD. Studies by Ishii, Hideki et al. and Takahashi, Hiroshi et al. found that the combination of PEW in patients with MHD increased the risk of cardiovascular events [27,28]. This is consistent with our findings.
Additionally, our research findings indicate that higher Kt/V urea is an independent protective factor against MACE in MHD patients. Single-pool Kt/V urea is used to assess dialysis adequacy. The effect of Kt/V urea on MACE incidence among MHD patients is somewhat controversial [29,30]. A prospective randomized controlled study of twice-weekly MHD patients in Shanghai showed a reduced incidence of cardiovascular disease in the high-dose group [31]. The malnutrition status associated with PEW and inadequate fluid removal may be the reasons for the correlation between Kt/V urea and MACE.
In our study, we found that PEW is not an independent risk factor for all-cause mortality in patients with MHD. However, previous studies have shown that PEW increases the risk of death and is mainly associated with systemic inflammation responses, muscle loss, and inadequate nutritional intake [32,33]. This may be related to the small sample size and short follow-up time of our study. Nonetheless, PEW was an independent risk factors for all-cause mortality in population aged ≥ 65 years in our study. Previous research indicated that PEW appeared to be more common in older (≥ 65 years) hemodialysis patients than in younger patients [34]. Firstly, elderly individuals may experience growth hormone-deficient, which contributes to muscle mass loss, reduced strength and decreased exercise capacity [35]. Secondly, mitochondrial dysfunction and oxidative stress are commonly present in the elderly population. These factors induce the accumulation of free radicals, resulting in age-related sarcopenia, accumulation of denatured proteins, and cellular inflammation [36]. Thus, it is even more important to focus on detecting PEW and providing early intervention in older patients (≥65 years).
TC, prealbumin, and albumin are biochemistry criteria used to diagnose PEW. We found that TC levels exhibited a risk factor for the incidence of MACE in MHD patients with PEW. Numerous previous studies have demonstrated a correlation between lowering TC and reducing the incidence of cardiovascular events, including heart attacks, revascularization, and ischemic stroke [37–42]. Moreover, there is no indication of a threshold within the cholesterol ranges studied, especially in hemodialysis patients [37]. In our study, the risk of MACE increased significantly when TC ≤ 3.4 mmol/L, and peaked when TC was at 2.8 mmol/L. It indicates that in the PEW population undergoing hemodialysis, simply lowering TC does not reduce the incidence of MACE and may accelerate it. After TC drops below 2.8, MACE occurrence starts decreasing again, possibly due to the scarcity of individuals with extremely low TC. This phenomenon may be attributed to the association between TC and nutrition in CKD, which reflects the body’s energy status [17,43]. Hypocholesterolemia is a risk factor for poor prognosis in dialysis patients, and a certain level of TC has a cardiovascular protective effect on the PEW population [44,45]. Therefore, it is necessary to control the TC levels in a reasonable range and improve the nutritional risk of the PEW population, thereby reducing the incidence of MACE.
Serum albumin and prealbumin have been widely evaluated as nutritional biomarkers and prognostic indicators [46,47]. Albumin characterized by its anti-inflammatory, antioxidant, anticoagulant, antithrombotic, and colloid permeable properties, serves as a robust predictor of cardiovascular risk and all-cause mortality. Additionally, it holds prognostic significance in patients with coronary and peripheral arterial disease [48–50]. Low prealbumin levels have been shown to be associated with an increased risk of Killip class III, cardiovascular death, and in-hospital heart failure complicated by MACE [46]. Our study confirmed that prealbumin serves not only as a diagnostic indicator for PEW but also as a risk factor for MACE in hemodialysis patients with PEW. Maintaining prealbumin ≥ 280 mg/L and albumin ≥ 38 g/L in MHD patients with PEW may significantly reduce the incidence of MACE.
In our study, the use of antiplatelet medications is associated with a higher risk of MACE in hemodialysis patients with PEW. It is inconsistent with conventional findings and may be related to the CKD itself [51–54], which remains somewhat controversial. Patients with CKD exhibit increased platelet activation, and an attenuated response to dual antiplatelet therapy compared with patients without renal insufficiency [54]. The status of dialysis altered the pharmacokinetics and coagulation function [55,56]. These factors might expose the CKD population to a different spectrum of risks and benefits from antiplatelet agents. Additionally, due to the small number of events in our study, confounding cannot be eliminated, which should be addressed in future research to explore the reasonable application of such drugs in hemodialysis patients with PEW.
Our study addresses the gap in understanding the relationship between PEW and CVD. Through an analysis of associated prognostic factors, we aimed to provide an improved survival guide for patients with PEW undergoing hemodialysis. The major limitations of this study were the small sample size and the short duration of follow-up. Nevertheless, despite the one-year study duration, the incidence of MACE remains noteworthy. Since the study was a retrospective study, we can’t fully control for confounding factors. However, our adjustment for dialysis duration and cardiovascular medications enhances the credibility of the results. Obviously, a subsequent expansion of the study and extension of the observation period are needed, which can be further validated from multiple perspectives.
5. Conclusion
Our retrospective study demonstrates that PEW is an independent risk factor for MACE in adult MHD patients. Among patients aged 65 years and over, PEW is also associated with a significantly increased risk of all-cause mortality. Furthermore, strategies aimed at optimizing TC, albumin, and prealbumin levels may improve cardiovascular outcomes in hemodialysis patients with PEW.
Supplementary Material
Funding Statement
This study was supported by Pudong Foundation for Development of Science and Technology (PKJ2022-Y56), the Key Discipline Construction Project of Shanghai Pudong New Area Health Commission (PWZxk2022-05), the Shanghai Municipal Health Committee of China (202340001), the New Quality Clinical Specialties of High-end Medical Disciplinary Construction in Pudong New Area (2025-PWXZ-09), the Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (PWR12021-02), the Shanghai Pudong New Area summit (Emergency Medicine and Critical Care) Construction Project (PWYgf2021-03) and the Clinical Investigation Grant of Shanghai East Hospital (DFLC2022016).
Authors’ contributions
Na Liu: research idea and study design. Xiaoyan Ma, Danying Yan, Canxin Zhou, Yingfeng Shi, Yi Wang, Jinqing Li, Qin Zhong, Xialin Li, Yan Hu, Weiwei Liang, Daofang Jiang, Yishu Wang, Ting Zhang, Yilin Ruan, and Shasha Zhang: data acquisition. Xiaoyan Ma and Danying Yan: statistical analysis. Xiaoyan Ma, Danying Yan, Canxin Zhou, Yingfeng shi, Shougang Zhuang and Na Liu: manuscript drafting or revision. Na Liu: supervision or mentorship. All authors: read and approved the final manuscript.
Ethics statement
This study was conducted according to the guidelines of the Helsinki Declaration. The study protocol was approved by the Human Research Ethics Committee of Shanghai East Hospital Affiliated with Tongji University School of Medicine (ChiCTR2000038127).
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Collaboration GCKD. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/s0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koppe L, Fouque D, Kalantar-Zadeh K.. Kidney cachexia or protein-energy wasting in chronic kidney disease: facts and numbers. J Cachexia Sarcopenia Muscle. 2019;10(3):479–484. doi: 10.1002/jcsm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84(6):1096–1107. doi: 10.1038/ki.2013.147. [DOI] [PubMed] [Google Scholar]
- 4.Herselman M, Esau N, Kruger JM, et al. Relationship between serum protein and mortality in adults on long-term hemodialysis: exhaustive review and meta-analysis. Nutrition. 2010;26(1):10–32. doi: 10.1016/j.nut.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Piccoli GB, Cederholm T, Avesani CM, et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease - implications for low protein intake and nutritional care: a critical review endorsed by ERN-ERA and ESPEN. Clin Nutr. 2023;42(4):443–457. doi: 10.1016/j.clnu.2023.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Carrero JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the international society of renal nutrition and metabolism. J Ren Nutr. 2018;28(6):380–392. doi: 10.1053/j.jrn.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Chan W. Chronic kidney disease and nutrition support. Nutr Clin Pract. 2021;36(2):312–330. doi: 10.1002/ncp.10658. [DOI] [PubMed] [Google Scholar]
- 8.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the international society of renal nutrition and metabolism (ISRNM). J Ren Nutr. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.El Chamieh C, Liabeuf S, Massy Z.. Uremic toxins and cardiovascular risk in chronic kidney disease: what have we learned recently beyond the past findings? Toxins (Basel). 2022;14(4):280. doi: 10.3390/toxins14040280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foley RN, Parfrey PS, Sarnak MJ.. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–S9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 11.de Mutsert R, Grootendorst DC, Axelsson J, et al. Excess mortality due to interaction between protein-energy wasting, inflammation and cardiovascular disease in chronic dialysis patients. Nephrol Dial Transplant. 2008;23(9):2957–2964. doi: 10.1093/ndt/gfn167. [DOI] [PubMed] [Google Scholar]
- 12.Kaysen GA. Association between inflammation and malnutrition as risk factors of cardiovascular disease. Blood Purif. 2006;24(1):51–55. doi: 10.1159/000089437. [DOI] [PubMed] [Google Scholar]
- 13.Stenvinkel P, Heimbürger O, Lindholm B.. Wasting, but not malnutrition, predicts cardiovascular mortality in end-stage renal disease. Nephrol Dial Transplant. 2004;19(9):2181–2183. doi: 10.1093/ndt/gfh296. [DOI] [PubMed] [Google Scholar]
- 14.Dinh QN, Drummond GR, Sobey CG, et al. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed Res Int. 2014;2014:406960–406911. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 16.Sjöström M, Wretling ML, Karlberg I, et al. Ultrastructural changes and enzyme activities for energy production in hearts concomitant with tumor-associated malnutrition. J Surg Res. 1987;42(3):304–313. doi: 10.1016/0022-4804(87)90148-x. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Ma X, Zhou X, et al. An updated clinical prediction model of protein-energy wasting for hemodialysis patients. Front Nutr. 2022;9:933745. doi: 10.3389/fnut.2022.933745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das SR, Abdullah SM, Leonard D, et al. Association between renal function and circulating levels of natriuretic peptides (from the Dallas Heart Study). Am J Cardiol. 2008;102(10):1394–1398. doi: 10.1016/j.amjcard.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Vickery S, Price CP, John RI, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46(4):610–620. doi: 10.1053/j.ajkd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein–energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 21.Huang YC, Wahlqvist ML, Lo YC, et al. A non-invasive modifiable Healthy Ageing Nutrition Index (HANI) predicts longevity in free-living older Taiwanese. Sci Rep. 2018;8(1):7113. doi: 10.1038/s41598-018-24625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLaughlin HL, Friedman AN, Ikizler TA.. Nutrition in kidney disease: core curriculum 2022. Am J Kidney Dis. 2022;79(3):437–449. doi: 10.1053/j.ajkd.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Obi Y, Qader H, Kovesdy CP, et al. Latest consensus and update on protein-energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18(3):254–262. doi: 10.1097/mco.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenvinkel P, Heimbürger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999;55(5):1899–1911. doi: 10.1046/j.1523-1755.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 25.Vernooij LM, van Klei WA, Moons KG, et al. The comparative and added prognostic value of biomarkers to the Revised Cardiac Risk Index for preoperative prediction of major adverse cardiac events and all-cause mortality in patients who undergo noncardiac surgery. Cochrane Database Syst Rev. 2021;12(12):Cd013139. doi: 10.1002/14651858.CD013139.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab S, Pörner D, Kleine CE, et al. NT-proBNP as predictor of major cardiac events after renal transplantation in patients with preserved left ventricular ejection fraction. BMC Nephrol. 2023;24(1):32. doi: 10.1186/s12882-023-03082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi H, Ito Y, Ishii H, et al. Geriatric nutritional risk index accurately predicts cardiovascular mortality in incident hemodialysis patients. J Cardiol. 2014;64(1):32–36. doi: 10.1016/j.jjcc.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Ishii H, Takahashi H, Ito Y, et al. The association of ankle brachial index, protein-energy wasting, and inflammation status with cardiovascular mortality in patients on chronic hemodialysis. Nutrients. 2017;9(4):416. doi: 10.3390/nu9040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69(7):1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 30.Locatelli F. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 31.Liu SX, Wang ZH, Zhang S, et al. The association between dose of hemodialysis and patients mortality in a prospective cohort study. Sci Rep. 2022;12(1):13708. doi: 10.1038/s41598-022-17943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hara H, Nakamura Y, Hatano M, et al. Protein energy wasting and sarcopenia in dialysis patients. Contrib Nephrol. 2018;196:243–249. doi: 10.1159/000485729. [DOI] [PubMed] [Google Scholar]
- 33.Ran Y, Wu QN, Long YJ, et al. Association of systemic immune-inflammation index with protein-energy wasting and prognosis in patients on maintenance hemodialysis. Zhonghua Yi Xue Za Zhi. 2021;101(28):2223–2227. doi: 10.3760/cma.j.cn112137-20210220-00445. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi AR, Alvestrand A, Danielsson A, et al. Factors predicting malnutrition in hemodialysis patients: a cross-sectional study. Kidney Int. 1998;53(3):773–782. doi: 10.1046/j.1523-1755.1998.00812.x. [DOI] [PubMed] [Google Scholar]
- 35.Perrini S, Laviola L, Carreira MC, et al. The GH/IGF1 axis and signaling pathways in the muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205(3):201–210. doi: 10.1677/joe-09-0431. [DOI] [PubMed] [Google Scholar]
- 36.Dalle-Donne I, Rossi R, Giustarini D, et al. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1-2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 37.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/s0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. Jama. 2018;319(15):1566–1579. doi: 10.1001/jama.2018.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 40.Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 41.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Bhatt DL, Pradhan AD, et al. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. 2023;401(10384):1293–1301. doi: 10.1016/s0140-6736(23)00215-5. [DOI] [PubMed] [Google Scholar]
- 43.Al-Sabti H, Al-Hinai AT, Al-Zakwani I, et al. The achievement of non-high-density lipoprotein cholesterol target in patients with very high atherosclerotic cardiovascular disease risk stratified by triglyceride levels despite statin-controlled low-density lipoprotein cholesterol. Oman Med J. 2022;37(2):e367–e367. doi: 10.5001/omj.2022.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalantar-Zadeh K, Block G, Humphreys MH, et al. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoppe K, Schwermer K, Dopierała M, et al. Can overnutrition lead to wasting?-The paradox of diabetes mellitus in end-stage renal disease treated with maintenance hemodialysis. Nutrients. 2022;14(2):247. doi: 10.3390/nu14020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan J, Si J, Xiao KL, et al. Association of prealbumin with short-term and long-term outcomes in patients with acute ST-segment elevation myocardial infarction. J Geriatr Cardiol. 2024;21(4):421–430. doi: 10.26599/1671-5411.2024.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SH. Using albumin and prealbumin to assess nutritional status. Nursing. 2017;47(4):65–66. doi: 10.1097/01.NURSE.0000511805.83334.df. [DOI] [PubMed] [Google Scholar]
- 48.Manolis AA, Manolis TA, Melita H, et al. Low serum albumin: a neglected predictor in patients with cardiovascular disease. Eur J Intern Med. 2022;102:24–39. doi: 10.1016/j.ejim.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi: 10.1016/j.ejim.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 50.Kalantar-Zadeh K, Cano NJ, Budde K, et al. Diets and enteral supplements for improving outcomes in chronic kidney disease. Nat Rev Nephrol. 2011;7(7):369–384. doi: 10.1038/nrneph.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim H, Rao SV.. Oral antiplatelet drugs in patients with chronic kidney disease (CKD): a review. J Thromb Thrombolysis. 2017;43(4):519–527. doi: 10.1007/s11239-017-1483-3. [DOI] [PubMed] [Google Scholar]
- 52.Natale P, Palmer SC, Saglimbene VM, et al. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst Rev. 2022;2(2):Cd008834. doi: 10.1002/14651858.CD008834.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harmon JP, Zimmerman DL, Zimmerman DL.. Anticoagulant and antiplatelet therapy in patients with chronic kidney disease: risks versus benefits review. Curr Opin Nephrol Hypertens. 2013;22(6):624–628. doi: 10.1097/MNH.0b013e328365adca. [DOI] [PubMed] [Google Scholar]
- 54.Gremmel T, Müller M, Steiner S, et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013;28(8):2116–2122. doi: 10.1093/ndt/gft103. [DOI] [PubMed] [Google Scholar]
- 55.Scheen AJ. Medications in the kidney. Acta Clin Belg. 2008;63(2):76–80. doi: 10.1179/acb.2008.63.2.003. [DOI] [PubMed] [Google Scholar]
- 56.Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19(1):135–140. doi: 10.1681/asn.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.