Abstract
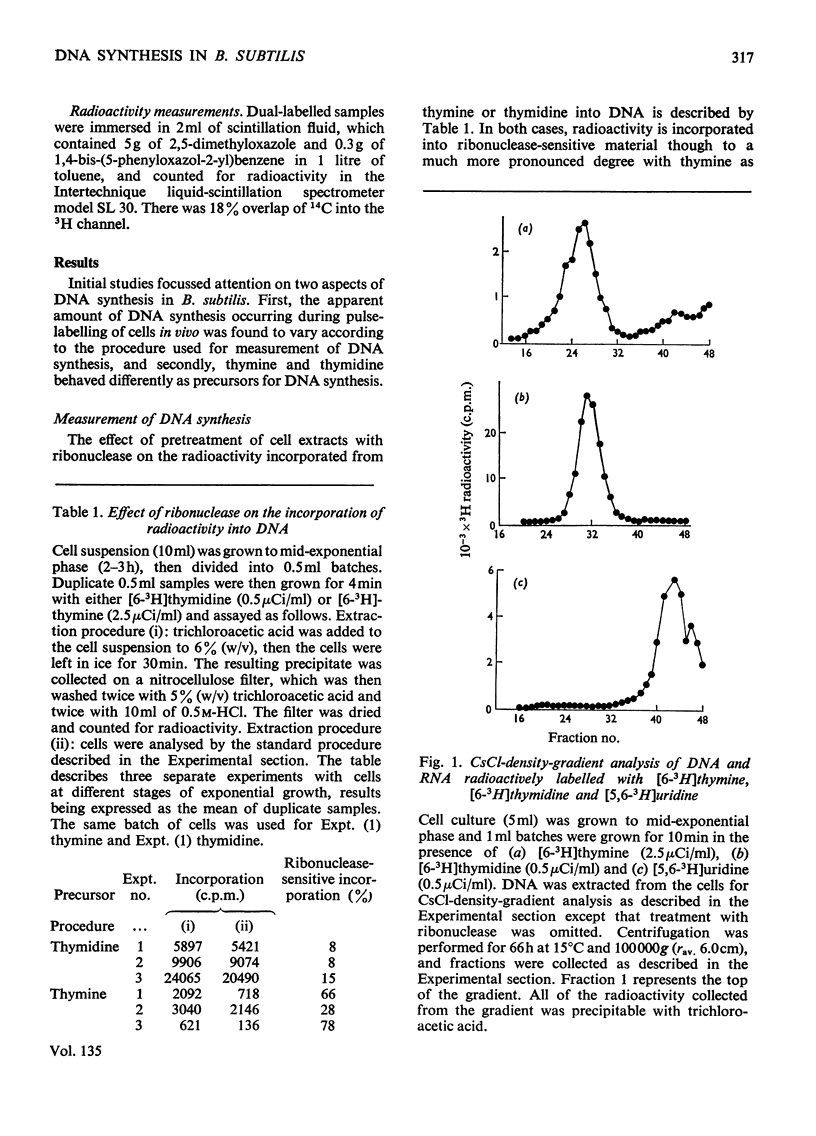
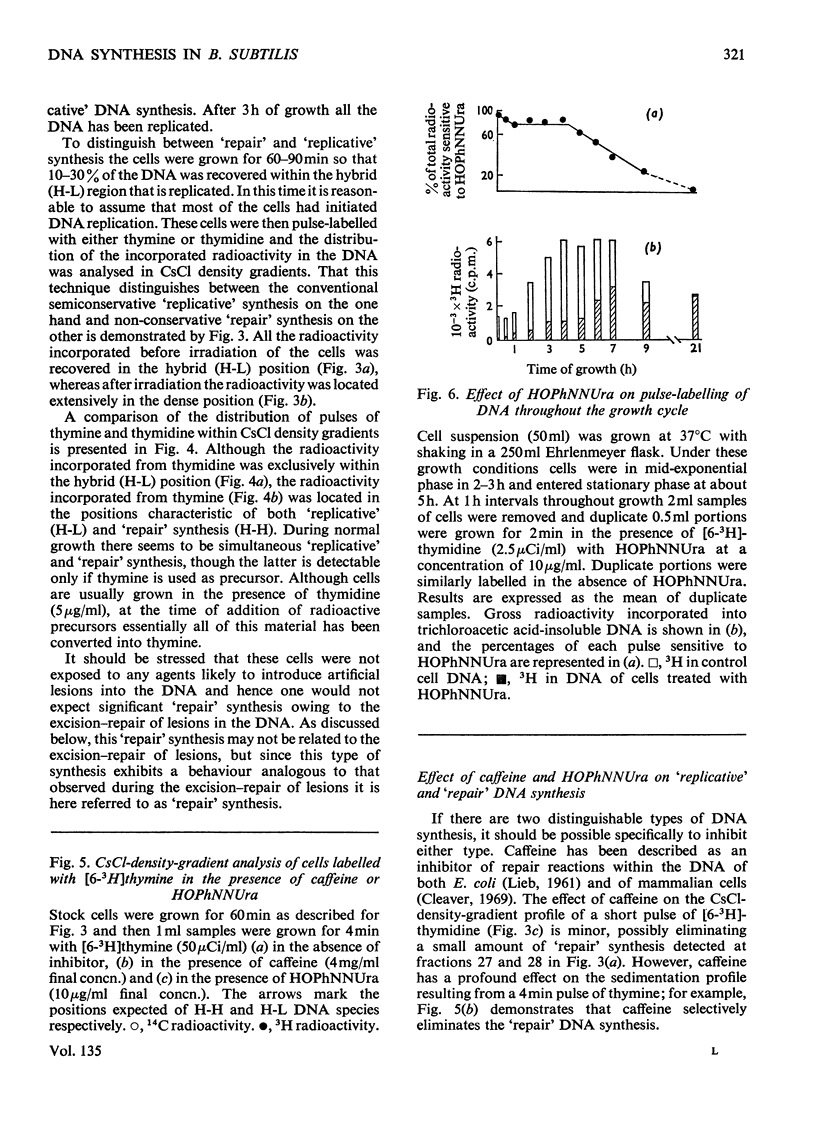
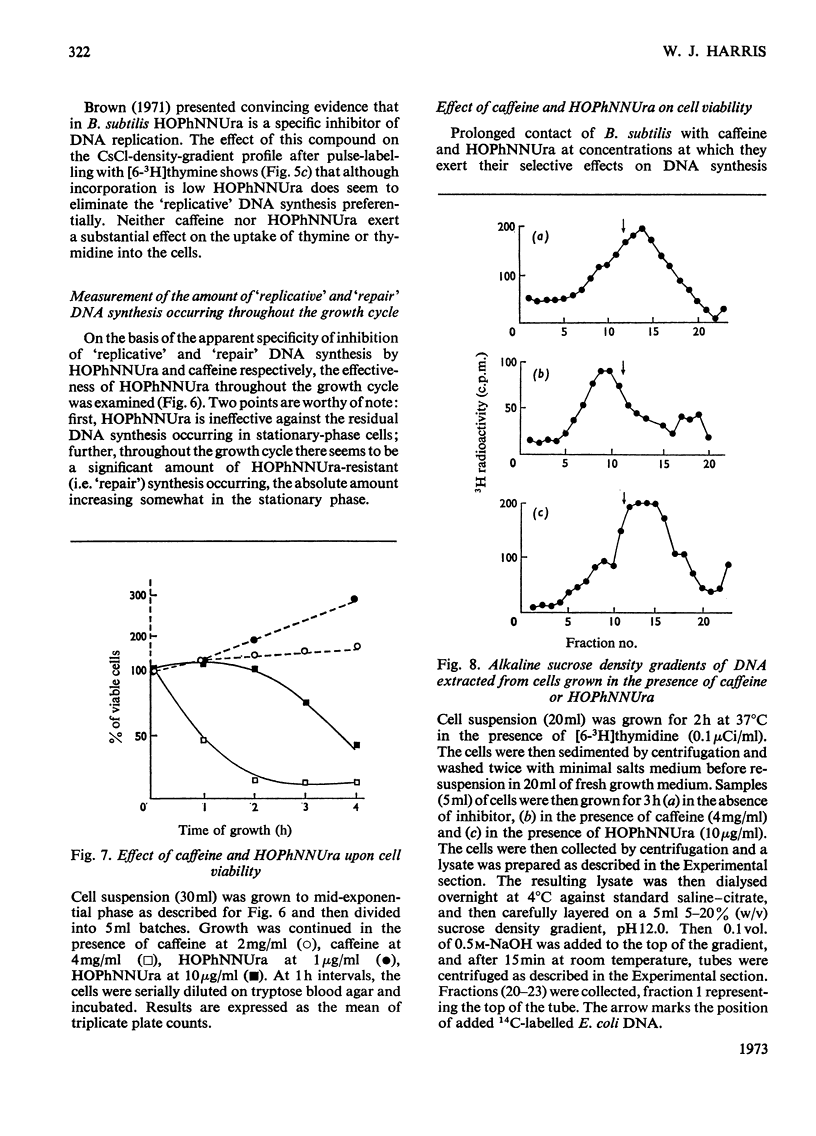
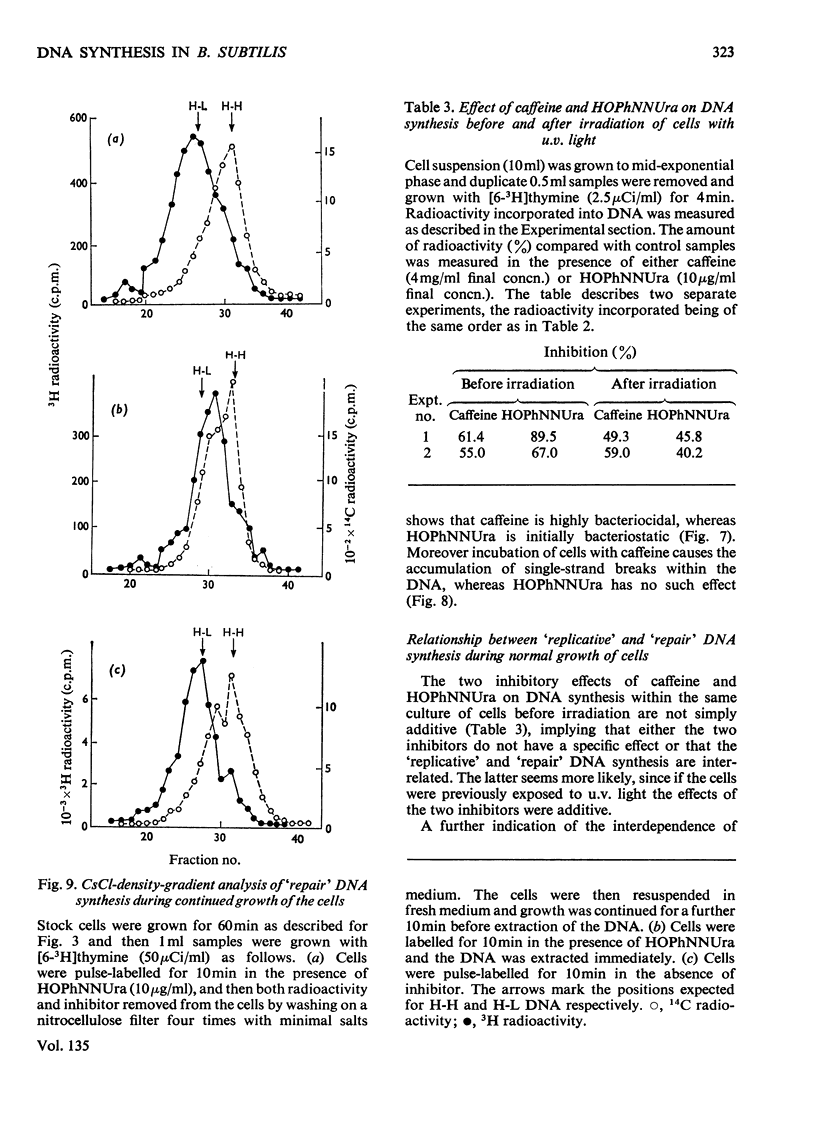
A study of the relative utilization of thymine and thymidine as precursors for DNA synthesis during normal growth in Bacillus subtilis showed that thymine serves preferentially as a precursor for `repair' synthesis, whereas thymidine is used preferentially for `replicative' synthesis. Further, evidence was obtained which suggests that during normal growth both `replicative' and `repair' DNA syntheses occur simultaneously. `Repair' synthesis is distinguished not only on the basis of its preferential utilization of thymine but also by its selective inhibition by caffeine. `Replicative' synthesis, however, is selectively inhibited by 6-(p-hydroxyphenylazo)-uracil. `Repair' synthesis would seem to be a `pre-fork' phenomenon and its inhibition is highly lethal to the cell.
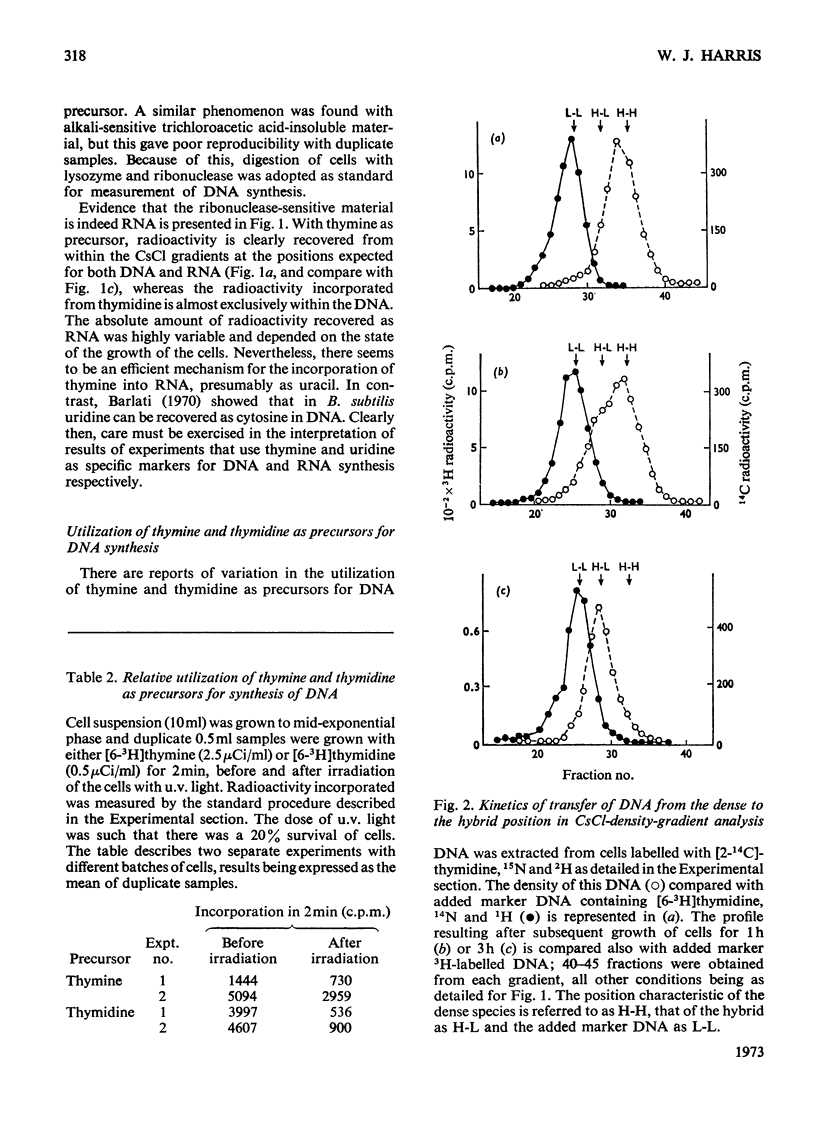
Full text
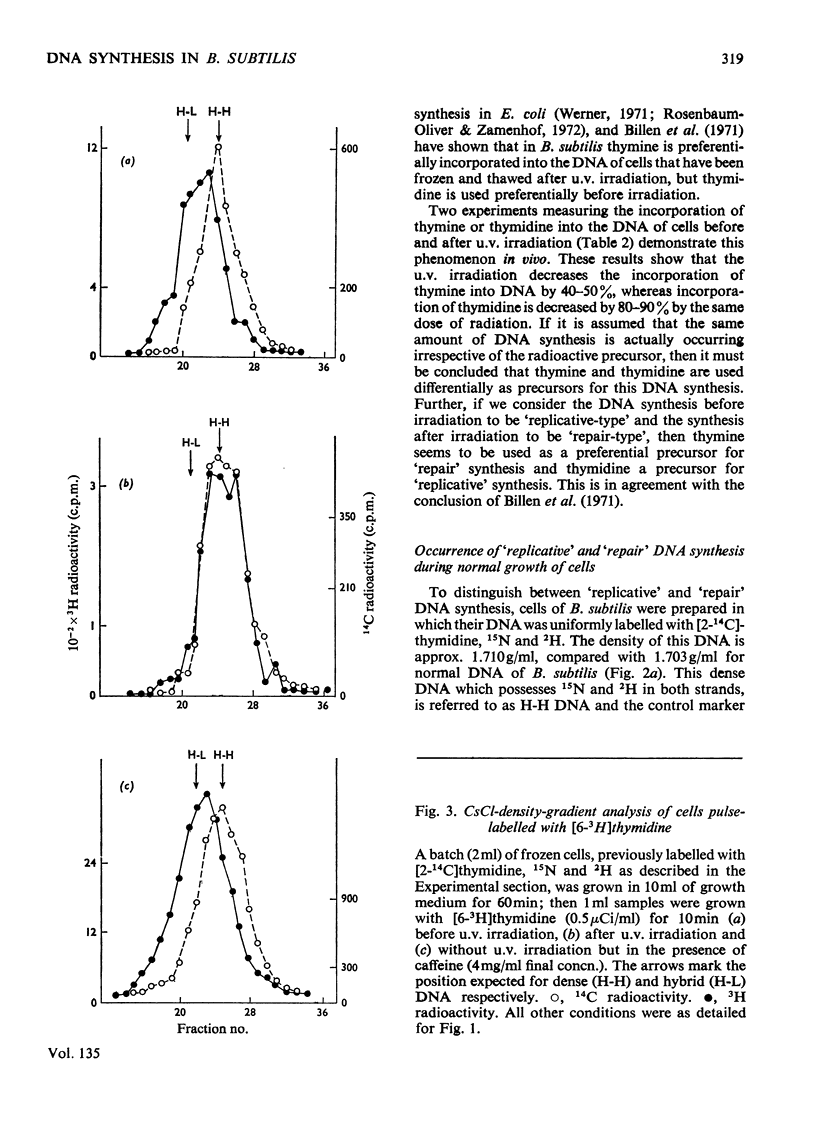
PDF

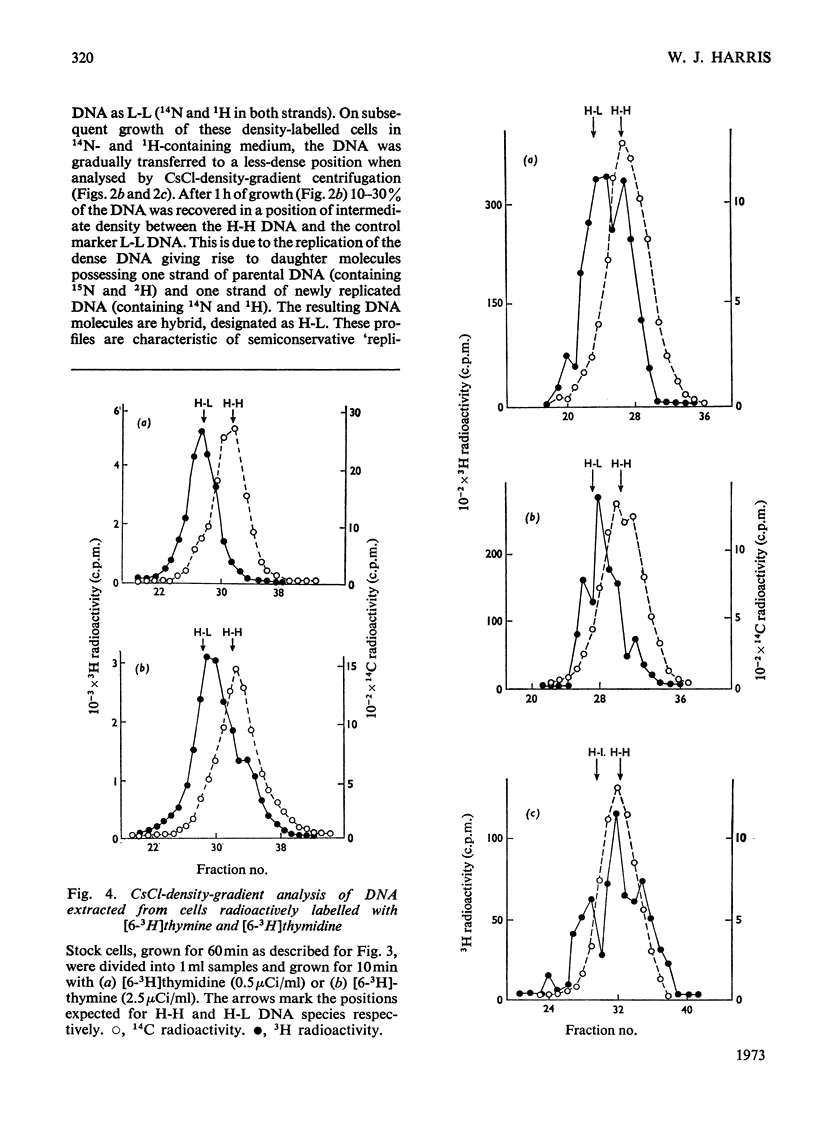


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlati S. Incorporation of uridine into Bacillus subtilis and SPP1 bacteriophage deoxyribonucleic acid. J Bacteriol. 1970 Jan;101(1):330–332. doi: 10.1128/jb.101.1.330-332.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen D., Carreira L. B., Hadden C. T., Silverstein S. J. Evidence suggestive of compartmentalization of deoxyribonucleic acid-synthesizing systems in freeze-treated Bacillus subtilis. J Bacteriol. 1971 Dec;108(3):1250–1256. doi: 10.1128/jb.108.3.1250-1256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. C. Inhibition of bacterial DNA replication by 6-(p-hydroxyphenylazo)-uracil: differential effect on repair and semi-conservative synthesis in Bacillus subtilis. J Mol Biol. 1971 Jul 14;59(1):1–16. doi: 10.1016/0022-2836(71)90409-8. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication of mammalian cell DNA: effects of compounds that inhibit DNA synthesis or dark repair. Radiat Res. 1969 Feb;37(2):334–348. [PubMed] [Google Scholar]
- De Lucia P., Cairns J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1164–1166. doi: 10.1038/2241164a0. [DOI] [PubMed] [Google Scholar]
- Ganesan A. T., Yehle C. O., Yu C. C. DNA replication in a polymerase I deficient mutant and the identification of DNA polymerases II and 3 in Bacillus subtilis. Biochem Biophys Res Commun. 1973 Jan 4;50(1):155–163. doi: 10.1016/0006-291x(73)91077-2. [DOI] [PubMed] [Google Scholar]
- Grigg G. W. Caffeine-death in Escherichia coli. Mol Gen Genet. 1968;102(4):316–335. doi: 10.1007/BF00433723. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Mechanism of transformation in B. subtilis. Mol Gen Genet. 1971;113(4):331–344. doi: 10.1007/BF00272333. [DOI] [PubMed] [Google Scholar]
- Harris W. J., Barr G. C. Some properties of DNA in competent Bacillus subtilis. J Mol Biol. 1969 Jan;39(2):245–255. doi: 10.1016/0022-2836(69)90314-3. [DOI] [PubMed] [Google Scholar]
- Haskell E. H., Daverin C. I. Pre-fork synthesis: a model for DNA replication. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1065–1071. doi: 10.1073/pnas.64.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M. S., PRITCHARD R. H. UNSTABLE LINKAGE BETWEEN GENETIC MARKERS IN TRANSFORMATION. J Bacteriol. 1965 May;89:1314–1321. doi: 10.1128/jb.89.5.1314-1321.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laipis P. J., Ganesan A. T. A deoxyribonucleic acid polymerase I-deficient mutant of Bacillus subtilis. J Biol Chem. 1972 Sep 25;247(18):5867–5871. [PubMed] [Google Scholar]
- Pato M. L. Regulation of chromosome replication and the bacterial cell cycle. Annu Rev Microbiol. 1972;26:347–368. doi: 10.1146/annurev.mi.26.100172.002023. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum-Oliver D., Zamenhof S. Degree of participation of exogenous thymidine in the overall deoxyribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):585–591. doi: 10.1128/jb.110.2.585-591.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R. Mechanism of DNA replication. Nature. 1971 Apr 30;230(5296):570–572. doi: 10.1038/230570a0. [DOI] [PubMed] [Google Scholar]


