Abstract
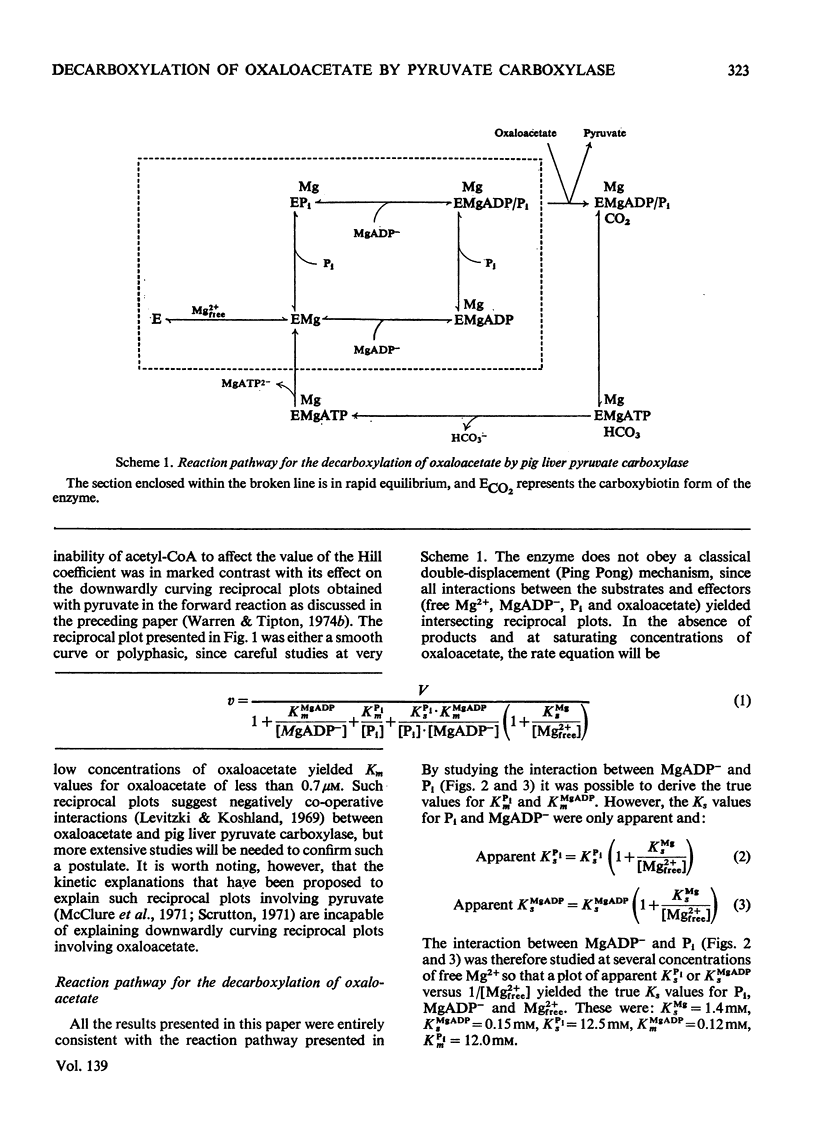
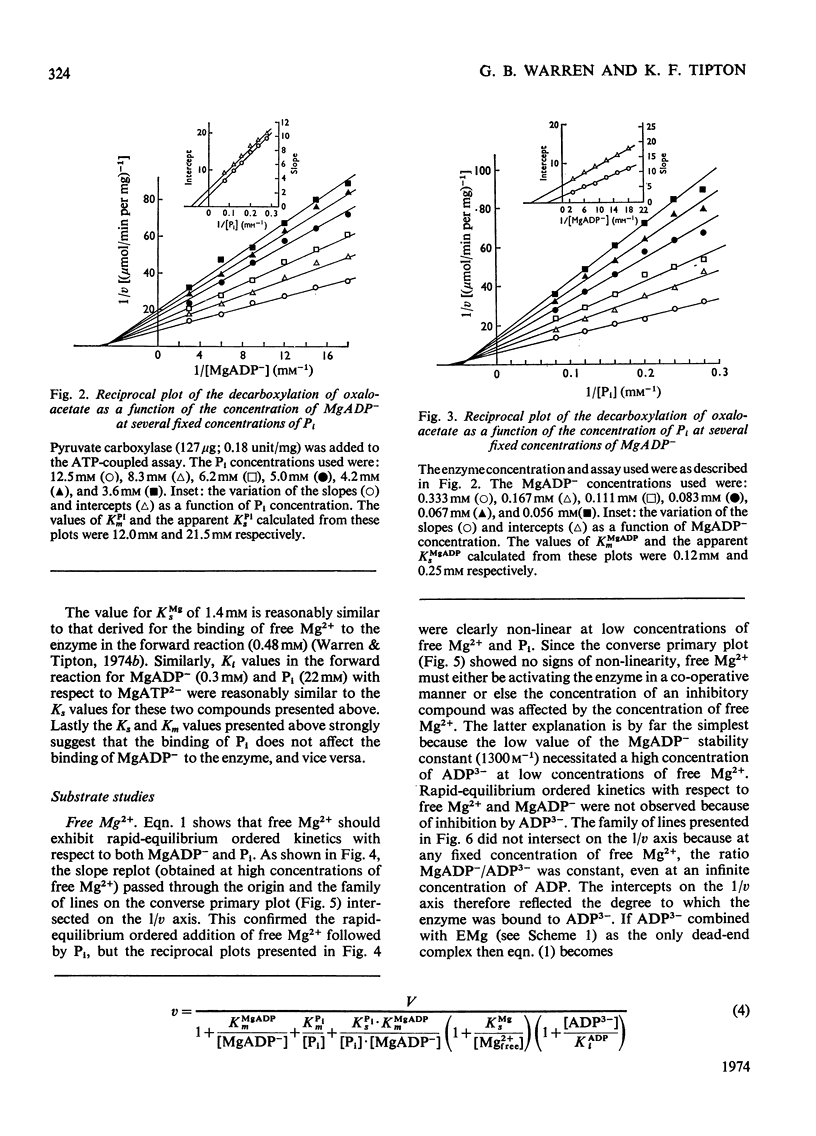
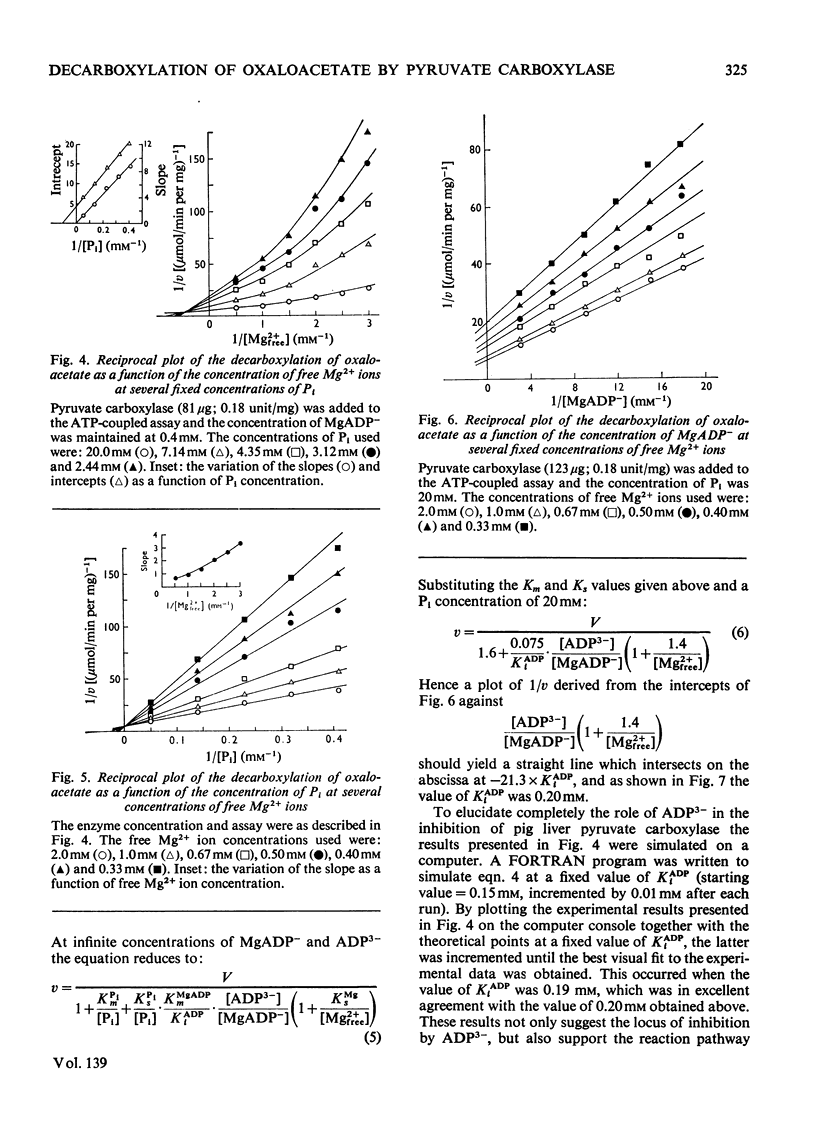
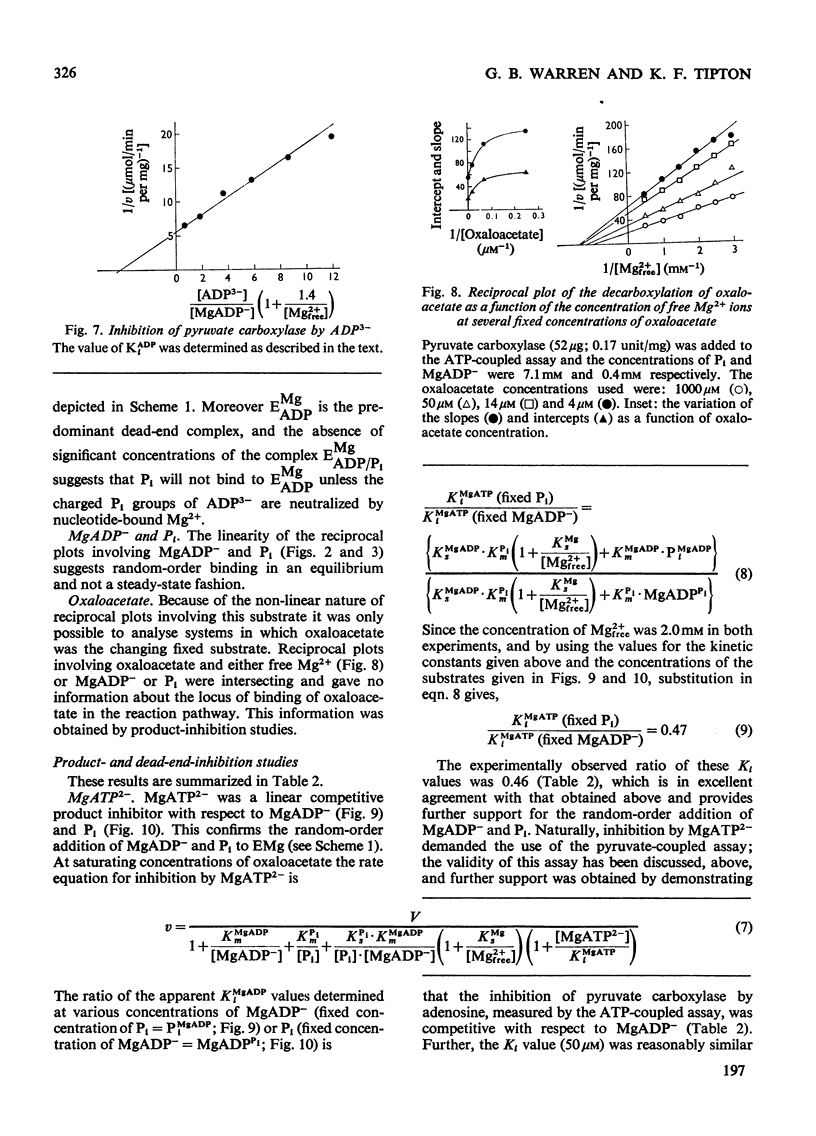
1. The reaction pathway for the decarboxylation of oxaloacetate, catalysed by pig liver pyruvate carboxylase, was studied in the presence of saturating concentrations of K+ and acetyl-CoA. 2. Free Mg2+ binds to the enzyme in an equilibrium fashion and remains bound during all further catalytic cycles. MgADP− and Pi bind randomly, at equilibrium, followed by the binding of oxaloacetate. Pyruvate is released before the ordered steay-state release of HCO3− and MgATP2−. 3. These results are entirely consistent with studies on the carboxylation of pyruvate presented in the preceding paper (Warren & Tipton, 1974b) and together they allow a quantitative description of the reaction mechanism of pig liver pyruvate carboxylase. 4. In the absence of other substrates of the back reaction pig liver pyruvate carboxylase will decarboxylate oxaloacetate in a manner that is not inhibited by avidin. 5. Reciprocal plots involving oxaloacetate are non-linear curves, which suggest a negatively co-operative interaction between this substrate and the enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barden R. E., Fung C. H., Utter M. F., Scrutton M. C. Pyruvate carboxylase from chicken liver. Steady state kinetic studies indicate a "two-site" ping-pong mechanism. J Biol Chem. 1972 Feb 25;247(4):1323–1333. [PubMed] [Google Scholar]
- Cha S. A simple method for derivation of rate equations for enzyme-catalyzed reactions under the rapid equilibrium assumption or combined assumptions of equilibrium and steady state. J Biol Chem. 1968 Feb 25;243(4):820–825. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- Gumaa K. A., McLean P., Greenbaum A. L. Compartmentation in relation to metabolic control in liver. Essays Biochem. 1971;7:39–86. [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure W. R. A kinetic analysis of coupled enzyme assays. Biochemistry. 1969 Jul;8(7):2782–2786. doi: 10.1021/bi00835a014. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A. Rat liver pyruvate carboxylase. IV. Factors affeing the regulation in vivo. J Biol Chem. 1971 Jun 10;246(11):3591–3596. [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Wagner M., Cleland W. W. Rat liver pyruvate carboxylase. II. Kinetic studies of the forward reaction. J Biol Chem. 1971 Jun 10;246(11):3579–3583. [PubMed] [Google Scholar]
- Moss J., Lane M. D. Acetyl coenzyme A carboxylase. IV. Biotinyl prosthetic group-independent malonyl coenzyme A decarboxylation and carbosyl transfer: generalization to other biotin enzymes. J Biol Chem. 1972 Aug 25;247(16):4952–4959. [PubMed] [Google Scholar]
- Scrutton M. C., Mildvan A. S. Pyruvate carboxylase. XI. Nuclear magnetic resonance studies of the properties of the bound manganese after interaction of the biotin residues with avidin. Biochemistry. 1968 Apr;7(4):1490–1505. doi: 10.1021/bi00844a036. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C. Possible regulatory factors for pyruvate carboxylase with particular reference to enzyme from chicken liver. Metabolism. 1971 Feb;20(2):168–186. doi: 10.1016/0026-0495(71)90090-4. [DOI] [PubMed] [Google Scholar]
- Walker W. D., Goodwin F. J., Cohen R. D. Mean intracellular hydrogen ion activity in the whole body liver, heart and skeletal muscle of the rat. Clin Sci. 1969 Jun;36(3):409–417. [PubMed] [Google Scholar]
- Warren G. B., Tipton K. F. Pig liver pyruvate carboxylase. Purification, properties and cation specificity. Biochem J. 1974 May;139(2):297–310. doi: 10.1042/bj1390297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. B., Tipton K. F. Pig liver pyruvate carboxylase. The reaction pathway for the carboxylation of pyruvate. Biochem J. 1974 May;139(2):311–320. doi: 10.1042/bj1390311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


