Summary
Biomarkers have been instrumental in population selection and disease monitoring in clinical trials of recently FDA-approved drugs targeting amyloid-β to slow the progression of Alzheimer's disease (AD). As new therapeutic strategies and biomarker techniques emerge, the importance of biomarkers in drug development is growing exponentially. In this emerging landscape, biomarkers are expected to serve a wide range of contexts of use in clinical trials focusing on AD and related dementias. The joint FDA-NIH BEST (Biomarkers, EndpointS, and other Tools) framework provides standardised terminology to facilitate communication among stakeholders in this increasingly complex field. This review explores various applications of biomarkers relevant to AD clinical trials, using the BEST resource as a reference. For simplicity, we predominantly provide contextual characterizations of biomarkers use from the perspective of drugs targeting amyloid-β and tau proteins. However, general definitions and concepts can be extrapolated to other targets.
Keywords: Alzheimer's disease, Clinical trials, Biomarkers, Disease-modifying therapies, BEST glossary
Key messages.
-
•
Clinicobiological selection of participants for AD clinical trials involves determining the presence of cognitive impairment, excluding individuals with clinical syndromes indicative of other brain conditions, and utilizing diagnostic biomarkers to confirm underlying pathology.
-
•
Biological disease staging using biomarkers can provide a framework for predicting participants' disease progression (prognostic biomarker) and therapeutic benefit (predictive biomarker).
-
•
Response biomarkers can offer inferential evidence that the drug engaged its target, produced the expected biological effect, and induced a downstream modification in the AD pathway.
-
•
Although it is difficult to fully invalidate a drug mechanism in the presence of biomarker evidence of disease modification, clinical trials can support that the drug is unable to produce a meaningful clinical benefit in the respective clinicobiological contexts tested.
-
•
Validated surrogate biomarkers offer a means to reduce resource utilization and accelerate drug development, particularly when detecting changes in clinical outcomes is challenging. Putative surrogate biomarkers that have not yet been validated should be used only after full consideration of their limitations and potential for misleading results.
Introduction
Alzheimer's disease (AD) is often conceptualised as a sequential cascade of pathophysiological events, beginning with the impact of amyloid-β (Aβ) on tau phosphorylation and aggregation, which when associated with neuronal and glial dysfunctions, eventually leads to dementia.1,2 The absence of biomarkers to identify the presence of these pathophysiological processes has limited the ability to interpret the negative results of early clinical trials targeting Aβ.3 The lack of biomarkers for population selection has raised the possibility that their negative results could be because other brain pathologies, unaffected by the drugs being studied, were causing symptoms in many participants.3 Furthermore, the lack of biomarkers to track therapeutic response has raised questions about whether drugs reach their targets and produce the expected biological effects.3 The lessons learned from these clinical trials have highlighted that it is imperative to use biomarkers for more informative drug development in the field of AD. Biomarkers can be defined as quantifiable characteristics of the body, serving as objective indicators of biological processes or pathological conditions.4,5 A joint initiative led by the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH) named BEST (Biomarkers, EndpointS, and other Tools) classifies biomarkers into diagnostic, predictive, prognostic, susceptibility, response, monitoring, and safety categories.4 The FDA defines the context of use (COU) of biomarkers for drug development as the combination of their BEST category and their specific use in the clinical trial.6 In AD trials, biomarkers can serve as inferential indicators of pathophysiology for several possible COUs.7 This review will discuss some COUs that biomarkers can play in refining population selection and tracking drug response (Table 1). While we recognise the importance of clinical trials targeting a variety of pathophysiological processes to mitigate AD,8 such as those associated with the neuroimmune system and metabolism, this review will primarily discuss COUs with a focus on drugs targeting Aβ and tau proteins. We chose not to delve into other targets as they encompass a multitude of different proteins, each necessitating unique COU considerations. The primary purpose of this review is to discuss the adaptation of the BEST concepts to the AD field, rather than to provide an exhaustive description of all possible targets and COUs.
Table 1.
Contexts of use of biomarkers for AD clinical trials.
| BEST category | Specific use in clinical trials |
|---|---|
| 1. Diagnostic | Select individuals with brain pathology |
| 2. Predictive | Select individuals most likely to respond to treatment |
| 3. Prognostic | Select individuals most likely to progress |
| 4. Susceptibility | Select individuals most likely to develop pathology |
| 5. Response | a. Target engagement (drug engaged its target) |
| b. Biologic response (drug effect on the target) | |
| c. Disease modification (drug effect on the AD pathway) | |
| d. Surrogate Endpoint (likelihood of clinical benefit) | |
| 6. Monitoring | Serially monitor treatment response or toxicity |
| 7. Safety | Indicate the likelihood, presence, or extent of a side effect |
Clinicobiological diagnosis
Clinical assessment
Clinical steps in selecting AD participants for clinical trials include identification of (1) cognitive disorder and characterization of (2) clinical syndrome. (1) Cognitive Disorder (i.e., mild cognitive impairment (MCI) or dementia). A disorder can be defined as a set of problems that impair the body's functioning, without necessarily indicating a specific disease or condition.9 The presence of both subjective and objective cognitive deficits has often been used to characterise individuals with perceived and confirmed cognitive impairment.10 After a thorough medical interview, if neither the patient, family, nor physician subjectively notes cognitive decline compared to a prior state, an objective abnormality on cognitive testing alone does not strongly support a classification of cognitive impairment. This is because the abnormality may be indicative of premorbid conditions or a variety of challenges the patient may have faced on the day of testing. The fact that identification of cognitive impairment requires both subjective and objective evidence inevitably leads to two additional groups of individuals who meet only one of the two criteria (Table 2). Individuals with cognitive complaints but no objective deficit can be categorised as having subjective cognitive decline,11 while individuals with an objective deficit that is not subjectively perceived may be categorized as having a subtle objective cognitive deficit.12 Individuals with either subjective cognitive decline or subtle objective cognitive deficit have demonstrated an increased risk of progression to MCI.11, 12, 13 If the subtle objective deficit is detected based on a single assessment relative to population test norms, it could be referred to as subtle objective cognitive impairment, while evidence of an abnormal longitudinal decline relative to the patient's own baseline testing could be referred to as subtle objective cognitive decline. The combination of subtle objective impairment and decline, which indicate participants' deficits relative to population norms and their own baseline, respectively, likely increases the chance that subtle objective cognitive deficit represents a transitional phase to MCI. Individuals with confirmed cognitive impairment who maintain functional independence in activities of daily living (ADL) are typically classified as MCI, while those who struggle with ADL are classified as having dementia.10 Dementia can be further stratified into mild (difficulties with instrumental ADLs), moderate (difficulties with basic ADLs), and severe (fully dependent).14 The FDA's recently updated draft guidance on this topic outlines a six-stage clinical classification for individuals with biomarker evidence of Alzheimer's pathology (Stage 1: no evidence of clinical impact; Stage 2: subtle objective or subjective deficits; Stage 3: mild but detectable functional impairment; Stages 4, 5, and 6: mild, moderate, and severe dementia).15 Recent AD trials have often included patients with MCI or mild dementia,16, 17, 18 whereas earlier trials tended to include individuals with mild to moderate dementia.19,20 Some recent clinical trials have also focused on cognitively unimpaired (CU) populations, in which the presence of a cognitive disorder is clinically excluded.21 (2) A Clinical Syndrome Reasonably Supporting AD as the Cause of Cognitive Disorder. A syndrome can be defined as a group of signs and symptoms that collectively suggest a disease or condition, even when the direct pathological cause has not yet been confirmed.9,22 When specific signs and symptoms of a cognitive disorder (i.e., MCI or dementia) suggest AD, it may be called Alzheimer's clinical syndrome,23 which also includes atypical presentations.24 During the initial syndromic evaluation of participants for clinical trials utilizing diagnostic biomarkers, it is not necessary for AD to be the most probable differential diagnosis; rather, it may be sufficient for AD to be reasonably postulated as the main cause of symptoms if the participant tests positive for the supportive diagnostic biomarkers. Therefore, when diagnostic biomarkers are available, more important than identifying Alzheimer's clinical syndrome is excluding individuals with pathognomonic features of other brain conditions. This process involves the analysis of participants' clinical characteristics by a clinician with the support of lab tests (e.g., vitamin B12, thyroid-stimulating hormone) and brain magnetic resonance imaging (MRI) to exclude other causes of cognitive deficits. There are several circumstances in which even a positive result for diagnostic biomarkers would not reasonably support AD as the main driver of clinical symptoms. These include, but are not limited to, atypical parkinsonian syndromes or a clear stepwise decline with a brain MRI showing major vascular pathology.25,26 Neuropsychiatric symptoms could support clinical trial selection by providing a richer phenotypic characterization of Alzheimer's clinical syndrome or by indicating a distinct cognitive disorder characterised by mild behavioural impairment.27
Table 2.
Cognitive disorders relevant for AD clinical trials.
| Impairment | Cognitively unimpaired |
Cognitively impaired |
|||||
|---|---|---|---|---|---|---|---|
| Cognitively normal | Subjective cognitive decline | Subtle objective cognitive deficit | MCI | Dementia |
|||
| Mild | Moderate | Severe | |||||
| Subjective | No | Yes | No | Yes | Yes | Yes | Yes |
| Objective | No | No | Yes | Yes | Yes | Yes | Yes |
| IADL | No | No | No | No | Yes | Yes | Yes |
| BADL | No | No | No | No | No | Yes | Yes |
| Fully Dependent | No | No | No | No | No | No | Yes |
BADL: Basic Activities of Daily Living; IADL: Instrumental Activities of Daily Living; MCI: mild cognitive impairment.
Diagnostic biomarkers
Diagnostic biomarkers can be used to select individuals with the disease or medical condition, or a subtype thereof.4,5 In AD, diagnostic biomarkers are commonly used in the context of identifying the hallmark pathological features of the disease, Aβ and tau pathologies.28 Aβ (A+) and tau (T+) brain pathologies have been measured using CSF Aβ42 and phosphorylated tau (p-tau), respectively, as well as PET techniques in clinical trials and practice for many years.29 Blood biomarkers of Aβ and tau have been proposed for screening patients who will later undergo CSF/PET confirmation,30 while some recent studies suggest that they may have already achieved the accuracy necessary to replace CSF/PET as diagnostic tools.31 It is important to emphasise that the T+ status discussed in this review is determined by biomarkers that identify AD-related tau pathology and would not consider tau biomarkers specific to other tauopathies. The performance of a biomarker in detecting brain pathology depends on the technique employed (i.e., PET differs from fluid-based methods) and the assumptions made to establish its abnormality cut-off.29 Consequently, the resulting biomarker status may vary based on the analytical idiosyncrasies used and may not always truly reflect the underlying pathological environment of the brain tissue. For example, A+/T− individuals may have minimal levels of tau pathology in the brain that may not yet be detected by in vivo biomarkers.32,33 Despite this limitation, an A+/T− profile can be useful for selecting a relatively homogeneous group of participants with absent or at least low levels of brain tau pathology. Biomarker studies support the existence of a significant proportion of older adults who are CU A+/T− and that their rates of clinical progression over five years are more akin to A−/T−than A+/T+.34 This suggests that abnormality in Aβ biomarkers alone is not an optimal indicator of clinical progression within typical clinical trial periods. Therefore, to enrich clinical trial populations, it could be more informative to categorise individuals into A+/T− (i.e., Aβ pathology) who progress slowly, separately from A+/T+ (i.e., Alzheimer's pathology) who tend to progress at a faster rate.34 Furthermore, when we consider the long clinical stability of CU A+/T− and the fact that Aβ is unlikely to protect these individuals against the development of other brain pathologies, it is reasonable to assume that some CU A+/T− will progress to cognitive impairment due to factors other than Aβ pathology. Thus, clinical trials including CI A+ without evidence of tau biomarkers could potentially select CI A+/T− in whom it is unclear whether Aβ is associated with cognitive symptoms. Use of anti-Aβ therapy in these individuals may lead to Aβ reduction that is less likely to result in imminent clinical benefit. Therefore, in line with the idea that AD is a cascade of events in which Aβ effects on tau lead to dementia,1 the likelihood of an accurate AD diagnosis increases with the presence of both A+ and T+. This is consistent with earlier notions of preclinical AD,13 cognitive impairment due to AD,35 as well as the neuropathological diagnosis of AD.36 As previous studies have shown that changes in both soluble and insoluble tau species can occur in response to Aβ pathology and may represent different facets of the same process,1 we postulate that in the presence of A+, evidence of tau abnormality measured using either fluid or PET indicates Alzheimer's pathology. Among individuals who are A+/T+, potential discrepancies in tau positivity resulting from the use of fluid or PET could be attributed to different stages of the disease. While individuals at an earlier stage may show abnormal tau phosphorylation in the absence of detectable tau PET abnormality,37 later stages are characterised by the regional progression of tau PET accumulation.33,38,39 Differentiating T+ status, whether defined by fluid or PET, may be relevant for potential anti-tau trials targeting A−/T+ populations, as this differentiation could highlight individuals following distinct pathophysiological pathways. Although cognitive changes in individuals who are A−/T+, as determined by abnormal CSF p-tau, appear to mirror that of A−/T−,40 those identified as A−/T+ through tau tangle PET consistently demonstrate more rapid neurodegeneration and cognitive impairment.33,41Together, these results suggest that it can be useful for clinical trials to measure AD-related tau biomarkers using both fluid and PET to better stage A+/T+ and classify A−/T+ participants. Table 3 shows pathological profiles of individuals classified using Aβ and tau biomarkers. Clinical trials that did not use diagnostic biomarkers for population selection enrolled a high proportion of participants who were found not to have AD pathology.42, 43, 44, 45 Most recent clinical trials used only Aβ biomarkers to enrich their populations,16, 17, 18 whereas Donanemab trials were enriched with participants who had an A+/T+ biomarker profile.46,47
Table 3.
Aβ and tau biomarker profiles.
| Biomarker profile | Pathology |
|---|---|
| A−/T− | Normal biomarkers |
| A+/T− | Aβ pathology |
| A−/T+ | Abnormal tau phosphorylation and/or tau tangle pathology |
| A+/T+ | Alzheimer's pathology |
A: Aβ status; AD: Alzheimer's disease; T: tau status. Aβ pathology indicates an abnormal Aβ biomarker with normal fluid p-tau and tau tangle PET. Abnormal tau phosphorylation consists of abnormal fluid p-tau with normal tau tangle PET. Tau tangle pathology consists of an abnormal tau tangle PET biomarker regardless of fluid p-tau levels. Alzheimer's pathology consists of abnormal Aβ plus fluid p-tau and/or tau tangle PET.
Clinicobiological assessment
As mentioned above, selecting participants for AD clinical trials involves (1) identifying the cognitive disorder or its absence (e.g., CU), (2) assessing whether signs and symptoms are compatible with AD, and (3) using diagnostic biomarkers to support the presence of AD pathology. Together, these three steps provide clinicobiological profiles that enable the selection of participants most appropriate for different clinical trial designs. Here, we will use the term “with” either in the absence of a cognitive disorder or when the likelihood that the pathology indicated by the biomarker is causing the clinical symptoms is moderate to low. In contrast, we will use the term “due to” when there is a high likelihood that the pathology indicated by the biomarker is causing the symptoms. It is worth emphasizing that the “due to” label should be determined not only by biomarkers but also by assessing whether the medical history and physical examination are consistent with the disease. Table 4 summarises the groups defined following the notions described above, which can provide insights into the eligibility of participants for clinical trials. For example, CU with Aβ pathology (A+/T−) or Alzheimer's pathology (A+/T+) could be the target of anti-Aβ or anti-tau trials to prevent or reduce tau pathology, respectively. Individuals with cognitive impairment and Aβ pathology alone may not be the ideal group for anti-Aβ or anti-tau trials due to the high probability that pathologies other than Aβ and tau are the main cause of their current symptoms. Individuals with cognitive impairment who are A−/T+ due to an abnormal tau tangle PET scan, suggesting tangle-predominant pathology, exhibit accelerated cognitive decline and could therefore potentially be selected for trials aimed at mitigating tangle formation.33,41 Cognitive impairment due to AD (A+/T+) is the natural clinicobiological profile for trials testing anti-Aβ and anti-tau therapies, as it is the most likely to identify participants on the AD pathway. However, individuals with cognitive impairment and an A+/T+ biomarker profile, in whom the clinical syndrome is pathognomonic of other forms of dementia (considered here as “due to” [another disease] “with” concomitant Alzheimer's pathology), should probably not be enrolled into anti-Aβ or anti-tau trials under normal circumstances. For example, individuals with an A+/T+ biomarker profile who experience fluctuating cognition, recurrent visual hallucinations, and parkinsonism will likely have their symptoms largely influenced by alpha-synuclein. In fact, most patients with Lewy body dementia are positive for Aβ and/or tau markers.48 Further studies are needed to clarify the benefit that drugs designed to mitigate Alzheimer's pathology may have in individuals A+/T+ in whom other diseases are the primary driver of symptoms. When robust biomarkers to identify the multiple brain pathologies associated with cognitive deficits are available, we will no longer need to exclude A+/T+ individuals with clinical features of other diseases, as negative biomarkers for these conditions will do so reliably.
Table 4.
Clinicobiological categorization relevant for AD clinical trials.
| Cognitive disorder | Clinical syndrome | A/T biomarker profile | Condition/disease |
|---|---|---|---|
| CU | No | A+/T− | with Aβ pathology |
| A−/T+ | with abnormal tau phosphorylation | ||
| with tau tangle pathology | |||
| A+/T+ | with Alzheimer's pathology | ||
| MCI or dementia | AD in the differential | A+/T− | with Aβ pathology |
| A−/T+ | with abnormal tau phosphorylation | ||
| due to tau tangle-predominant pathology | |||
| A+/T+ | due to Alzheimer's disease | ||
| A−/T+ (post-treatment) | due to Alzheimer's disease with Aβ in remissiona | ||
| Main characteristics pathognomonic of other disease | A+/T+ | due to [other disease] with Alzheimer's pathology |
A: Aβ status; AD: Alzheimer's disease; CU: cognitively unimpaired; MCI: mild cognitive impairment; T: tau status. The term “Alzheimer's pathology” is used to indicate the presence of A+/T+ biomarker abnormalities, whereas the term “Alzheimer's disease” is used when A+/T+ abnormalities are the most likely cause of cognitive symptoms.
We use the term “remission” to indicate the reduction of Aβ after therapy because this may be a transient state as Aβ pathology may return. The term remission can also be accompanied by “partial” or “complete”, which can be used to indicate the degree of response to therapy.
Predictive biomarkers
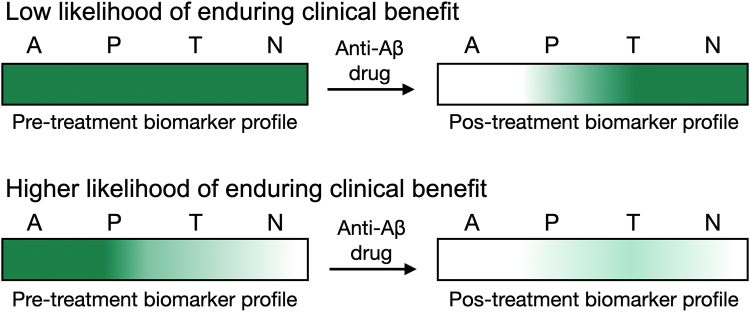
Predictive biomarkers can be used to select individuals most likely to benefit from a therapeutic intervention.4 Predictive biomarkers may be characteristics of the participant, such as genetics, or of the disease or condition, such as tissue protein level.4 The identification of predictive biomarkers typically requires a comparison of the drug's effects on the outcome between individuals with and without the biomarker abnormality in randomised clinical trials.4 The predictive biomarker could initially be inferred from the drug mechanism and/or investigated post hoc in completed trials, and subsequently used as an a priori hypothesis.4 It would be advantageous if the predictive biomarker used for trial enrichment were not overly complex or costly, as its use might be necessary to identify the intended population in clinical practice following regulatory approval. Given the current uncertainty about whether remission of one pathology in the AD cascade will resolve subsequent pathologies that are more closely related to cognitive deficits, it might be beneficial to enrol trial participants who exhibit minimal levels of pathologies that occur downstream from the pathology being targeted by the drug to increase the chance of clinical benefit. In this context, biological disease staging using biomarkers can offer a framework for predicting therapeutic benefit. In practical terms, if the drug cannot resolve downstream pathologies, the trial should enrol participants at a biomarker stage that is equal to or earlier than the stage indicated by the targeted pathology, to increase the chance of clinically meaningful results. Within this framework, the probability of a therapy yielding enduring clinical benefits could best be understood by the biomarker abnormalities that will remain after treatment (Fig. 1). Most AD clinical trials have not used predictive biomarkers to increase the likelihood of clinical benefit.16, 17, 18 On the other hand, the TRAILBLAZER-ALZ 2 trial segregated individuals into low and high tau PET groups under the assumption that participants with low tau PET would benefit the most from the anti-Aβ therapy.46
Fig. 1.
Biomarkers profile predictive of anti-Aβ therapy benefit. A (Aβ); P (tau Phosphorylation), T (tau Tangle) N (Neurodegeneration). Green = pathological burden.
Prognostic biomarkers
Prognostic biomarkers can be used to select among participants with the disease or medical condition those most likely to progress.4 Under the premise that AD occurs in a sequence of pathological events, the abnormality of a biomarker will have greater imminent prognostic value for an immediately subsequent process in the cascade. A wide range of biomarkers have been associated with an increased risk of pathological and clinical progression in AD. In the early stages of the AD cascade, studies indicate that CU populations with abnormal Aβ and plasma glial fibrillary acidic protein (GFAP) biomarkers are at an increased risk of accelerated tau phosphorylation and aggregation.49 Thus, clinical trials could enrol CU individuals with abnormalities in both Aβ and GFAP biomarkers to increase the likelihood that they will experience AD-related progression. CSF or plasma p-tau biomarker abnormalities appear to precede and are therefore good prognostic markers for tau-PET accumulation.50 Braak stages derived from tau PET were associated with an increased chance of deposition of tau tangle PET in brain regions comprising subsequent but not earlier Braak stages.51 Tau-PET and neurodegeneration (e.g., fluid neurofilament light chain (NfL), structural MRI) biomarkers are expected to become abnormal later in the disease,52 making them suitable prognostic biomarkers for imminent cognitive impairment. Interestingly, the use of cut-offs in diagnostic biomarkers other than those used for determining the presence of brain pathology has allowed these biomarkers to also be used in the context of prognosis. For example, AHEAD A3 (Centiloid 20–40) and 45 (Centiloid >40) trials were enriched with Centiloid Aβ PET levels that increase the likelihood that their participants will show progression on the trials' proposed endpoints, Aβ PET and cognition, respectively.53 Similarly, optimised cut-off values for CSF Aβ and p-tau were related to brain hypometabolism in an AD-like pattern among CU A+/T+.54 APOEε4 status has also been associated with a higher chance of tau PET accumulation in CU individuals with Aβ pathology or Alzheimer's pathology.55 Clinical trials targeting Aβ,56 neuroinflammation,57 lipid metabolism,58 and the GABAergic system59 have used the APOEε4 genotype to enrich their populations with participants more likely to experience AD-related progression. Recently, trialists and researchers have delved into complex models using multidimensional data and machine learning algorithms to identify individuals most prone to progress within clinical trial periods.60
Susceptibility/risk biomarkers
Susceptibility biomarkers can be used to select among participants who do not have the disease or medical condition those who are more likely to develop it.4 A biomarker is classified into the susceptibility rather than prognostic category based on the absence of the disease or condition.4 Consequently, the classification of a biomarker into the susceptibility category may vary based on the criteria utilised to define who has the disease or condition. Depending on the COU established, susceptibility biomarkers can help to indicate who will develop clinical AD or biological AD. In clinical settings, AI-based biomarkers derived from electronic health record data could be used to identify individuals without cognitive impairment who are susceptible to developing clinical AD. This could help flag people within the health system who would benefit most from more intensive diagnostic monitoring or preventive strategies.4 On the other hand, in clinical trials, susceptibility biomarkers could be used to indicate the likelihood of developing biological AD among populations negative for Aβ and/or tau markers to test drugs to prevent Alzheimer's pathology. If Alzheimer's pathology is characterised by A+ plus T+, Aβ biomarkers could be considered indicators of susceptibility to Alzheimer's pathology in individuals with an A+/T− biomarker profile. Recent studies have suggested that the presence of an abnormal plasma GFAP biomarker may indicate an even greater susceptibility of individuals A+/T− to develop Alzheimer's pathology (A+/T+).49 Genetic variants associated with Alzheimer's pathology have been utilised for years as susceptibility biomarkers in clinical trials and practice. The presence of mutations in the amyloid precursor protein and presenilin 1 and 2 genes are the strongest susceptibility biomarkers for AD, with nearly 100% of mutation carriers manifesting autosomal-dominant AD.61 APOEε4 genotype increases the risk for sporadic AD,62 especially among homozygous carriers who have been reported to develop Alzheimer's pathology in most cases.63
Response biomarkers
Biomarkers that change in response to a therapeutic intervention can be classified as response biomarkers.5 In AD trials, changes in biomarkers can provide inferential evidence to determine whether: (a) the drug engages its target (target engagement), (b) the drug-target interaction induces the expected biological activity (biological response), (c) the biological response is linked to a modification in the AD pathway (disease modification), and (d) clinical benefit is likely (surrogate).
Target engagement
Response biomarkers can be used to provide evidence that the drug engaged its target in living patients.4 Verification of target engagement in clinical studies is important as they may face challenges not always seen in vitro and in animal models, such as dose limitation due to safety and differences in tissue penetrance and physiological and pathological environments.64,65 Target engagement can be directly assessed using assays that identify drug–protein interactions in biofluids.66 Although some advanced PET techniques can directly verify drug-target occupancy, various methodological challenges limit their application in AD drug development.67 Most AD clinical trials rely on observations of the drug's biological activity on the target as indirect evidence of target engagement, because to elicit this effect the drug must first engage its target in the expected compartment/organ.68,69 For example, the biological activity of an anti-Aβ therapy resulting in a reduction of Aβ pathology in CSF or PET indicates an effective interaction between the anti-Aβ agent and Aβ in the brain.46 For anti-tau therapies, changes in CSF total/phosphorylated tau70,71 or markers of tau aggregation70 have been used to support an interaction between drugs and target.72 While evidence related to drug biological activity can reliably indicate successful target engagement,66 its interpretation is limited when the drug does not elicit the predicted effect on the biomarker. For instance, if a hypothetical anti-tau therapy fails to modify a tau biomarker, it becomes challenging to identify whether this can be attributed to inadequate target engagement, the inability of the biomarker to represent the drug-target pathway, or a flaw in the drug mechanism itself.73,74 Pfizer and AstraZeneca concluded that more than a third of their failed Phase II trials across multiple areas could not effectively invalidate the drug's mechanism due to a limited understanding of target exposure and engagement.65,74,75 Notably, the fact that the progression of Aβ and tau proteins occurs across different pathophysiological species, from oligomers to aggregates,76,77 imposes an additional layer of complexity in ascertaining target engagement in AD. The assessment of target engagement for drugs targeting these proteins may be more informative using biomarkers that represent or are as proximal as possible to the target protein species. For example, for drugs that aim to mitigate Aβ pre-plaque conformations, target engagement can be tested more reliably using biomarkers for oligomeric Aβ,68 whereas for drugs targeting fibrillar Aβ using Aβ PET.78 Tau-lowering drugs can target a variety of tau-related processes that, broadly speaking, can be related to proximal tau expression and modifications or distal tau aggregation.8 The use of tau biomarkers for target engagement that are distant from, or not in equilibrium with, the targeted tau species can produce inconclusive results. For instance, a reduction in tau PET uptake when evaluating a therapy that targets tau expression can support target engagement and an equilibrium between the drug target and distal tau aggregation. Conversely, the absence of change in tau PET in the same trial could represent a lack of target engagement or simply that the drug target did not sufficiently contribute to the tau aggregation measured by the tau PET tracer. This distinction can be important in deciding whether the drug should be abandoned due to pharmacodynamic or pharmacokinetic concerns, or whether it can be used in combination therapies or repurposed for other conditions. The assessment of target engagement for the emerging tau immunotherapies could benefit from the use of fluid assays that measure the specific epitope targeted or those in the same domain.79 For instance, the microtubule-binding region (MTBR) of tau represents a small fraction of total tau in CSF,73 suggesting that MTBR-specific assays can potentially increase the sensitivity of target engagement for MTBR-directed therapies compared to N-terminal or total tau assays.80 AD clinical trials that failed to slow clinical progression and did not produce evidence of target engagement had a limited ability to establish mechanism failure.3 Most recent clinical trials testing anti-Aβ or anti-tau drugs used evidence of effects on CSF/PET Aβ and tau, respectively, to support drug-target interaction.19,20,69 Clinical trial reports showing population-level mean biomarker reductions in the treatment group provide good insights into the potential for target engagement.81 However, population-level observations may obscure interindividual heterogeneities in target engagement, such as those caused by differences in the integrity of the blood–brain barrier and other factors associated with drug tissue distribution and elimination.64,82 The most informative studies have reported target engagement as the percentage of individuals showing evidence of drug-target interaction in the brain compartment.68
Biological response
Response biomarkers can be used to confirm that the drug produced the expected biological effect on the target.4,83 Changes in fluid or PET biomarkers, regardless of their magnitude, can substantiate the engagement of anti-Aβ and anti-tau therapies with their respective targets. Although this may be difficult to achieve, the ideal outcome when assessing biological response would be the complete remission of protein biomarkers to their normal levels. This is due to the current lack of data that excludes the possibility that residual levels of pathological proteins may continue to cause the symptoms. It could be argued that a reduction in protein levels measured directly in the brain using PET biomarkers provides a more robust indicator of biological response compared to reductions in brain proteins measured indirectly through CSF and blood biomarkers. This is because post-treatment changes in biofluids are more likely than changes in PET to reflect transient shifts in equilibrium between compartments (i.e., brain and fluids), which may not always represent the pathological environment of brain tissue. On the other hand, a remission of the PET biomarker to normal levels is more likely to indicate the physical absence of the proteinopathy in the brain tissue. Although assessing biological response at the population level using mean biomarker reduction in the treatment group is important for estimating the net effect of the drug on biomarkers,68 understanding the proportion of participants who achieved complete remission of the pathology can help determine whether the effect of eliminating the target pathology was thoroughly tested in most participants in the trial.47 Furthermore, individual-level assessments can facilitate the understanding of biological response in the context of characteristics of people (e.g., demographics) and disease (e.g., severity, duration). The anti-Aβ monoclonal antibodies that showed the highest proportion of complete biological remission leading to Aβ PET negativity were Lecanemab (81%),84 Gantenerumab (80%) in a 36-month extension,85 Donanemab low (80%) and high (68%) tau PET groups,46 and Aducanumab EMERGE (48%) and ENGAGE (31%).16 Some anti-Aβ drugs have shown negligible remissions of Aβ PET to normal levels in their Phase II or III trials.21,86, 87, 88, 89, 90, 91 There is currently no consensus regarding cut-off values for determining Aβ PET positivity. Therefore, the use of different cut-offs to determine Aβ PET positivity in some of these trials limits the comparability of the proportions of participants who achieved complete remission of Aβ plaques after treatment.
Disease modification
Response biomarkers can be used to determine whether the biological effect on the target induced a downstream effect in the AD pathway. If we consider that AD progresses in a cascade of events,1 evidence of a biological response on the target, together with an associated effect on downstream biomarkers in the disease pathway, could increase the likelihood of a true modification of AD progression. A clear distinction between biological response and downstream AD modification has the potential to provide valuable information for interpreting clinical trial outcomes. For example, a drug that exclusively reduces Aβ plaques could decrease Aβ PET without having a downstream effect in the AD pathway, if Aβ pre-plaque conformations are responsible for triggering tau and neurodegeneration.92 Similarly, it could be postulated that a reduction in tau PET uptake after an anti-tau therapy could have been artificially produced by reducing off-target tracer.93 In both cases, changes in biomarkers of downstream pathologies may support that the drug's mechanism lies in the pathway leading to dementia. Clinical trials in which an adequate biological response did not translate into an effect on the disease pathway could be interpreted as an indication that the drug mechanism was adequately examined and found to be ineffective. In contrast, it can be challenging to invalidate a mechanism based on trials that show no clinical benefit but strong evidence of downstream modification in the AD pathway due to a lack of knowledge about the timing, magnitude, and duration of the effect needed to alter clinical outcomes. Although it is difficult to fully invalidate a drug target in the presence of robust disease modification, a trial may support that the drug is unable to produce a meaningful clinical benefit in the respective clinicobiological context tested. For example, evidence indicates the inability of adequate Aβ plaque removal to translate into a significant clinical benefit over 1.5 years in individuals with already high levels of tau tangle pathology.46 The collective observation of a set of clinical trials demonstrating negligible clinical benefits across the clinicobiological spectrum of AD, despite complete remission of the targeted pathology, may support contextual invalidation of the mechanism even in the presence of disease modification. Still, negative outcomes from open-label extensions and registries may be required to substantiate the invalidation of the mechanism over longer periods of pathological remission. The anti-Aβ monoclonal antibodies Lecanemab and Donanemab reduced CSF and/or plasma p-tau and GFAP.18,46,94 Aducanumab decreased p-tau levels measured in CSF and plasma.16 Lecanemab, but not Donanemab, decreased plasma NfL.18,94 Lecanemab and Aducanumab, but not Donanemab, reduced temporal lobe tau PET uptake.16,46,95 Gantenerumab reduced CSF p-tau and did not alter tau PET uptake.17 In a recent post hoc analysis, Gantenerumab did not modify the trajectory of CSF NfL but was associated with an increase in CSF sTREM2 and a decrease in plasma GFAP levels.96 Bapineuzumab reduced CSF p-tau only among APOEε4 carriers.90 Solanezumab and Crenezumab showed no evidence of a reduction in tau biomarkers in CSF and PET.19,21,87,91 Solanezumab did not decrease but increased CSF NfL neurodegeneration and did not affect microglial (TREM2) or astrocytic (GFAP) biomarkers.96 While some anti-tau therapies have shown downstream effects on NfL (e.g., AADvac1,97 HMTM98), others have not (e.g., Semorinemab99).
Surrogate endpoint
Response biomarkers can be used as substitutes for clinical endpoints.4,5 Randomised clinical trials that test drug effects on clinical outcome assessments provide the highest level of evidence for a clinical benefit.4,100 To reduce resource utilization and/or accelerate drug development, indirect measures such as biomarkers are often considered surrogates or substitutes for clinical outcomes.4 The FDA reported that between 2010 and 2012, approximately 45% of new drugs were approved based on the results of surrogate endpoints.101 FDA classifies surrogate endpoints into validated, candidate, and reasonably likely.4 A validated surrogate endpoint is supported by clear mechanistic rationale and robust clinical data.4 Clinical validation of a surrogate endpoint for a specific COU requires both (1) a strong correlation with the clinical outcome and (2) statistical inference supporting that the net effect of the intervention on the surrogate endpoint predicts its net effect on the clinical outcome.102, 103, 104, 105, 106, 107 Analysis of data from multiple randomised controlled trials is generally necessary to confidently meet the second requirement, ensuring that the surrogate endpoint is generalizable across drugs in the class.5,108 Thus, while observational studies can provide supportive data, they typically cannot validate a surrogate endpoint.4 Due to the complexities involved in meeting the second requirement, there are currently no validated surrogate biomarkers in the field of AD. Yet, putative surrogate endpoints that are not fully validated are often utilised to support clinical trials. A candidate surrogate endpoint is still being evaluated for its capacity to predict the clinical benefit.4 A reasonably likely surrogate endpoint has a strong mechanistic and/or epidemiological rationale but lacks robust clinical validation, such as that derived from the analysis of multiple randomised controlled trials.109 They should be used only after full consideration of their limitations and the fact that they may produce misleading conclusions.110 Reasonably likely surrogate endpoints can be used for FDA accelerated approvals, but due to the residual uncertainty regarding clinical benefit that is typical of this approval route, post-market confirmatory trials are generally required.4,108 The FDA accelerated approval program was created in the 1990s in response to the HIV pandemic to bring drugs to market more quickly based on changes to unvalidated surrogates that were reasonably likely to predict clinical benefit.111 Over the past decade, the majority of accelerated approvals have occurred in the field of oncology.112 In the realm of neurodegenerative conditions, a drug designed to halt the progression of amyotrophic lateral sclerosis recently received accelerated approval based on reducing plasma NfL.113,114 In AD, it has been proposed that a reduction in Aβ PET uptake is reasonably likely to predict clinical benefit when testing anti-Aβ therapies.115 In practice, some recent clinical trials have demonstrated that reducing Aβ PET parallels clinical benefit,18,46 whereas others have shown that no clinical benefit was observed with Aβ PET reduction.17 Changes in biomarkers that indicate distant pathologies in the causal pathway of clinical symptoms, such as Aβ, are known to have the potential to produce conflicting conclusions about clinical benefit.106,107 This may be attributed to the fact that symptomatic patients already present varying degrees of downstream pathologies that are more closely associated with their clinical symptoms. Given the strong correlation between tau tangles and neurodegeneration with clinical symptoms,116,117 it can be postulated that biomarkers for these pathologies have the potential to be used as surrogates for clinical outcomes. The longitudinal hierarchical accumulation of tau tangles PET following Braak stages suggests that trials using tau PET as an endpoint could select participants at similar Braak stages to avoid misinterpretation of their results.33,118 Suppose that two participants, one with baseline tau PET indicating Braak II and another indicating Braak VI, participate in the same trial testing drug effects on tau PET regions encompassing Braak I-III. In this circumstance, the participant at Braak VI would naturally exhibit a decelerated rate of tau accumulation in the region compared to the participant at Braak II. This difference, which is related to their different stages of tau propagation, could be misinterpreted as a therapeutic effect.51 Several recent clinical trials have used changes in CSF and blood biomarkers, such as p-tau and NfL, as exploratory endpoints.18,46,70,97 There are clear advantages to using fluid biomarkers as endpoints in clinical trials, such as the possibility of concomitantly evaluating drug effects on multiple biological processes. However, the utility of changes in fluid biomarkers as surrogates for clinical outcomes in AD trials is unclear. In fact, it remains unclear whether pathological changes measured in biofluids following drug exposure can even be surrogates for changes in brain tissue pathology. Evidence suggests that drug-induced reduction in fluid p-tau does not necessarily equate to a reduction in brain tau PET uptake.46 Furthermore, a decrease in NfL, theoretically representing less brain degeneration in the treatment group, has been observed in parallel with a reduction in brain volume.84,119 Other current limitations primarily involving the use of blood biomarkers as endpoints include a poor understanding of factors associated with biological and analytical variation of available assays which, if not taken into consideration, could greatly affect their longitudinal quantification in clinical trials.120,121
Monitoring biomarkers
Monitoring biomarkers can be used to serially assess the status of a disease or medical condition or the effects of exposure to therapy.4 This includes repeated measurements of biomarkers across a wide range of BEST categories, including those related to population selection, drug response, and safety.4 Monitoring biomarkers are most commonly used in contexts of tracking ongoing response to treatment or the emergence of toxicity. Biomarkers can also be repeatedly measured to quantify rates of change and magnitudes of drug effects over time points.4 Serial biomarkers collected after drug discontinuation can potentially inform expected periods of pathology/biomarker remission after treatment, which can help to clarify the need for additional drug exposure. Serial Aβ PET measurements have been used to monitor continued biological response to anti-Aβ therapies.18,85 Serial tau PET can investigate continued biological response or disease modification in the context of anti-tau and anti-Aβ therapies, respectively.18,46 Repeated brain MRI acquisitions to detect amyloid-related imaging abnormalities (ARIA) represent an intersection between monitoring and safety biomarkers, helping to control drug toxicity and the need for drug discontinuation.122
Safety biomarkers
Safety biomarkers can be used to indicate the probability, presence, or extent of an adverse event.1 The use of brain MRI to monitor the presence of brain bleeding and swelling, which can contraindicate or lead to the discontinuation of anti-Aβ therapies, is currently the best-known example of the use of safety biomarkers in AD clinical trials.123 APOEε4 genotype may be considered a safety biomarker in the context where homozygous carriers are at greater risk of developing ARIA when treated with anti-Aβ therapies.124 The need for additional drug- or class-specific safety biomarkers may arise with different drug targets/mechanisms. For example, BACE inhibitors have been shown to decrease lymphocytes and increase alanine transaminase,125,126 which should be monitored when testing these medications.
Conclusion
Biomarkers hold significant potential for enhancing and expediting the development of drugs for AD and related dementias. The BEST glossary provides a means to standardise the terminology used for various biomarker applications, fostering discussions within and beyond the AD field. Baseline biomarker values can help identify participants with AD (diagnostic) or those likely to develop AD (susceptibility), who are most likely to progress during the clinical trial period (prognosis), or who could potentially benefit most from therapy (predictive) with less chance of side effects (safety). Changes in biomarkers following drug exposure can provide evidence of drug-target interaction (target engagement), biological effect on the target (biological response), and whether the biological effect is linked to a downstream effect in the AD pathway (disease modification) or possible clinical benefit (surrogate). Biomarkers can also be repeatedly measured (monitoring) to track changes in treatment response or toxicity. While the baseline levels of both biofluid and PET protein markers provide a robust foundation for population selection, additional data are needed to better understand the significance of changes in fluid levels in response to drug therapies. It is crucial to separately characterise, whenever possible, the different COU of biomarkers for accurate interpretation of positive and negative clinical trial results. For example, if a trial selected the appropriate population and confirmed target engagement with a sufficient biological response but without evidence of downstream disease modification or clinical benefit, this would support an interpretation that the mechanism was adequately tested and invalidated. Conversely, if the same clinical trial had shown robust modification in biomarkers downstream in the AD pathway, the notion that the trial adequately tested the mechanism could be questioned, given that the timing and duration of effects necessary to influence cognition remain uncertain. In this case, it may be necessary to collectively observe the results of numerous trials that span the clinicobiological spectrum of AD, including long-term extensions and registries, to comprehensively evaluate the drug mechanism. In clinical trials that do not use biomarkers, invalidating mechanisms will always be challenging due to uncertainties about whether the appropriate population was selected, and whether the drug reached the brain, sustained target engagement, and exerted the expected effect.
Outstanding questions
The use of biomarkers in AD clinical trials has broadened, resulting in more complex interpretations of their outcomes. This has heightened the demand for standardised terminologies and interpretations to streamline communication. However, certain definitions and concepts, such as those pertaining to population selection and enrichment, target engagement, drugs’ biological activity, disease modification, and surrogacy, are frequently used interchangeably, leaving room for variations in their interpretation across studies.
Contributors
Conceptualisation: TAP; Literature search: TAP and BB; Figure and tables: TAP; Writing - original draft: TAP. Writing - review & editing: CSA, FL, LC, CKS, JF, PR-N, ERZ, PCLF and BB. All authors approved the final version of the manuscript for submission.
Declaration of interests
TAP received support to attend meetings from the Alzheimer's Association. LC has received support to attend meetings from the Alzheimer's Association and IMPACT-AD. CKS has received support to attend meetings from the Alzheimer's Association and payment for lectures from the Busse Research Award for Biomedical Research. CSA has received support to attend meetings from the Alzheimer's Association. JF reported receiving personal fees for service on the advisory boards, adjudication committees, or speaker honoraria from AC Immune, Adamed, Alzheon, Biogen, Esteve, Laboratorios Carnot, Ionis, Lilly, LMI, Lundbeck, Perha, Roche, Zambon and, outside the submitted work. JF reports holding a patent for markers of synaptopathy in neurodegenerative disease (licence to Adx, EPI8382175.0). PR-N received payment for lectures and has served on scientific advisory boards and/or as a consultant for Novo Nordisk, and Eisai. ERZ is on the scientific advisory board of Nintx, Novo Nordisk and is on the scientific advisory board and is a co-founder of MASIMA. MASIMA is a Brazilian company that provides brain scan analytical tools to hospitals. ERZ has never received royalties of financial gains from MASIMA. ERZ has received support to attend meetings from Alzheimer's Association, CNPq, and CAPES. PCLF has received support to attend meetings from the Alzheimer's Association. BB has received support to attend meetings from the Alzheimer's Association.
Acknowledgements
TAP is supported by the National Institute on Aging (R01AG075336, R01AG073267). JF was supported by Fondo de Investigaciones Sanitario, Carlos III Health Institute (INT21/00073, PI20/01473 and PI23/01786) and the Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas Program 1, partly jointly funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una Manera de Hacer Europa. JF was also supported by the National Institutes of Health grants (R01 AG056850; R21 AG056974, R01 AG061566, R01 AG081394 and R61AG066543), the Department de Salut de la Generalitat de Catalunya, Pla Estratègic de Recerca Innovació en Salut (SLT006/17/00119), by Fundación Tatiana Pérez de Guzmán el Bueno (IIBSP-DOW-2020-151) and Horizon 2020–Research and Innovation Framework Programme from the European Union (H2020-SC1-BHC-2018-2020). PRN is supported by the Fonds de Recherche du Québec – Santé (FRQS; Chercheur Boursier, 2020-VICO-279314) and Colin J. Adair Charitable Foundation. ERZ is supported by CNPq (435642/2018-9; 312410/2018- 2; 409066/2022-2; 312306/2021-0; 409595/2023-3), Instituto Serrapilheira (Serra-1912-31365), Brazilian National Institute of Science and Technology in Excitotoxicity and Neuroprotection (465671/2014-4), FAPERGS/MS/CNPq/SESRS–PPSUS (30786.434.24734.231120170), ARD/FAPERGS (21/2551-0000673-0) and Alzheimer's Association [AARGD-21-850670]. PCLF is supported by the Alzheimer's Association (AARFD-22-923814). BB receives financial support from the Alzheimer's Association (AARFD-22-974627). Funders had no role in the paper design, data collection, data analysis, interpretation, writing, or the decision to submit it for publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105322.
Appendix A. Supplementary data
References
- 1.Jack C.R., Jr., Knopman D.S., Jagust W.J., et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heneka M.T., Carson M.J., El Khoury J., et al. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings J. Lessons learned from Alzheimer disease: clinical trials with negative outcomes. Clin Transl Sci. 2018;11(2):147–152. doi: 10.1111/cts.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BEST (Biomarkers, EndpointS, and other Tools) resource. Silver Spring; Bethesda (MD): 2016. [PubMed] [Google Scholar]
- 5.Califf R.M. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018;243(3):213–221. doi: 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . 2021. Context of use.https://www.fda.gov/drugs/biomarker-qualification-program/context-use Accessed May, 2024. [Google Scholar]
- 7.Cummings J., Kinney J. Biomarkers for Alzheimer's disease: context of use, qualification, and roadmap for clinical implementation. Medicina (Kaunas) 2022;58(7):952. doi: 10.3390/medicina58070952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings J., Zhou Y., Lee G., Zhong K., Fonseca J., Cheng F. Alzheimer's disease drug development pipeline: 2024. Alzheimers Dement (N Y) 2024;10(2) doi: 10.1002/trc2.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Health NIo . Dictionary of Cancer Terms; 2011. National cancer institute. [Google Scholar]
- 10.Petersen R.C. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 11.Jessen F., Amariglio R.E., van Boxtel M., et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas K.R., Bangen K.J., Weigand A.J., et al. Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology. 2020;94(4):e397–e406. doi: 10.1212/WNL.0000000000008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sperling R.A., Aisen P.S., Beckett L.A., et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association D., Association A.P. American psychiatric association; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders: DSM-5. [Google Scholar]
- 15.US Food and Drug Administration . 2024. Early Alzheimer's disease: developing drugs for treatment.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-alzheimers-disease-developing-drugs-treatment Accessed May, 2024. [Google Scholar]
- 16.Budd Haeberlein S., Aisen P.S., Barkhof F., et al. Two randomized phase 3 studies of Aducanumab in early Alzheimer's disease. J Prev Alzheimers Dis. 2022;9(2):197–210. doi: 10.14283/jpad.2022.30. [DOI] [PubMed] [Google Scholar]
- 17.Bateman R.J., Smith J., Donohue M.C., et al. Two phase 3 trials of gantenerumab in early Alzheimer's disease. N Engl J Med. 2023;389(20):1862–1876. doi: 10.1056/NEJMoa2304430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Dyck C.H., Swanson C.J., Aisen P., et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 19.Salloway S., Honigberg L.A., Cho W., et al. Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE) Alzheimers Res Ther. 2018;10(1):96. doi: 10.1186/s13195-018-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cummings J.L., Cohen S., van Dyck C.H., et al. ABBY: a phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90(21):e1889–e1897. doi: 10.1212/WNL.0000000000005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperling R.A., Donohue M.C., Raman R., et al. Trial of solanezumab in preclinical Alzheimer's disease. N Engl J Med. 2023;389(12):1096–1107. doi: 10.1056/NEJMoa2305032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo F., Karras B.T., Phillips R., Kimball A.M., Wolf F. Diagnoses, syndromes, and diseases: a knowledge representation problem. AMIA Annu Symp Proc. 2003;2003:802. [PMC free article] [PubMed] [Google Scholar]
- 23.Jagust W., Jack C.R., Jr., Bennett D.A., et al. “Alzheimer's disease” is neither “Alzheimer's clinical syndrome” nor “dementia”. Alzheimers Dement. 2019;15(1):153–157. doi: 10.1016/j.jalz.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Graff-Radford J., Yong K.X.X., Apostolova L.G., et al. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222–234. doi: 10.1016/S1474-4422(20)30440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFarland N.R. Diagnostic approach to atypical parkinsonian syndromes. Continuum (Minneap Minn) 2016;22(4 Movement Disorders):1117–1142. doi: 10.1212/CON.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien J.T., Erkinjuntti T., Reisberg B., et al. Vascular cognitive impairment. Lancet Neurol. 2003;2(2):89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 27.Ismail Z., Aguera-Ortiz L., Brodaty H., et al. The mild behavioral impairment checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56(3):929–938. doi: 10.3233/JAD-160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloom G.S. Amyloid-beta and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 29.Jack C.R., Jr., Bennett D.A., Blennow K., et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87(5):539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brum W.S., Cullen N.C., Janelidze S., et al. A two-step workflow based on plasma p-tau217 to screen for amyloid β positivity with further confirmatory testing only in uncertain cases. Nature Aging. 2023;3(9):1079–1090. doi: 10.1038/s43587-023-00471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barthelemy N.R., Salvado G., Schindler S., et al. Highly accurate blood test for Alzheimer's disease comparable or superior to clinical CSF tests. Nat Med. 2024;30(4):1085–1095. doi: 10.1038/s41591-024-02869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H., Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 33.Pascoal T.A., Therriault J., Benedet A.L., et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain. 2020;143(9):2818–2830. doi: 10.1093/brain/awaa180. [DOI] [PubMed] [Google Scholar]
- 34.Ossenkoppele R., Pichet Binette A., Groot C., et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat Med. 2022;28(11):2381–2387. doi: 10.1038/s41591-022-02049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albert M.S., DeKosky S.T., Dickson D., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montine T.J., Phelps C.H., Beach T.G., et al. National institute on aging-Alzheimer's association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123(1):1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattsson-Carlgren N., Andersson E., Janelidze S., et al. Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease. Sci Adv. 2020;6(16) doi: 10.1126/sciadv.aaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Therriault J., Pascoal T.A., Lussier F.Z., et al. Biomarker modeling of Alzheimer's disease using PET-based Braak staging. Nature Aging. 2022;2(6):526–535. doi: 10.1038/s43587-022-00204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwarz A.J., Yu P., Miller B.B., et al. Regional profiles of the candidate tau PET ligand 18F-AV-1451 recapitulate key features of Braak histopathological stages. Brain. 2016;139(Pt 5):1539–1550. doi: 10.1093/brain/aww023. [DOI] [PubMed] [Google Scholar]
- 40.Erickson P., Simren J., Brum W.S., et al. Prevalence and clinical implications of a beta-amyloid-negative, tau-positive cerebrospinal fluid biomarker profile in alzheimer disease. JAMA Neurol. 2023;80(9):969–979. doi: 10.1001/jamaneurol.2023.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon B., Guo T., Provost K., et al. Abnormal tau in amyloid PET negative individuals. Neurobiol Aging. 2022;109:125–134. doi: 10.1016/j.neurobiolaging.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vellas B., Carrillo M.C., Sampaio C., et al. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimers Dement. 2013;9(4):438–444. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Karran E., Hardy J. A critique of the drug discovery and phase 3 clinical programs targeting the amyloid hypothesis for Alzheimer disease. Ann Neurol. 2014;76(2):185–205. doi: 10.1002/ana.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gauthier S., Feldman H.H., Schneider L.S., et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer's disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388(10062):2873–2884. doi: 10.1016/S0140-6736(16)31275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beach T.G., Monsell S.E., Phillips L.E., Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at national Institute on aging Alzheimer disease centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sims J.R., Zimmer J.A., Evans C.D., et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–527. doi: 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mintun M.A., Lo A.C., Duggan Evans C., et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691–1704. doi: 10.1056/NEJMoa2100708. [DOI] [PubMed] [Google Scholar]
- 48.Ferreira D., Przybelski S.A., Lesnick T.G., et al. β-Amyloid and tau biomarkers and clinical phenotype in dementia with Lewy bodies. Neurology. 2020;95(24):e3257–e3268. doi: 10.1212/WNL.0000000000010943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellaver B., Povala G., Ferreira P.C.L., et al. Astrocyte reactivity influences amyloid-beta effects on tau pathology in preclinical Alzheimer's disease. Nat Med. 2023;29(7):1775–1781. doi: 10.1038/s41591-023-02380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groot C., Smith R., Stomrud E., et al. Phospho-tau with subthreshold tau-PET predicts increased tau accumulation rates in amyloid-positive individuals. Brain. 2022;146(4):1580–1591. doi: 10.1093/brain/awac329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pascoal T.A., Benedet A.L., Tudorascu D.L., et al. Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages. Brain. 2021;144(11):3517–3528. doi: 10.1093/brain/awab248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith R., Cullen N.C., Pichet Binette A., et al. Tau-PET is superior to phospho-tau when predicting cognitive decline in symptomatic AD patients. Alzheimers Dement. 2023;19(6):2497–2507. doi: 10.1002/alz.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rafii M.S., Sperling R.A., Donohue M.C., et al. The AHEAD 3-45 study: design of a prevention trial for Alzheimer's disease. Alzheimers Dement. 2023;19(4):1227–1233. doi: 10.1002/alz.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pascoal T.A., Mathotaarachchi S., Shin M., et al. Amyloid and tau signatures of brain metabolic decline in preclinical Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2018;45(6):1021–1030. doi: 10.1007/s00259-018-3933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrari-Souza J.P., Bellaver B., Ferreira P.C.L., et al. APOEepsilon4 potentiates amyloid beta effects on longitudinal tau pathology. Nat Aging. 2023;3(10):1210–1218. doi: 10.1038/s43587-023-00490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Riviere M.E., Langbaum J.B., Turner R.S., et al. Effects of the active amyloid beta immunotherapy CAD106 on PET measurements of amyloid plaque deposition in cognitively unimpaired APOE epsilon4 homozygotes. Alzheimers Dement. 2024;20(3):1839–1850. doi: 10.1002/alz.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ClinicalTrials.gov . 2024. MCLENA-1: a clinical trial for the assessment of lenalidomide in amnestic MCI patients.https://classic.clinicaltrials.gov/show/NCT04032626 Accessed May, 2024. [Google Scholar]
- 58.ClinicalTrials.gov . 2024. Safety and pharmacokinetics of single ascending doses and multiple ascending doses of CS6253 in healthy volunteers.https://classic.clinicaltrials.gov/show/NCT05965414 Accessed May, 2024. [Google Scholar]
- 59.ClinicalTrials gov . 2023. Allopregnanolone regenerative therapeutic for mild Alzheimer's disease.https://classic.clinicaltrials.gov/show/NCT04838301 Accessed May, 2024. [Google Scholar]
- 60.Lee G., Nho K., Kang B., Sohn K.A., Kim D. For Alzheimer's disease neuroimaging I. predicting Alzheimer's disease progression using multi-modal deep learning approach. Sci Rep. 2019;9(1):1952. doi: 10.1038/s41598-018-37769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu S., Gordon B.A., Hornbeck R., et al. Discovery and validation of autosomal dominant Alzheimer's disease mutations. Alzheimers Res Ther. 2018;10(1):67. doi: 10.1186/s13195-018-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Polvikoski T., Sulkava R., Haltia M., et al. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333(19):1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 63.Fortea J., Pegueroles J., Alcolea D., et al. APOE4 homozygozity represents a distinct genetic form of Alzheimer's disease. Nat Med. 2024;30(5):1284–1291. doi: 10.1038/s41591-024-02931-w. [DOI] [PubMed] [Google Scholar]
- 64.Di L., Rong H., Feng B. Demystifying brain penetration in central nervous system drug discovery. Miniperspective. J Med Chem. 2013;56(1):2–12. doi: 10.1021/jm301297f. [DOI] [PubMed] [Google Scholar]
- 65.Cook D., Brown D., Alexander R., et al. Lessons learned from the fate of AstraZeneca's drug pipeline: a five-dimensional framework. Nat Rev Drug Discov. 2014;13(6):419–431. doi: 10.1038/nrd4309. [DOI] [PubMed] [Google Scholar]
- 66.Simon G.M., Niphakis M.J., Cravatt B.F. Determining target engagement in living systems. Nat Chem Biol. 2013;9(4):200–205. doi: 10.1038/nchembio.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matthews P.M., Rabiner E.A., Passchier J., Gunn R.N. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol. 2012;73(2):175–186. doi: 10.1111/j.1365-2125.2011.04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang T., Dang Y., Ostaszewski B., et al. Target engagement in an alzheimer trial: crenezumab lowers amyloid beta oligomers in cerebrospinal fluid. Ann Neurol. 2019;86(2):215–224. doi: 10.1002/ana.25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mummery C.J., Borjesson-Hanson A., Blackburn D.J., et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer's disease: a phase 1b, randomized, placebo-controlled trial. Nat Med. 2023;29(6):1437–1447. doi: 10.1038/s41591-023-02326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng E., Manser P.T., Pickthorn K., et al. Safety and efficacy of Semorinemab in individuals with prodromal to mild Alzheimer disease: a randomized clinical trial. JAMA Neurol. 2022;79(8):758–767. doi: 10.1001/jamaneurol.2022.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shulman M., Kong J., O'Gorman J., et al. TANGO: a placebo-controlled randomized phase 2 study of efficacy and safety of the anti-tau monoclonal antibody gosuranemab in early Alzheimer's disease. Nat Aging. 2023;3(12):1591–1601. doi: 10.1038/s43587-023-00523-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moloney C.M., Lowe V.J., Murray M.E. Visualization of neurofibrillary tangle maturity in Alzheimer's disease: a clinicopathologic perspective for biomarker research. Alzheimers Dement. 2021;17(9):1554–1574. doi: 10.1002/alz.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bespalov A., Courade J.P., Khiroug L., Terstappen G.C., Wang Y. A call for better understanding of target engagement in Tau antibody development. Drug Discov Today. 2022;27(11) doi: 10.1016/j.drudis.2022.103338. [DOI] [PubMed] [Google Scholar]
- 74.Bespalov A., Steckler T., Altevogt B., et al. Failed trials for central nervous system disorders do not necessarily invalidate preclinical models and drug targets. Nat Rev Drug Discov. 2016;15(7):516. doi: 10.1038/nrd.2016.88. [DOI] [PubMed] [Google Scholar]
- 75.Morgan P., Van Der Graaf P.H., Arrowsmith J., et al. Can the flow of medicines be improved? Fundamental pharmacokinetic and pharmacological principles toward improving phase II survival. Drug Discov Today. 2012;17(9-10):419–424. doi: 10.1016/j.drudis.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 76.Selkoe D.J. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192(1):106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niewiadomska G., Niewiadomski W., Steczkowska M., Gasiorowska A. Tau oligomers neurotoxicity. Life (Basel) 2021;11(1) doi: 10.3390/life11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tolar M., Abushakra S., Hey J.A., Porsteinsson A., Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ-801-the first wave of amyloid-targeting drugs for Alzheimer's disease with potential for near term approval. Alzheimer's Res Ther. 2020;12(1):95. doi: 10.1186/s13195-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Congdon E.E., Ji C., Tetlow A.M., Jiang Y., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease: current status and future directions. Nat Rev Neurol. 2023;19(12):715–736. doi: 10.1038/s41582-023-00883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Horie K., Barthelemy N.R., Sato C., Bateman R.J. CSF tau microtubule binding region identifies tau tangle and clinical stages of Alzheimer's disease. Brain. 2021;144(2):515–527. doi: 10.1093/brain/awaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu E., Schmidt M.E., Margolin R., et al. Amyloid-beta 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85(8):692–700. doi: 10.1212/WNL.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rezai A.R., D'Haese P.F., Finomore V., et al. Ultrasound blood-brain barrier opening and Aducanumab in Alzheimer's disease. N Engl J Med. 2024;390(1):55–62. doi: 10.1056/NEJMoa2308719. [DOI] [PubMed] [Google Scholar]
- 83.Plenge R.M., Scolnick E.M., Altshuler D. Validating therapeutic targets through human genetics. Nat Rev Drug Discov. 2013;12(8):581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- 84.Swanson C.J., Zhang Y., Dhadda S., et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer's disease with lecanemab, an anti-Abeta protofibril antibody. Alzheimers Res Ther. 2021;13(1):80. doi: 10.1186/s13195-021-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Klein G., Delmar P., Kerchner G.A., et al. Thirty-six-month amyloid positron emission tomography results show continued reduction in amyloid burden with subcutaneous gantenerumab. J Prev Alzheimers Dis. 2021;8(1):3–6. doi: 10.14283/jpad.2020.68. [DOI] [PubMed] [Google Scholar]
- 86.Honig L.S., Vellas B., Woodward M., et al. Trial of solanezumab for mild dementia due to Alzheimer's disease. N Engl J Med. 2018;378(4):321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 87.Doody R.S., Thomas R.G., Farlow M., et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370(4):311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 88.Ostrowitzki S., Lasser R.A., Dorflinger E., et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer's disease. Alzheimers Res Ther. 2017;9(1):95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salloway S., Farlow M., McDade E., et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer's disease. Nat Med. 2021;27(7):1187–1196. doi: 10.1038/s41591-021-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salloway S., Sperling R., Fox N.C., et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N Engl J Med. 2014;370(4):322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ostrowitzki S., Bittner T., Sink K.M., et al. Evaluating the safety and efficacy of crenezumab vs placebo in adults with early alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurol. 2022;79(11):1113–1121. doi: 10.1001/jamaneurol.2022.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sengupta U., Nilson A.N., Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. eBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ng K.P., Pascoal T.A., Mathotaarachchi S., et al. Monoamine oxidase B inhibitor, selegiline, reduces 18F-THK5351 uptake in the human brain. Alzheimer's Res Ther. 2017;9(1):25. doi: 10.1186/s13195-017-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pontecorvo M.J., Lu M., Burnham S.C., et al. Association of Donanemab treatment with exploratory plasma biomarkers in early symptomatic alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA Neurol. 2022;79(12):1250–1259. doi: 10.1001/jamaneurol.2022.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knopman D.S. Lecanemab reduces brain amyloid-beta and delays cognitive worsening. Cell Rep Med. 2023;4(3) doi: 10.1016/j.xcrm.2023.100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wagemann O., Liu H., Wang G., et al. Downstream biomarker effects of gantenerumab or solanezumab in dominantly inherited alzheimer disease: the DIAN-TU-001 randomized clinical trial. JAMA Neurol. 2024;81(6):582–593. doi: 10.1001/jamaneurol.2024.0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cullen N.C., Novak P., Tosun D., et al. Efficacy assessment of an active tau immunotherapy in Alzheimer's disease patients with amyloid and tau pathology: a post hoc analysis of the "ADAMANT" randomised, placebo-controlled, double-blind, multi-centre, phase 2 clinical trial. eBioMedicine. 2024;99 doi: 10.1016/j.ebiom.2023.104923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wischik C.M., Schelter B., Penny L.K., et al. Significant dose-dependent reduction in neurofilament light chain concentration in plasma with oral tau aggregation inhibitor hydromethylthionine mesylate. Alzheimers Dementia. 2023;19(S24) [Google Scholar]
- 99.AlzForum . 2021. N-terminal tau antibodies fade, Mid-domain ones push to the fore.https://www.alzforum.org/news/conference-coverage/n-terminal-tau-antibodies-fade-mid-domain-ones-push-fore Accessed May, 2024. [Google Scholar]
- 100.Walton M.K., Powers J.H., 3rd, Hobart J., et al. Clinical outcome assessments: conceptual foundation-report of the ISPOR clinical outcomes assessment - emerging good practices for outcomes research task force. Value Health. 2015;18(6):741–752. doi: 10.1016/j.jval.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.US Food and Drug Administration . 2018. Surrogate endpoint resources for drug and biologic development.https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development Accessed May, 2024. [Google Scholar]
- 102.Weir C.J., Taylor R.S. Informed decision-making: statistical methodology for surrogacy evaluation and its role in licensing and reimbursement assessments. Pharm Stat. 2022;21(4):740–756. doi: 10.1002/pst.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buyse M., Molenberghs G., Paoletti X., et al. Statistical evaluation of surrogate endpoints with examples from cancer clinical trials. Biom J. 2016;58(1):104–132. doi: 10.1002/bimj.201400049. [DOI] [PubMed] [Google Scholar]
- 104.Sargent D.J., Goldberg R.M., Jacobson S.D., et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 105.DeMets D.L., Psaty B.M., Fleming T.R. When can intermediate outcomes Be used as surrogate outcomes? JAMA. 2020;323(12):1184–1185. doi: 10.1001/jama.2020.1176. [DOI] [PubMed] [Google Scholar]
- 106.Prentice R.L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 107.Fleming T.R., Powers J.H. Biomarkers and surrogate endpoints in clinical trials. Stat Med. 2012;31(25):2973–2984. doi: 10.1002/sim.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fleming T.R. Surrogate endpoints and FDA's accelerated approval process. Health Aff (Millwood) 2005;24(1):67–78. doi: 10.1377/hlthaff.24.1.67. [DOI] [PubMed] [Google Scholar]
- 109.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1(2):189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedman L.M., Furberg C.D., DeMets D.L., Reboussin D.M., Granger C.B. Springer; 2015. Fundamentals of clinical trials. [Google Scholar]
- 111.Beaver J.A., Howie L.J., Pelosof L., et al. A 25-year experience of US food and drug administration accelerated approval of malignant hematology and oncology drugs and biologics: a review. JAMA Oncol. 2018;4(6):849–856. doi: 10.1001/jamaoncol.2017.5618. [DOI] [PubMed] [Google Scholar]
- 112.Beakes-Read G., Neisser M., Frey P., Guarducci M. Analysis of FDA's accelerated approval program performance December 1992-December 2021. Ther Innov Regul Sci. 2022;56(5):698–703. doi: 10.1007/s43441-022-00430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller T.M., Cudkowicz M.E., Genge A., et al. Trial of antisense oligonucleotide Tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099–1110. doi: 10.1056/NEJMoa2204705. [DOI] [PubMed] [Google Scholar]
- 114.US Food and Drug Administration . 2024. FDA approves treatment of amyotrophic lateral sclerosis associated with a mutation in the SOD1 gene.https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-amyotrophic-lateral-sclerosis-associated-mutation-sod1-gene Accessed May, 2024. [Google Scholar]
- 115.US Food and Drug Administration . 2023. FDA grants accelerated approval for Alzheimer's disease treatment.https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment Accessed May, 2024. [Google Scholar]
- 116.Bejanin A., Schonhaut D.R., La Joie R., et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer's disease. Brain. 2017;140(12):3286–3300. doi: 10.1093/brain/awx243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nelson P.T., Alafuzoff I., Bigio E.H., et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 119.Novak G., Fox N., Clegg S., et al. Changes in brain volume with bapineuzumab in mild to moderate Alzheimer's disease. J Alzheimers Dis. 2016;49(4):1123–1134. doi: 10.3233/JAD-150448. [DOI] [PubMed] [Google Scholar]
- 120.Ashton N.J., Suarez-Calvet M., Karikari T.K., et al. Effects of pre-analytical procedures on blood biomarkers for Alzheimer's pathophysiology, glial activation, and neurodegeneration. Alzheimers Dement (Amst) 2021;13(1) doi: 10.1002/dad2.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brum W.S., Ashton N.J., Simren J., et al. Biological variation estimates of Alzheimer's disease plasma biomarkers in healthy individuals. Alzheimers Dement. 2024;20(2):1284–1297. doi: 10.1002/alz.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Salloway S., Chalkias S., Barkhof F., et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating Aducanumab in patients with early alzheimer disease. JAMA Neurol. 2022;79(1):13–21. doi: 10.1001/jamaneurol.2021.4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hampel H., Elhage A., Cho M., Apostolova L.G., Nicoll J.A.R., Atri A. Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain. 2023;146(11):4414–4424. doi: 10.1093/brain/awad188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Filippi M., Cecchetti G., Spinelli E.G., Vezzulli P., Falini A., Agosta F. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a Systematic review. JAMA Neurol. 2022;79(3):291–304. doi: 10.1001/jamaneurol.2021.5205. [DOI] [PubMed] [Google Scholar]
- 125.Wessels A.M., Tariot P.N., Zimmer J.A., et al. Efficacy and safety of lanabecestat for treatment of early and mild alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 2020;77(2):199–209. doi: 10.1001/jamaneurol.2019.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sperling R., Henley D., Aisen P.S., et al. Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical alzheimer disease: a truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 2021;78(3):293–301. doi: 10.1001/jamaneurol.2020.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.