Abstract
This work explores the use of a cross-shaped organic framework that is used as a template for the investigation of multi-functionalized chromophores. We report the design and synthesis of a universal cross-shaped building block bearing two bromines and two iodines on its peripheral positions. The template can be synthesized on a gram scale in a five-step reaction comprising an oxidative homo-coupling macro-cyclization. The formed scaffold was selectively functionalized via Suzuki cross-coupling reactions with methoxynaphthalene, naphthalimide and BODIPY derivatives, yielding a library of cross-shaped and chromophore-decorated model compounds, all of which were fully characterized. The formed racemic bis- and tetra-substituted crosses were resolved via chiral stationary phase HPLC, and assignment of the enantiomers was done via comparison of experimental and simulated electronic circular dichroism spectra as well as enantiomer single-crystal analysis. Additionally, the hybrid naphthalimide/BODIPY chromophore was found to be acting as an intramolecular Förster energy resonance transfer pair, which was investigated in more detail. With this easy-to-functionalize universal building block, we believe it might prove to be useful in the study of different sets of chromophores.
The design and synthesis of an easily functionalizable cross-shaped organic framework is presented. The template is bis- and tetra-decorated with achiral chromophores, offering a platform to study fundamental properties such as chirality and FRET.
Introduction
Unravelling the chemistry and photophysics of dyes has occupied the scientific world from the time of discovering the first synthetic colorant about 150 years ago.1 Ever since, prediction of and control over color and luminescence have become important due to the rising applications of dyes and pigments in our everyday life. To understand these apparent properties, scientist have studied the underlying processes that explain the features and characteristics of these optically active molecules.
Researchers found that the influence of two or more optically active molecules in close proximity can result in a variety of photophysical effects, e.g., excimer-induced red shifts, aggregation-induced fluorescence, etc.2,3 Understanding these phenomena proves to be critical due to their use in a variety of fields such as solar cells,4,5 sensing,6 catalysis7,8 and biomedical studies,9 for example, Förster Energy Resonance Transfer (FRET), which is used to study molecular dynamics in biological systems like substrate protein binding.10 Here, the donor chromophore or fluorophore, when in close enough proximity, transfers its potential fluorescence light to the next chromophore (acceptor).11 As a result, the overall fluorescence will vary depending on the distance and alignment between the donor and acceptor pair, providing insights into the spatial displacement and arrangement of the species e.g. molecular binding events. Understanding these photophysical phenomena, by studying how properties of distinct chromophores near each other influence one another, requires suitable model compounds with a fixed arrangement of their dye subunits.
Recently, there have been some examples investigating separated chromophores, attached to well-defined molecular superstructures.12–15 Crassous and co-workers synthesized helicene conjugates with naphthalimide, porphyrin or diketopyrrolopyrrole (DPP) derivatives.12–14 They showed that the chiral information of the helicenes was successfully transferred to the otherwise achiral optically active subunits. In addition, they showed that the measured circular dichroism directly relates to the exciton coupling between the chromophores. Another work, by Sidler et al., showed the importance of the spatial arrangement of chromophores by fixing a-chiral dyes (6-methoxy naphthalene) in a chiral structure.16 The confined arrangement in space resulted in induced axial chirality based on the intramolecular Davydov splitting assignment of excimers.17,18 Both studies showed how important the structural alignment of the chromophores is for the resulting properties of the system.
Inspired by these works and our recently developed cross-shaped motif, we envisioned that we could use this as a new organic superstructure. The rigid conformation of the cross-shaped motif, approaching close to a 90° angle, makes it a perfect candidate to supplement the already existing library of molecular frameworks.19 To make this motif even more appealing, it consists of two thermally stable enantiomers. A particularly interesting feature broadening the playing field is that the rigid cross-shaped motif can be split into a pair of rigid rod-type subunits by cleavage of both ester bonds.20
Here, we present a universal cross-shaped building block consisting of a pair of rigid rods, which can easily be functionalized and distinguished by common cross-coupling protocols. A library of systems consisting of up to four chromophores, including an intramolecular FRET pair, was synthesized to demonstrate its potential. These new multi-chromophore model compounds were analyzed in terms of optical and chiro-optical properties, which were attributed to the variety of structural features the cross-shaped framework provides.
Results and discussion
Design and retrosynthesis
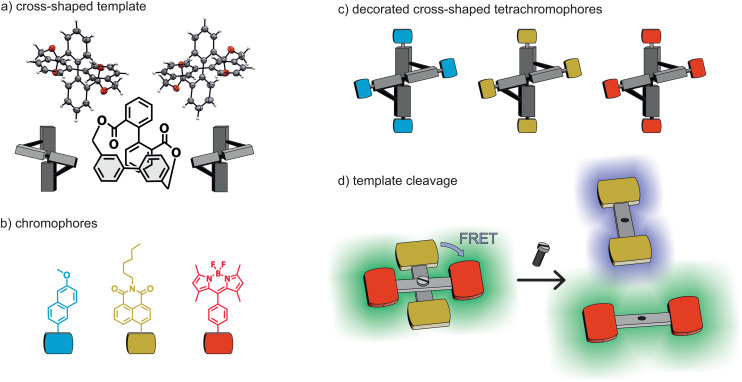
Our design principle is depicted in Fig. 1. The template consists of two biphenyls bridged over two esters, in 1-1′ and 2-2′, respectively, resulting in a cross-shaped conformation. We envisioned that functionalizing the peripheral positions with achiral chromophores would allow us to transfer the chiral information from the template to the chromophores and that we could investigate different sets of chromophores in close proximity. We opted for a set of three chromophores: the first being the 6-methoxynaphthalene chosen due to its well-studied optical and electronic properties as well as its previous use in a chiral architecture reported by our group.16 The latter two – a naphthalimide (NI) derivative and a BODIPY (BY) derivative – were chosen due to their potential of forming an intramolecular (FRET) pair as the emission spectrum (λem) of the NI overlaps with the absorption spectrum (λabs) of the BODIPY.21,22 The intention was to profit from the FRET experiment to demonstrate the cleavage of the template. As sketched in Fig. 1(d), a FRET is expected in the cross-shaped model compound, which should disappear upon template cleavage, liberating terminally NI and BODIPY decorated rigid rods.
Fig. 1. Schematic representation of the functionalization of a cross-shaped molecule with chromophores to obtain a library of mono- or hybrid chromophores. (a) Cross-shaped motif consisting of both (M) and (P) conformers adopting a distorted tetrahedron conformation. (b) Target chromophores for functionalization. (c) Library of decorated cross-shaped tetrachromophores. (d) A hybrid chromophore showing intramolecular-FRET activity, which after template cleavage results in the liberation of two differently decorated rigid rods.
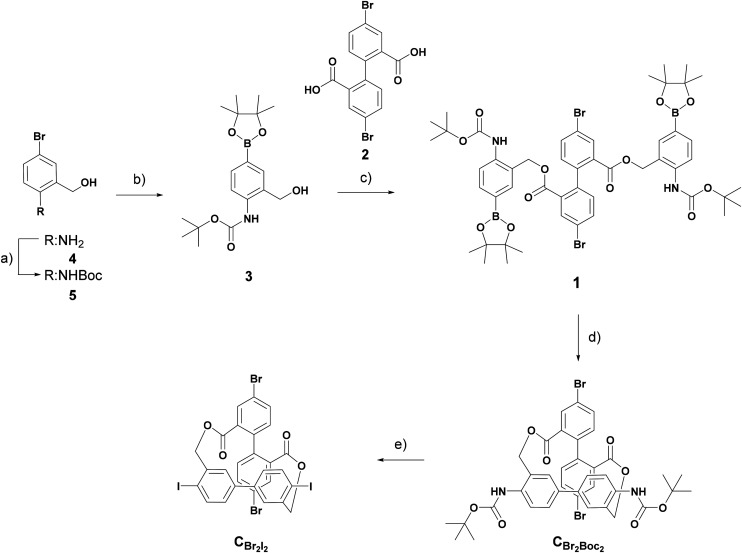
In order to access a variety of cross-shaped tetrachromophores, we revised the synthetic route of our previous work. The original synthetic route contained a number of parallel sequences equal to the amount of different target compounds. In this work, we present a modular approach with a universal building block which can be decorated via well-established cross-coupling chemistry, like, for example, the Suzuki and Sonogashira reactions. This on one hand reduces the number of reaction steps significantly, and on the other hand, allows for the implementation of the bis-biphenyl cross template into a variety of molecular designs. The universal building block CBr2I2 is displayed in Scheme 1, while further insights concerning its design (ESI, Fig. S1†) and retrosynthesis (ESI, Scheme S1†) are provided in the ESI.†CBr2I2 contains iodide and bromide functionalities, acting as two separate addressable motifs at the end of both bars of the cross. The retrosynthetic analysis (displayed in the ESI, Scheme S1,† because it basically duplicates the forward synthesis displayed in Scheme 1) of CBr2I2 was envisioned over bis-boronic ester intermediate 1via an oxidative homo-coupling and deprotection process, and the Sandmeyer sequence could be transformed into CBr2I2. 1 could be obtained from previously synthesized 4,4′-dibromo-diphenic acid 2 and boronic ester 3via a two-fold esterification reaction.19 After successful synthesis of CBr2I2, the more reactive aryl-iodide over aryl-bromide with regard to palladium-catalyzed cross-coupling chemistry enables the temperature-controlled sequential decoration with coupling partners and thus also the controlled assembly of the members of the library.23
Scheme 1. Synthesis of CBr2I2. Conditions: (a) Boc2O; DIPEA; THF; 0 °C–r.t.; 20 h; 66%. (b) PdCl2(dppf); B2Pin2; KOAc; dioxane; 85 °C; 22 h; 98%. (c) 2; DCC; DMAP; CH2Cl2/DMF; r.t.; 16 h; 95%. (d) PdCl2(PPh3)2; KF; B(OH)3, Tol/MeOH/H2O; r.t.; 16 h; 65%. (e) (i) p-TsOH; ACN/Tol; r.t./4 h; (ii) NaNO2; KI; H2O; −15 °C to r.t.; 1 h; 62%.
Synthesis and characterization of CBr2I2
The preparation of CBr2I2, as depicted in Scheme 1, started with the Boc protection of (2-amino-5-bromophenyl)methanol 4 by a Boc anhydride in THF, followed by Miyaura borylation to yield 3 in good yields over two steps. Di-acid 2, prepared as described in our previous work, was reacted with 3via a two-fold Steglich esterification using N,N′-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (DMAP) to yield 1 in excellent yields.19 The use of an excess amount of DCC (2 vs. 2.2 equiv.) turned out to be crucial, as the yield increased from a moderate level of 75% to almost a quantitative level of 95%. 1 was converted to CBr2Boc2 by applying palladium-catalyzed oxidative homo-coupling conditions. A slight modification of the solvent (THF vs. the toluene/MeOH mixture) improved the yield from ∼50% to 65% for this key step. After the usual development on a small scale, these conditions were also applied on a multi-gram scale, accessing CBr2Boc2 in large quantities. In the final step, the Boc-protected amines were converted to iodine in a one-pot deprotection and Sandmeyer reaction using para-toluene sulfonic acid (p-TsOH) to obtain target CBr2I2. The one-pot strategy was chosen over the stepwise approach due to the instability of the free di-amine intermediate. A small optimization process showed that applying lower temperatures and the use of toluene as a co-solvent allowed the synthesis of the target in 62% isolated yield. The protocol allowed the gram-scale synthesis of CBr2I2 in 6 steps with an overall yield of 25% from commercial building blocks.
Structural analysis
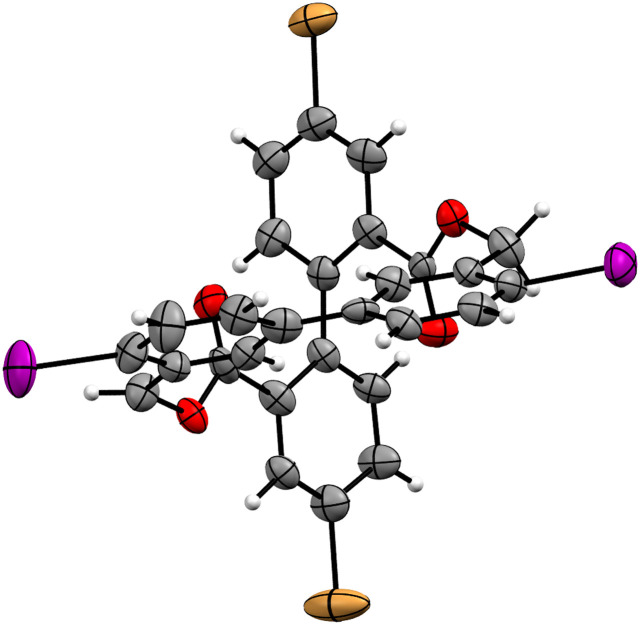
The identity of CBr2I2 was corroborated by its solid-state structure (ESI, Fig. S34†). Single crystals suitable for X-ray analysis were obtained by slow vapor diffusion of MeOH into a solution of CBr2I2 in toluene. The solid-state structure analysis revealed the racemic mixture of both enantiomers (M and P). Interestingly the crystals organized themselves in a layered fashion, containing solely one of the enantiomers per alternating layer. The crystal structure also showed an angle between 80° and 86° between the two biphenyl moieties, which is in line with the previously reported structures.19,20
A study on the optical properties of the racemic mixture (rac)-CBr2I2 and its respective enantiomers (M)-CBr2I2 and (P)-CBr2I2 was conducted. The enantiomers were separated using chiral stationary phase HPLC (heptane : ethyl acetate 1 : 1, Chiralpak IG column), and circular dichroism (CD) was measured. The good separation (ESI, Fig. S2†) of (rac)-CBr2I2 allowed for preparative isolation of tens of milligrams of the enantiomers. The CD spectra of the pure enantiomers indicated opposite signs of the Cotton bands (ESI, Fig. S3†) and allowed via DFT calculations the assignment of the first eluting enantiomer to be (M)-CBr2I2, while the second being (P)-CBr2I2 (ESI, Fig. S4†). This was further corroborated by growing enantiopure crystals of the second eluting enantiomer (Fig. 2). Slow diffusion of MeOH into a solution of E2-CBr2I2 rendered single crystals suitable for X-ray analysis, which were resolved as bearing only P enantiomers. Due to the fact that the obtained Flack parameter is nearly 0, the absolute configuration can be assigned with certainty and thus identifies the second eluting enantiomer as (P)-CBr2I2.
Fig. 2. Solid-state structure of enantiopure (P)-CBr2I2 plotted as an ORTEP plot with 50% probability, obtained as a second eluting enantiomer.
Selective chromophore decoration
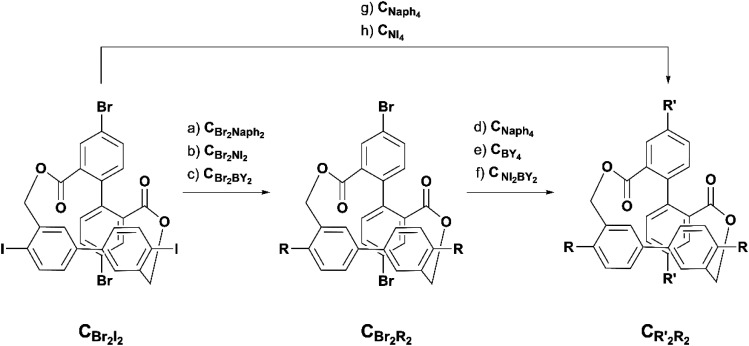
With CBr2I2 in hand, a library of cross-shaped tetrachromophores was synthesized, as depicted in Scheme 2. At first the bis- and tetra-functionalized methoxynaphthalenes (CBr2Naph2 and CNaph4), were synthesized via either a stepwise approach over CBr2Chromophore2 or a direct four-fold Suzuki reaction. CBr2I2 was reacted in the presence of 2 equivalents of commercially available 6-methoxynaphthalene boronic acid (Naph-B(OH)2), yielding CBr2Naph2 in good yields. CNaph4 could in its turn be obtained from CBr2Naph2 in the same catalytic system by simply adding an additional 2 equivalents of Naph-B(OH)2 and elevating the temperature to 85 °C. Alternatively, CBr2I2 was reacted at 85 °C with 4 equivalents of Naph-(BOH)2 to yield the four-fold substituted CNaph4 in one step from CBr2I2.
Scheme 2. Synthesis of CBr2Naph2, CNaph4, CBr2NI2, CNI4, CBr2BY2, CBY4, and CNI2BY2. Reagents and conditions: (a) Naph-BOH2; PdCl2(dppf); K2CO3; THF/H2O (4 : 1); 4.5 h; 55 °C; 80%; (b) NI-BPin; PdCl2(dppf); K2CO3; THF/H2O (4 : 1); 4.5 h; 55 °C; 61%; (c) BY-BPin; PdCl2(dppf); K2CO3; THF/H2O (4 : 1); 4.5 h; 55 °C; 90%; (d) CBr2Naph2; Naph-BOH2; PdCl2(dppf); K2CO3; dioxane/H2O (4 : 1); 24 h; 85 °C; 47%; (e) CBr2BY2; BY-BPin; dioxane/H2O (4 : 1); 24 h; 90 °C; 21%; (f) CBr2BY2; NI-BPin; dioxane/DMF/H2O (9 : 1 : 2.5); 16 h; 90 °C; 61%; (g) Naph-BOH2; PdCl2(dppf); K2CO3; dioxane/H2O (4 : 1); 20 h; 85 °C; 56%; (h) NI-BPin; PdCl2(dppf); K2CO3; Tol/EtOH/H2O (4 : 1 : 1); 18 h; 85 °C; 27%.
To access the naphthalimide (NI) and BODIPY (BY) functionalized model compounds, the corresponding literature known boronic ester coupling partners were synthesized (ESI, Page S6 and S7†).24,25NI-Br and BY-Br were synthesized via imide condensation and a one-pot condensation process, oxidation and a BF2 insertion reaction, respectively. The bromides were converted to boronic esters via a Miyaura borylation process, yielding NI-Bpin and BY-Bpin in good yields over two steps.
The corresponding bis- and tetra-functionalized chromophores (CBr2NI2, CNI4, CBr2BY2 and CBY4) as well as a hybrid system (CNI2BY2) were synthesized following the same pathway as the methoxynaphthalene chromophores (Scheme 2). Depending on the solubility of the involved reaction partners and target structures, the solvent mixtures were adapted, yielding all of the desired cross-shaped and chromophore-decorated targets after simple silica gel column chromatography in acceptable yields and good quality. The quick accessibility of the variety of cross-shaped tetrachromophores showcases the easy and selective peripheral decoration of CBr2I2via the common Suzuki cross-coupling protocols. Of particular interest was the subsequent optical investigation of the obtained chromophore-decorated model compounds.
Physicochemical analysis
Optical analysis
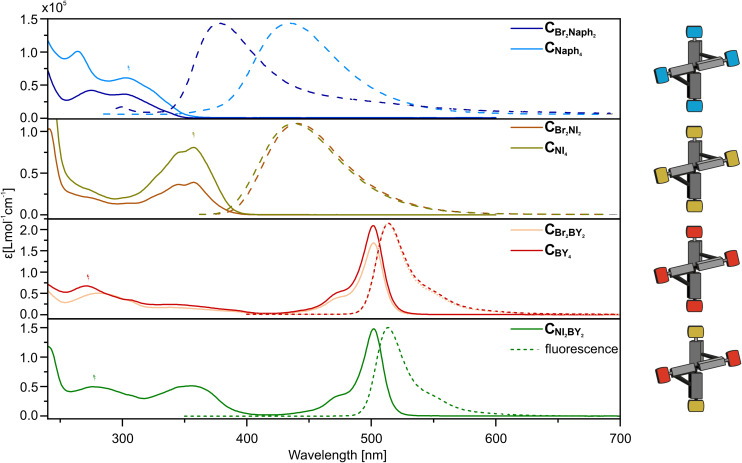
All cross-shaped tetrachromophores were characterized via absorption and fluorescence spectroscopy, as shown in Fig. 3 and Table S1.† Comparison of the absorption spectra between the two- and four-fold decorated crosses, respectively, showed in all cases the not-surprising increase of absorptivity in the spectral region associated with the absorption of the particular chromophore, namely methoxynaphthalene (300 nm), naphthalimide (360 nm) or BODIPY (500 nm). Upon comparison of the fluorescence spectra, it was seen that only the set of crosses with the Naph chromophores exhibited a large bathochromic shift, from 378 nm for CBr2Naph2 to 432 nm for CNaph4. This shift can be rationalized by the conjugation of the electron-donating methoxynaphthalene with the electron-withdrawing ester of the core when substituted on the bromine position. While substitution on the iodine position does not provide any conjugation with a withdrawing group, yielding comparable fluorescence maxima with earlier reported structures having the same methoxynaphthalene motifs.26,27 The almost non-existing fluorescence shift in the NI and BY decorated crosses can be explained by the isolation of the chromophores due to their more demanding steric nature twisting their π-system out of plane with respect to the bi-phenyl rod and the absence of significant exciton coupling. Telfer et al. correlated the magnitude of the coupling between two perpendicular dipoles and reported that this coupling almost disappears when approaching a 90° angle, as observed in the here-reported (almost 90°) cross-shaped model compounds.28
Fig. 3. Absorption spectra (solid line) and fluorescence spectra (dashed line) of CBr2Naph2 and CNaph4 (blue), CBr2NI2 and CNI4 (yellow), CBr2BY2 and CBY4 (red), and CNI2BY2 (green) in CH2Cl2. All spectra were recorded at 20 °C; c ∼10−6 mol L−1 for absorption and c ∼10−7 mol L−1 for emission.
By comparing the spectra of CNI2BY2 with the NI and BY series, the absorbance features of both chromophores at 360 and 500 nm, respectively, can clearly be identified. The fluorescent spectrum of CNI2BY2, however, has solely a BY characteristic with the exact same emission maxima (λem,max = 513 nm) as the other BY decorated model compounds. Even excitation of CNI2BY2 at 360 nm, which is the main NI absorption band, results in the same BY fluorescence spectra (ESI, Fig. S11†). As the emission of NI overlaps with the absorption region of BY, this is the expected optical feature of an intramolecular FRET, further corroborating the close proximity of both chromophores in the structure.
Structural analysis
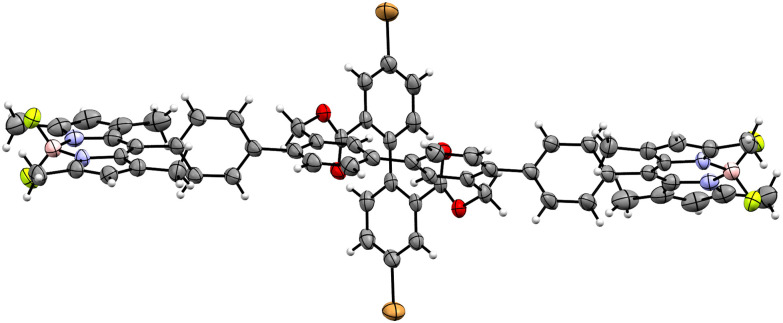
With the library of chromophore-decorated crosses in hand, their structural conformation was analyzed. To our delight, we were able to grow single crystals suitable for the X-ray analysis of CBr2BY2 (Fig. 4). Slow evaporation of heptane into a solution of CBr2BY2 in a toluene–CH2Cl2 mixture rendered bright red crystals. The X-ray structure analysis revealed an I2/a space group, where the unit cell consisted of a mixture of one M enantiomer and one P enantiomer. One can see that the BODIPY motif is twisted with respect to its phenyl spacer, resulting from the steric repulsion of the methyl groups. Furthermore, the center part of the molecule exhibits the earlier seen almost 90° cross shape between the two rods of the molecule.
Fig. 4. Solid-state structure of (M)-CBr2BY2 in the racemic crystal of CBr2BY2, plotted as ORTEP plots with 50% probability. Plotted as a single enantiomer and without solvent molecules for clarity.
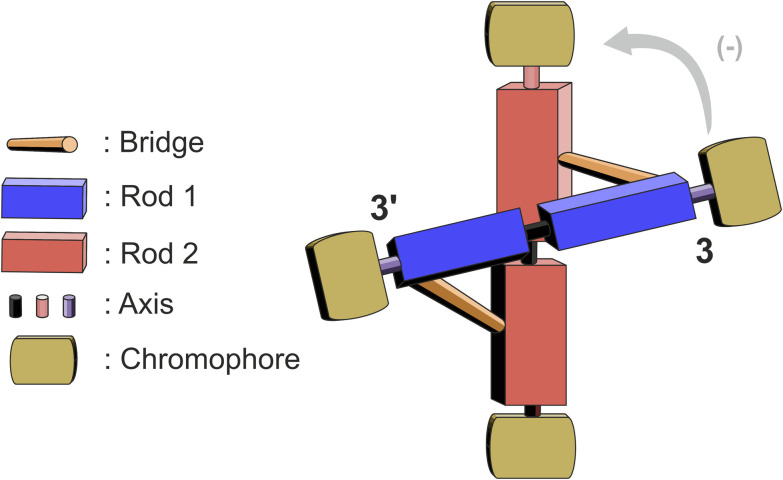
As we continued our structural investigation, we quickly encountered the complexity of these systems in terms of nomenclature and supposed chirality assignment. The difference in the substitution pattern on rod 1 (ortho) and rod 2 (meta) induces a size mismatch similar to banister type molecules, hence we describe the arising helicity with P and M.‡29 In Fig. 5, a schematic representation of the structure is displayed, with the corresponding nomenclature of the cross-shaped framework, acting as a guide for the following part.
Fig. 5. Schematic representation of the (P)-cross-shaped framework decorated with four chromophores. Rod 1 resembles the alcohol side of the molecule, while Rod 2 the ester side.
Analysis by 1H NMR spectroscopy revealed that the Naph- and BY-decorated crosses showed the characteristic peak splitting of the diastereotopic benzylic ester protons into two doublets (ESI, Page S75–S116†). However, in the case of the NI series (CBr2NI2 and CNI4), according to our interpretation, a set of eight doublets was found (ESI, Fig. S16†). In contrast, when NI was introduced on rod 2 (CNI2BY2), this was not observed.
We attributed this additional splitting to the presence of two additional conformational orientations of the bulkier NI in comparison with the Naph and phenyl spacers of BY. The steric repulsion of the CH2 bridge at the peripheral 3 and 3′ positions of rod 1 in combination with the rigid center motif forces the NI moiety to an additional set of conformers. Due to the hindered rotation of NI, 2 additional atropisomeric centers were created: Ra3/Sa3 and Ra3′/Sa3′.
Using VT-NMR, we investigated the rotational barrier of CBr2NI2 (Fig. S17†). To our surprise, upon heating to 120 °C in C2D2Cl4, we saw minor shifts in the proton signals, but the diastereotopic protons remained visible as 8 doublets. This points at a surprisingly high rotation barrier for the conformer interconversion. We hypothesize that this might arise from the structural entanglement of the center motif. As the NI motif clashes with the ester bridge on both sides simultaneously, the center motif probably cannot adopt the conformation required to let the NI pass.
To further shed light on the potential different conformers, DFT calculations were performed. The geometry optimized structures revealed that there were 4 potential conformers per enantiomer (ESI, Page S20 and Fig. S43–S45†), namely Ra3,R3′; Sa3,Sa3′; Ra3,Sa3′ and Sa3,Ra3′. In these calculated structures, the Ra3,Sa3′ and Sa3,Ra3′ conformers are identical, giving a total of 3 diastereoisomers; thus, 3 pairs of doublets would be expected. Instead, 4 pairs of doublets were observed (ESI, Fig. S16†), suggesting that, for the Ra3,Sa3′ (=Sa3,Ra3′) conformer, there is a symmetry loss. Thus, assuming that the chemical shielding for Ra3 and Ra3′ as well as for Sa3 and Sa3′ are identical or very close would lead to additional signal splitting, hence the additional set of doublets.
To gain further comprehension about the elaborate architecture, their chiral properties were studied by comparing the recorded spectra with the simulated ones. First, the enantiomers of all chromophore-decorated cross-shaped model compounds were separated using analytical scale chiral-stationary phase HPLC (ESI, Page S18, S22, S25 and S27†). In the case of CBr2NI2 and CNI4, the focus was on the separation of the (M) and (P) enantiomers. However, the HPLC traces (ESI, Fig. S18†) revealed that the enantiomers were indeed composed of several conformers. With in-line CD detection, the conformation of the center motif was assigned, assuring that the enantiomer sets were successfully separated (ESI, Page S22†). To corroborate this claim experimentally, enantiopure syntheses of (P)-CBr2NI2 and (P)-CNI4 were performed with (P)-CBr2I2 as the starting material. The samples obtained by the enantiopure assembly were identical to the separated ones from racemic synthesis, assuring the enantiopurity of the separated samples.
With all separated enantiomers in hand, their respective CD spectra were recorded (ESI, Page S18, S23, S25 and S27†). As expected, moderate Cotton bands of opposite sign were observed for all here-reported separated pairs of enantiomers. Simulation of all P isomers and comparison to the experimental spectra allowed the assignment of the helicity of all the resolved samples. With the here-applied separation conditions, the P isomers elute first during the resolution of CBr2Naph2, CBr2NI2, and CNaph4; they elute second in the case of CBr2I2, CBr2BY2, CNI4, CBY4 and CNI2BY2.
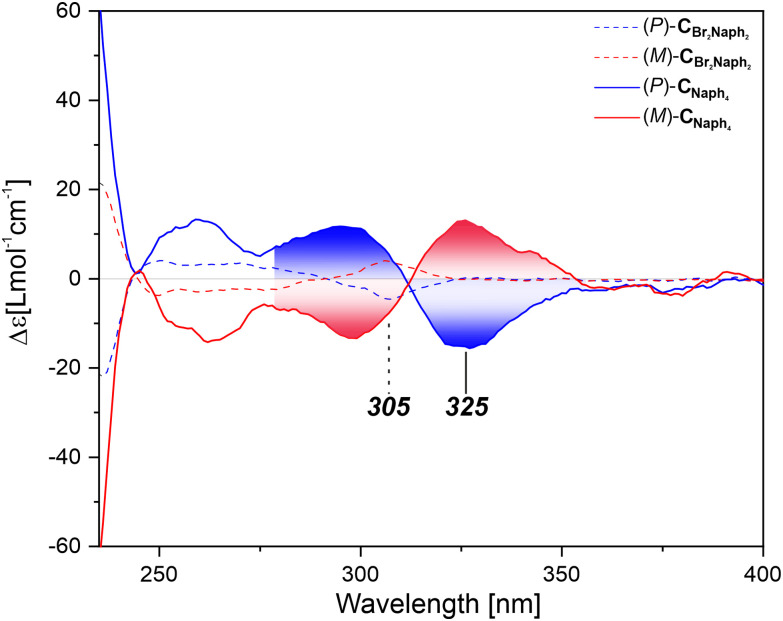
As an example, the CD spectra of the enantiomers of the Naph series are displayed in Fig. 6. Upon comparison of the CD spectra recorded for the two- and four-fold substituted chromophores, CBr2Naph2 and CNaph4, respectively, a significant red shift in the spectra was observed. While the first Cotton band of CBr2Naph2 appeared at 305 nm, it was at 325 nm in the case of CNaph4. This shift can be assigned to the communication between the methoxynaphthalene subunits on rod 1 and rod 2, respectively, as it is absent when only rod 1 is substituted. The observed bisignate signal for the CNaph4 enantiomers indicates an exciton coupled feature.18 Comparison of the CD signal to the earlier reported methoxynaphthalene architecture of Sidler et al. showed that these specific Cotton bands appear at exactly the same wavelengths, strengthening the argument for an exciton coupled signal.16 Inspired by this observation, we applied the exciton chirality method (ECM) to the four-fold decorated crosses: CNaph4, CNI4 and CBY4.
Fig. 6. ECD spectra of the (blue) (P) and (red) (M) enantiomers of CBr2Naph2 (dashed line) and CNaph4 (solid line). Bisignate signals of CNaph4 are displayed by the integrated area under the respective plots.
The orientation of rod 1 to rod 2 in the P isomers should lead to a so-called negative couplet, as indicated by the grey arrow in Fig. 5. This negative couplet was indeed observed in (P)-CNaph4, (P)-CNI4, and (P)-CBY4 (ESI, Fig. S31†), while in the case of the M enantiomers, the bisignate signal was first positive and then negative, hence, a positive couplet.
While CBr2Naph2 and CBr2NI2 lack this feature, CBr2BY2 showed a weaker but clear bisignate signal at around 500 nm. A positive couplet was observed for the P enantiomer, while the M enantiomer displayed a negative one. Obviously, in the case of CBr2BY2, the chiral information is communicated over rod 1 rather than between the terminal chromophores of both rods of the scaffold. Our current working hypothesis is that this is only detected for the BY chromophore because of the orientation of the excited dipole moment with a substantial contribution perpendicular to the rod's main axis, which is not the case for the other two chromophores. As the bi-phenyl axis of rod 1 is entangled with the helicity of the central part of the cross (adopting an Ra configuration‡), a positive couplet is expected for the P enantiomer (ESI, Fig. S32†).
According to the here-presented results, the ECM method is suited to assign the respective enantiomers for their four-fold substituted chromophores; however, the careful consideration of all chiral centers turned out to be crucial. The created library allowed us to dive into initially unexpected features, which via full-geometrical examination could be assigned to a variety of features the framework exhibits.
FRET system
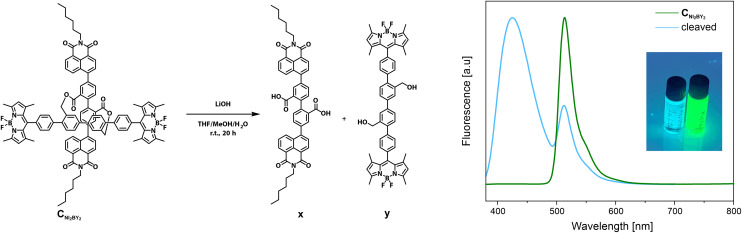
To explore the FRET activity of CNI2BY2, the template was cleaved in order to liberate the NI and BY decorated rods, respectively, as displayed in Fig. 7. Exposure of the chromophore to an aq. LiOH solution in THF/MeOH yielded overnight a change in the fluorescence signal visible by the bare eye from bright green to light faded blue. After acidic work-up, the cleaved pair of rods was dissolved in CH2Cl2, and fluorescence spectroscopy resolved two distinct emission peaks at 426 nm and 513 nm, respectively. As a control experiment, a reference sample of CNI2BY2 was stirred with NaCl instead of LiOH. As displayed in Fig. 7, the reference sample displayed exclusively emission at 513 nm, and the successful cleavage could easily be accessed by comparing the reaction mixtures under a UV lamp (inset in Fig. 7). Furthermore, to verify that the reaction conditions do not cause changes in the terminal chromophores, CNI4 and CBY4 were cleaved under identical conditions (ESI, Fig. S33†). Comparison of the cleaved reaction mixtures revealed that there were no changes in the fluorescence behavior of CNI4 and CBY4 upon cleavage, with a remaining deep blue-purple fluorescence for CNI4 and a bright green fluorescence for CBY4. The analysis of the fluorescence spectra showed for both cases a single emission peak, which are in agreement with the fluorescence spectra of the non-cleaved precursors (CNI4 and CBY4). The cleavage behaviour analysed by fluorescence spectroscopy corroborates the establishment of an intramolecular FRET pair in the cross-shaped architecture and demonstrates the potential of this template analyzing chromophores in a close confined space.
Fig. 7. Cleavage of the ester template of CNI2BY2 by exposure to LiOH, resulting in the fluorescence change. (left) Reaction conditions of the transformation of CNI2BY2 to yield NI-x and BY-y. (right) Fluorescence spectrum, in CH2Cl2 at 20 °C, of CNI2BY2 (green) and the crude product after cleavage (blue); λex (360 nm); (image) reaction mixture after the reaction (left) and reference with NaCl instead of LiOH (right) under a 366 nm UV lamp.
Conclusions
In conclusion, an organic cross-shaped framework is designed that acts as a template superstructure for the synthesis of (chiral) multi-chromophore architectures. A universal macrocyclic building block is synthesized on a gram scale that due to steric restrictions adopts a cross shape. The framework bears four attachment points that are used to selectively mount methoxynaphthalene, naphthalimide and BODIPY derivatives. The reactivity difference of aryl-iodine and aryl-bromine ensured the synthesis of a library of bi- and tetra-functionalized chromophores, including a hybrid intra-molecular FRET pair. NMR spectroscopy and (chiro)-optical analysis were conducted for all compounds and revealed in support of DFT calculations the geometrical configuration of the reported compounds. This was further supported by enantiopure solid-state structure analysis (CBr2I2) and the exciton chirality method (CR4). Lastly, removal of the template results in breaking the FRET pair of the hybrid chromophore (CNI2BY2), visualized by a change in its fluorescence properties.
The study shows that the organic framework acts as an excellent and versatile platform for studying a variety of chromophores in a confined space, allowing for the investigation of novel properties such as chirality and FRET. In addition, the presented halide-decorated precursor enables its integration as a functional unit in a variety of applications by C–C coupling chemistry.
Author contributions
C. C. E. K. conceptualized the project, performed the synthesis, characterized the compounds and wrote the manuscript; A. D'A. performed the DFT calculations and wrote the manuscript; A. P. analyzed the solid-state structures; D. H. performed the VT-NMR experiments; M. M. supervised the work and wrote the manuscript. All authors commented on the manuscript.
Data availability
The data supporting this article have been included as part of the ESI.† The crystallographic data for this paper can be found under the deposition number 2378892 for (rac)-CBr2I2, 2378893 for (P)-CBr2I2 and 2378894 for (rac)-CBr2BY2.† These data are provided by the joint Cambridge Crystallographic Data Centre and Fachinformations-zentrum Karlsruhe Access Structures service.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank Tim Henri Eggenweiler for his assistance in measuring quantum yield and fluorescence lifetime. We thank Aristide Coynel for his help during part of the synthesis. Generous support from the Swiss National Science Foundation (SNF) is acknowledged (200020_207744). M. M. acknowledges support from the 111 project (90002-18011002).
Electronic supplementary information (ESI) available. CCDC 2378892–2378894. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo01808g
Footnotes
The chirality could also be described by axial or planar chirality. In general, the P isomer of the crosses corresponds to an Ra,Ra or SP configuration.
References
- Benkhaya S. M'rabet S. El Harfi A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020;115:107891. doi: 10.1016/j.heliyon.2020.e03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitti A. Pasini D. Aggregation-Induced Circularly Polarized Luminescence: Chiral Organic Materials for Emerging Optical Technologies. Adv. Mater. 2020;32:1908021. doi: 10.1002/adma.201908021. [DOI] [PubMed] [Google Scholar]
- Luo Q. Li L. Ma H. Lv C. Jiang X. Gu X. An Z. Zou B. Zhang C. Zhang Y. Deep-Red fluorescence from isolated dimers: a highly bright excimer and imaging in vivo. Chem. Sci. 2020;11:6020–6025. doi: 10.1039/d0sc01873b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan V. Ganesan S. Rajakumar P. Synthesis and DSSC application of BODIPY decorated triazole bridged and benzene nucleus cored conjugated dendrimers. RSC Adv. 2020;10:18390–18399. doi: 10.1039/d0ra01672a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinchu I. Sreekala C. O. Sreelatha K. S. Dye Sensitized Solar Cell Using Natural Dyes as Chromophores. Mater. Sci. Forum. 2014;771:39–51. [Google Scholar]
- Schembri T. Kolb L. Stolte M. Würthner F. Polarized, color-selective and semi-transparant organic photodiode of aligned merocyanine H-aggregates. J. Mater. Chem. C. 2024;12:4948–4953. [Google Scholar]
- Gryszel M. Schlossarek T. Würthner F. Natali M. Głowacki E. D. Water-Soluble Cationic Perylene Diimide Dyes as Stable Photocatalysts for H2O2 Evolution. ChemPhotoChem. 2023;7:e202300070. [Google Scholar]
- Wang S. Xie Z. Zhu D. Fu S. Wu Y. Yu H. Lu C. Zhou P. Bonn M. Wang H. I. Liao Q. Xu H. Chen X. Gu C. Efficient photocatalytic production of hydrogen peroxide using dispersible and photoactive porous polymers. Nat. Commun. 2023;14:6891. doi: 10.1038/s41467-023-42720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Zhuang Z. Lou X. Zhao Z. Tang B. Z. Molecular Design and Biomedical Application of AiEgens with Photochemical Activity. Chem. Biomed. Imaging. 2023;1:785–795. doi: 10.1021/cbmi.3c00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha D. Jenei A. Nagy P. Vereb G. Szöllősi J. Understanding FRET as a Research Tool for Cellular Studies. Int. J. Mol. Sci. 2015;16:6718–6756. doi: 10.3390/ijms16046718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters B. R. Paths to Förster's resonance energy transfer (FRET) theory. Eur. Phys. J. H. 2014;39:87–139. [Google Scholar]
- Josse P. Favereau L. Shen C. Dabos-Seignon S. Blanchard P. Cabanetos C. Crassous J. Enantiopure versus Racemic Naphtaimide End-Capped Helicenic Non-Fullerene Electron Acceptors: Impact on Organic Phorovoltaics Performance. Chem. – Eur. J. 2017;23:6277–6281. doi: 10.1002/chem.201701066. [DOI] [PubMed] [Google Scholar]
- Dhbaibi K. Matozzo P. Abella L. Jean M. Vanthuyne N. Autschbach J. Favereau L. Crassous J. Exciton coupling chirality in helicene-porphyrin conjugates. Chem. Commun. 2021;57:10743–10746. doi: 10.1039/d1cc03314j. [DOI] [PubMed] [Google Scholar]
- Dhbaibi K. Favereau L. Srebro-Hooper M. Jean M. Vanthuyne N. Zinna F. Jamoussi B. Bari L. D. Autschbach J. Crassous J. Exciton coupling in diketopyrrolopyrorole-helicene derivatives leads to red an near-infrared circularly polarized luminescence. Chem. Sci. 2018;9:735–742. doi: 10.1039/c7sc04312k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhals H. Hofer A. Bernhard S. Siegel J. S. Mayer P. Axially Chiral Bichromophoric Fluorescent Dyes. J. Org. Chem. 2011;76:990–992. doi: 10.1021/jo102254a. [DOI] [PubMed] [Google Scholar]
- Sidler E. Malinčík J. Prescimone A. Mayor M. Induced axial chirality by a tight belt: naphtalene chromophores fixed in a 2,5-substituted cofacial para-phenylene-ethynylene framework. J. Mater. Chem. C. 2021;9:16199–16207. doi: 10.1039/d1tc02180j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N. Nakanishi K. Exciton chirality method and its application to configurational and conformational studies of natural products. Acc. Chem. Res. 1972;5:257–263. [Google Scholar]
- Hestand N. J. Spano F. C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018;118:7069–7163. doi: 10.1021/acs.chemrev.7b00581. [DOI] [PubMed] [Google Scholar]
- Kroonen C. C. E. D'Addio A. Prescimone A. Fuhr O. Fenske D. Mayor M. A Cross-Shaped Monomer as Building Block for Molecular Textiles. Helv. Chim. Acta. 2023;106:e202200204. [Google Scholar]
- Kroonen C. C. E. Hinaut A. D'Addio A. Prescimone A. Häussinger D. Navarro-Marín G. Fuhr O. Fenske D. Meyer E. Mayor M. Towards Molecular Textiles: Synthesis and Characterization of Molecular Patches. Chem. – Eur. J. 2024:e202402866. doi: 10.1002/chem.202402866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. Xu Y. Kim J. Lee J. Kim J. S. A rational design of AIE-active fluorophore for the fingerprint optical detection. Bull. Korean Chem. Soc. 2023;44:516–522. [Google Scholar]
- Bozzi Í. A. O. Machado L. A. Diogo E. B. T. Delolo F. G. Barros L. O. F. Graça G. A. P. Araujo M. H. Martins F. T. Pedrosa L. F. da Luz L. C. Moraes E. S. Rodembusch F. S. Guimarães J. S. F. Oliveira A. G. Röttger S. H. Werz D. B. Souza C. P. Fantuzzi F. Han J. Marder T. B. Braunschweig H. da Silva Júnior E. N. Electrochemical Diselenation of BODIPY Fluorophores for Bioimaging Applications and Sensitization of 1O2. Chem. – Eur. J. 2024;30:e202303883. doi: 10.1002/chem.202303883. [DOI] [PubMed] [Google Scholar]
- Bannwart L. M. Müntener T. Rickhaus M. Jundt L. Häussinger D. Mayor M. Bicyclic Phenyl-Ethynyl Architectures: Synthesis of a 1,4-Bis(phenylbuta-1,3-diyn-1-yl) Benzene Banister. Chem. – Eur. J. 2021;27:6295–6307. doi: 10.1002/chem.202005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M. Han J.-M. Zhang Y. Yang X. Zang L. A selective fluorescence turn-on sensor for trace vapor detection of hydrogen peroxide. Chem. Commun. 2013;49:11779–11781. doi: 10.1039/c3cc47631f. [DOI] [PubMed] [Google Scholar]
- Zhai J. Pan T. Zhu J. Xu Y. Chen J. Xie Y. Qin Y. Boronic Acid Functionalized Boron Dipyrromethene Fluorescent Probes: Preparation, Characterization, and Sacharides Sensing Applications. Anal. Chem. 2012;84:10214–10220. doi: 10.1021/ac301456s. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Muñiz G. M. Gomez-Mendoza M. Nuin E. Andreu I. Marin M. L. Miranda M. A. “Snorkelling” vs. “diving” in mixed micelles probed by means of a molecular bathymeter. Org. Biomol. Chem. 2017;15:10281–10288. doi: 10.1039/c7ob02595e. [DOI] [PubMed] [Google Scholar]
- Martínez L. J. Scaiano J. C. Characterization of the Transient Intermediates Generated from the Photoexcitation of Nabumetone: A Comparison with Naproxen. Photochem. Photobiol. 1998;68:646–651. [PubMed] [Google Scholar]
- Telfer S. G. McLean T. M. Waterland M. R. Excition coupling in coordination compounds. Dalton Trans. 2011;40:3097–3108. doi: 10.1039/c0dt01226b. [DOI] [PubMed] [Google Scholar]
- D'Addio A. Malinčik J. Fuhr O. Fenske D. Häussinger D. Mayor M. Geländer Molecules with Orthogonal Joints: Synthesis of Macrocyclic Dimers. Chem. – Eur. J. 2022;28:e202201678. doi: 10.1002/chem.202201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.† The crystallographic data for this paper can be found under the deposition number 2378892 for (rac)-CBr2I2, 2378893 for (P)-CBr2I2 and 2378894 for (rac)-CBr2BY2.† These data are provided by the joint Cambridge Crystallographic Data Centre and Fachinformations-zentrum Karlsruhe Access Structures service.