Abstract
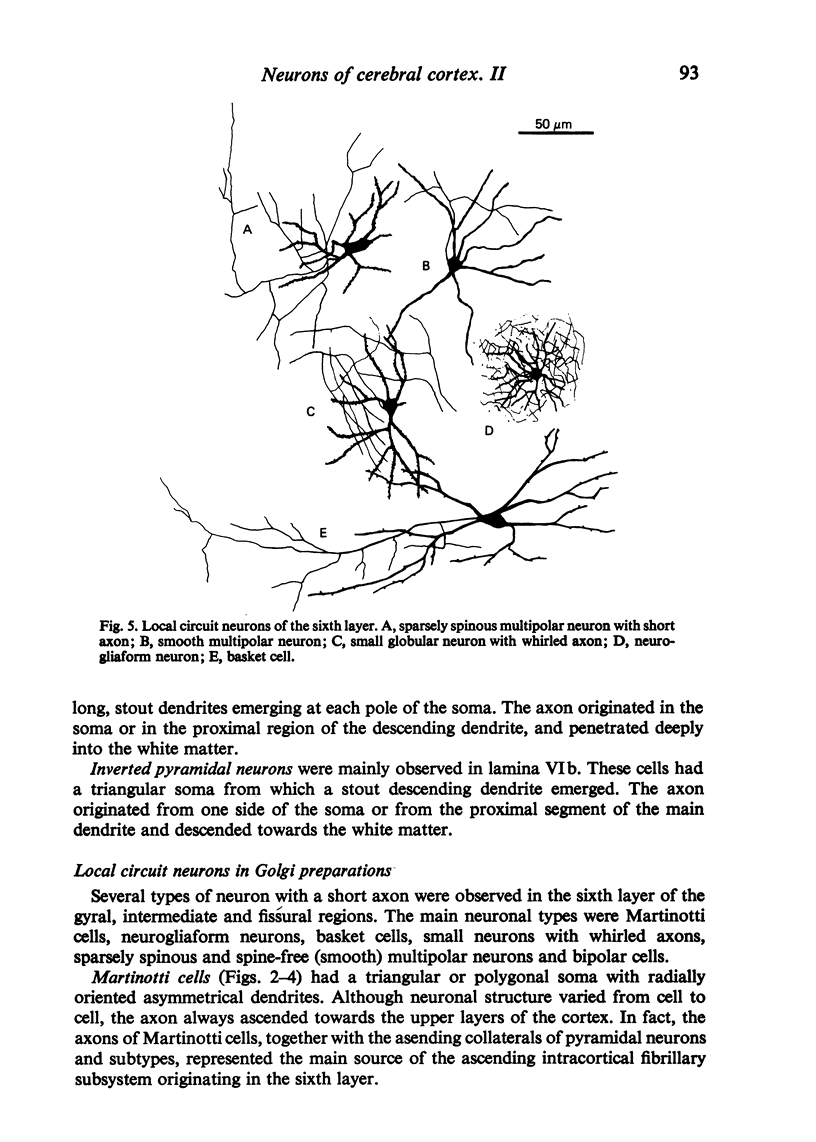
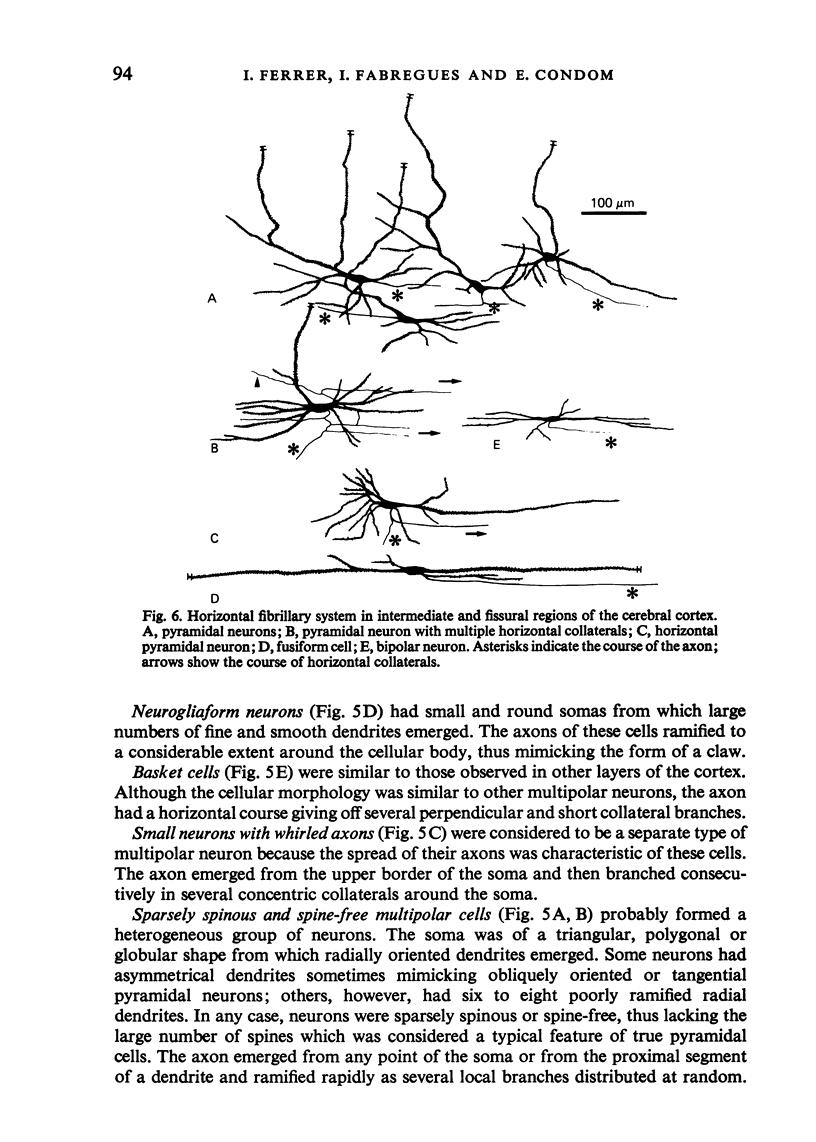
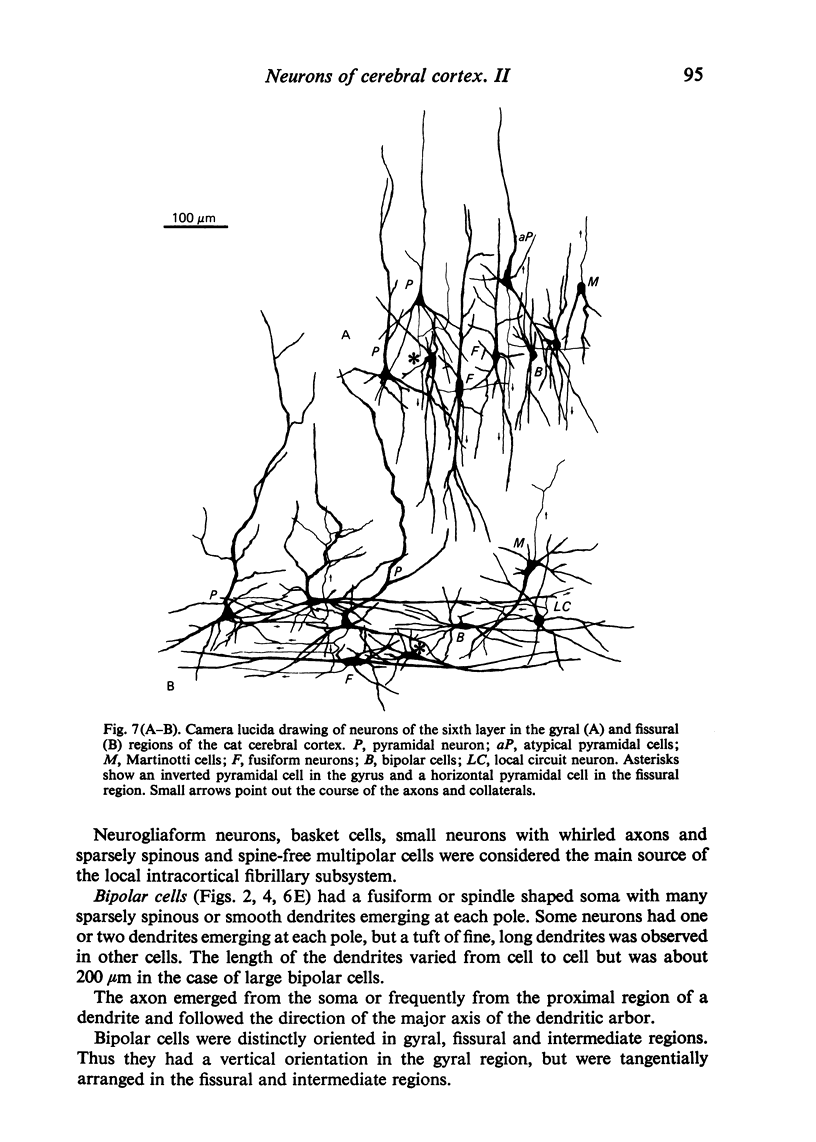
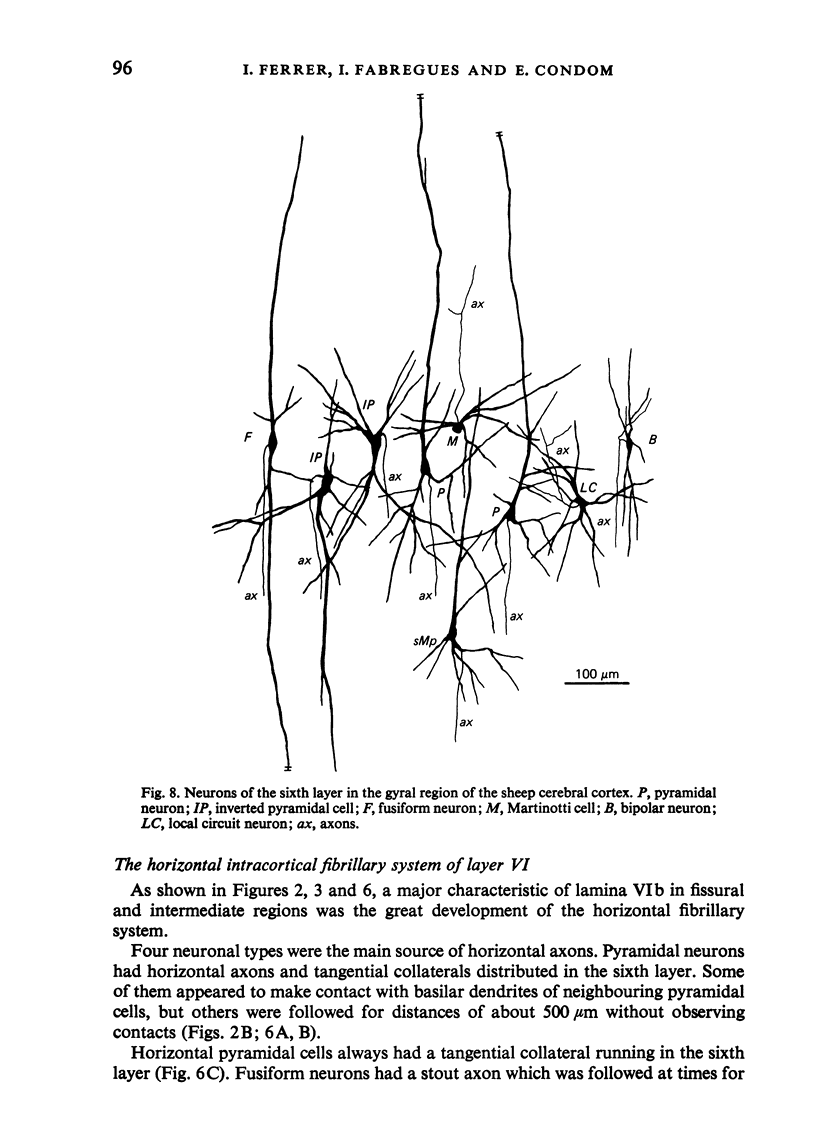
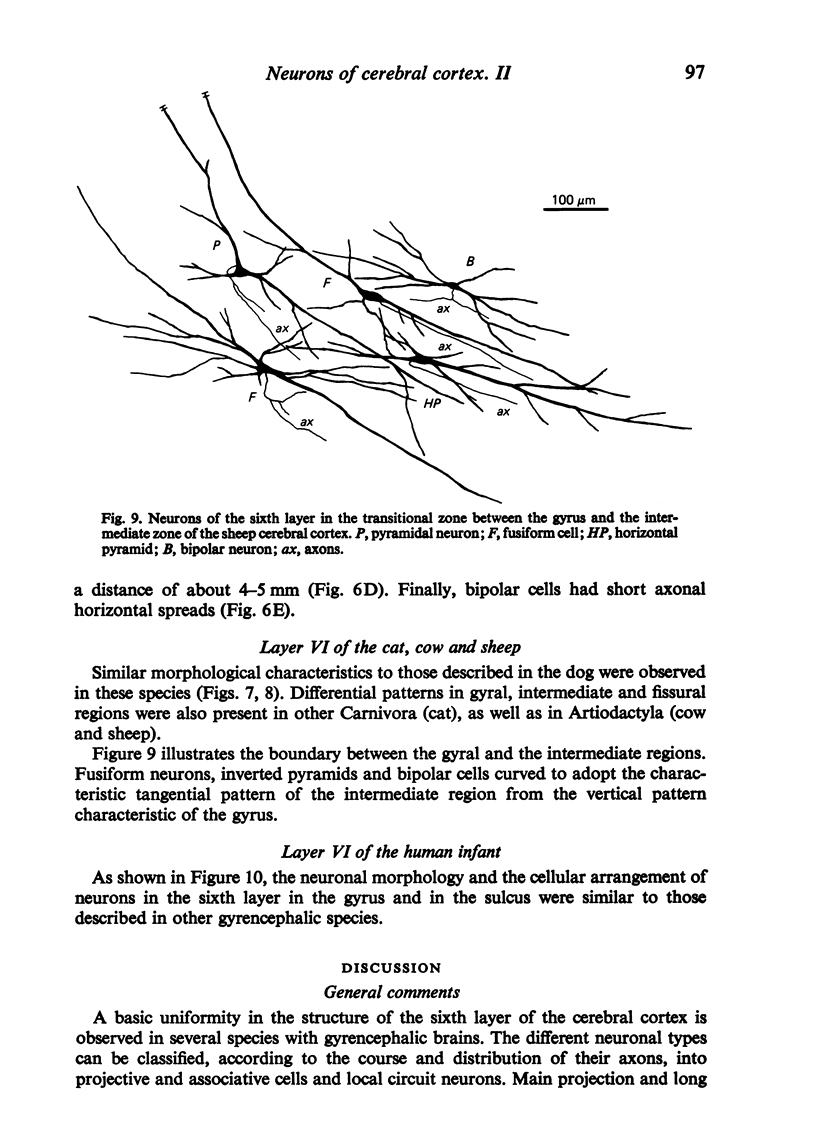
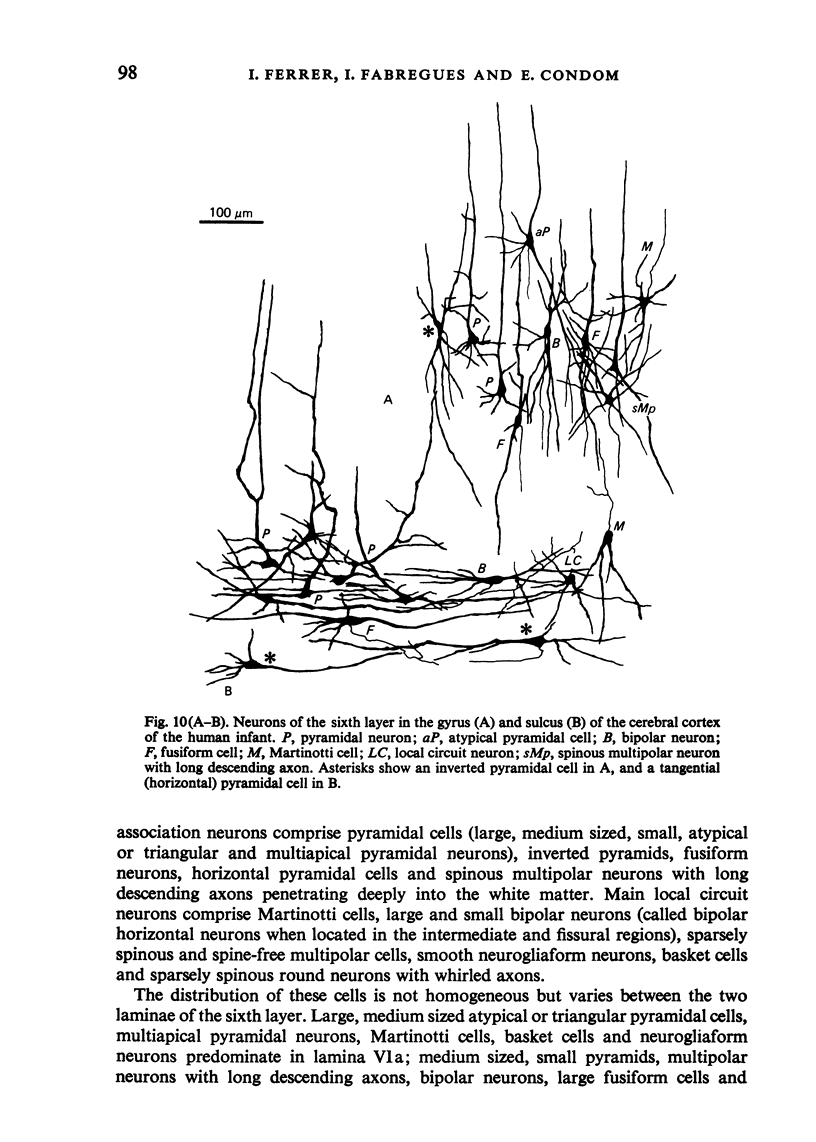
The sixth layer of the cerebral cortex has been studied by means of the Golgi method in Carnivora (dog and cat), Artiodactyla (cow and sheep), and Primates (human) brains; a basic structural uniformity being observed in all these species. Projection neurons of lamina VIa were large and medium sized pyramidal neurons (including atypical and multiapical), small pyramidal cells, and spinous multipolar neurons with long descending axons. Projection neurons of lamina VIb were medium sized pyramidal neurons and small pyramids, horizontal pyramids, inverted pyramidal cells, spinous multipolar neurons with long descending axons and large fusiform cells. Local circuit neurons of lamina VIa were Martinotti cells, basket neurons, neurogliaform cells, sparsely spinous neurons with whirled axons, spine-free multipolar cells and bipolar neurons. Local circuit neurons of lamina VIb were sparsely spinous and spine-free multipolar cells with short axons and bipolar neurons. Marked differences were observed between gyral, intermediate and fissural regions. Fusiform and bipolar neurons were vertically arranged in the former, but were tangentially orientated in intermediate and fissural regions; inverted pyramidal cells were present in the gyrus but horizontal pyramids were the respective cells in the intermediate and fissural zones. When compared with lissencephalic species, a great horizontal fibrillary system (which is vertically arranged in gyral regions) was observed in convoluted brains. Cells of origin were fusiform neurons, bipolar cells, horizontal and inverted pyramids and pyramidal neurons (the latter by means of horizontal axonal collaterals). The great development of this cortico-cortical association system in gyrencephalic species is considered to be a major step in neocortical evolution.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ferrer I., Fabregues I., Condom E. A Golgi study of the sixth layer of the cerebral cortex. I. The lissencephalic brain of Rodentia, Lagomorpha, Insectivora and Chiroptera. J Anat. 1986 Apr;145:217–234. [PMC free article] [PubMed] [Google Scholar]
- Friedman D. P. Laminar patterns of termination of cortico-cortical afferents in the somatosensory system. Brain Res. 1983 Aug 22;273(1):147–151. doi: 10.1016/0006-8993(83)91103-4. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Hornung J. P., Garey L. J. The thalamic projection to cat visual cortex: ultrastructure of neurons identified by Golgi impregnation or retrograde horseradish peroxidase transport. Neuroscience. 1981;6(6):1053–1068. doi: 10.1016/0306-4522(81)90070-1. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 1976 Jul 15;168(2):197–247. doi: 10.1002/cne.901680203. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Coulter J. D., Burton H., Porter R. Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol. 1977 May 1;173(1):53–80. doi: 10.1002/cne.901730105. [DOI] [PubMed] [Google Scholar]
- Jones E. G. Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J Comp Neurol. 1975 Mar 15;160(2):205–267. doi: 10.1002/cne.901600204. [DOI] [PubMed] [Google Scholar]
- Jones E. G., Wise S. P. Size, laminar and columnar distribution of efferent cells in the sensory-motor cortex of monkeys. J Comp Neurol. 1977 Oct 15;175(4):391–438. doi: 10.1002/cne.901750403. [DOI] [PubMed] [Google Scholar]
- Jouandet M. L., Garey L. J., Lipp H. P. Distribution of the cells of origin of the corpus callosum and anterior commissure in the marmoset monkey. Anat Embryol (Berl) 1984;169(1):45–59. doi: 10.1007/BF00300586. [DOI] [PubMed] [Google Scholar]
- Keller G., Innocenti G. M. Callosal connections of suprasylvian visual areas in the cat. Neuroscience. 1981;6(4):703–712. doi: 10.1016/0306-4522(81)90154-8. [DOI] [PubMed] [Google Scholar]
- Kosmal A., Stepniewska I., Markow G. Laminar organization of efferent connections of the prefrontal cortex in the dog. Acta Neurobiol Exp (Wars) 1983;43(2):115–127. [PubMed] [Google Scholar]
- Lin C. S., Weller R. E., Kaas J. H. Cortical connections of striate cortex in the owl monkey. J Comp Neurol. 1982 Oct 20;211(2):165–176. doi: 10.1002/cne.902110206. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Lund R. D., Hendrickson A. E., Bunt A. H., Fuchs A. F. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J Comp Neurol. 1975 Dec 1;164(3):287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Miodoński A. The angioarchitectonics and cytoarchitectonics (impregnation modo Golgi-Cox) structure of the fissural frontal neocortex in dog. Folia Biol (Krakow) 1974;22(3):237–279. [PubMed] [Google Scholar]
- Peters A., Regidor J. A reassessment of the forms of nonpyramidal neurons in area 17 of cat visual cortex. J Comp Neurol. 1981 Dec 20;203(4):685–716. doi: 10.1002/cne.902030408. [DOI] [PubMed] [Google Scholar]
- Rockland K. S., Pandya D. N. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979 Dec 21;179(1):3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Rosenquist A. C., Edwards S. B., Palmer L. A. An autoradiographic study of the projections of the dorsal lateral geniculate nucleus and the posterior nucleus in the cat. Brain Res. 1974 Nov 8;80(1):71–93. doi: 10.1016/0006-8993(74)90724-0. [DOI] [PubMed] [Google Scholar]
- Seltzer B., Pandya D. N. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978 Jun 23;149(1):1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Spatz W. B. Topographically organized reciprocal connections between areas 17 and MT (visual area of superior temporal sulcus) in the marmoset Callithrix jacchus. Exp Brain Res. 1977 Apr 21;27(5):559–572. doi: 10.1007/BF00239044. [DOI] [PubMed] [Google Scholar]
- Symonds L. L., Rosenquist A. C. Laminar origins of visual corticocortical connections in the cat. J Comp Neurol. 1984 Oct 10;229(1):39–47. doi: 10.1002/cne.902290104. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. The 'module-concept' in cerebral cortex architecture. Brain Res. 1975 Sep 23;95(2-3):475–496. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- Tigges J., Spatz W. B., Tigges M. Efferent cortico-cortical fiber connections of area 18 in the squirrel monkey (Saimiri). J Comp Neurol. 1974 Nov 15;158(2):219–235. doi: 10.1002/cne.901580208. [DOI] [PubMed] [Google Scholar]
- Valverde F. The organization of area 18 in the monkey. A Golgi study. Anat Embryol (Berl) 1978 Sep 27;154(3):305–334. doi: 10.1007/BF00345659. [DOI] [PubMed] [Google Scholar]
- Wall J. T., Symonds L. L., Kaas J. H. Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in galagos. J Comp Neurol. 1982 Oct 20;211(2):193–214. doi: 10.1002/cne.902110208. [DOI] [PubMed] [Google Scholar]




