Abstract
Mental stress may lead to ovarian dysfunction. Psychological stress disrupts ovarian function, leading to adverse in vitro fertilization outcomes, premature ovarian insufficiency and decreased ovarian reserve. Furthermore, psychological stress caused by decreased ovarian function and infertility can exacerbate the mental burden. In animals, psychological stress leads to ovarian insufficiency, resulting in irregular estrous cycles and decreased ovarian reserve. The present study summarizes effects of psychogenic stress on ovarian function and the underlying mechanisms, highlighting involvement of the hypothalamic-pituitary-adrenal, sympathetic-adrenal-medullary and hypothalamic-pituitary-ovarian axes, as well as the neuroendocrine-metabolic network. Moreover, the present review outlines psychological intervention and metabolic strategies for improving ovarian function, offering potential new approaches for treating ovarian hypofunction. The present study aims to clarify understanding of psychological stress-induced ovarian dysfunction and propose alternative intervention strategies for ovarian dysfunction and infertility.
Key words: stress, ovarian function, HPA axis, SAM axis, HPO axis, neuroendocrine-metabolic disease
1. Introduction
Globally, stress is a prevalent issue. Mental health obstacles, such as social isolation, fear, loneliness, unemployment, financial instability and loss of family or friends, all contribute to psychological stress (1). Mental stress causes physical health problems, including weakened immune function, hypertension, gastrointestinal issues, cardiovascular disease, sleep disturbance and ovarian dysfunction (2,3). Stress has been widely acknowledged as a key factor that impacts the advancement and onset of ovarian damage (2,4). To the best of our knowledge, however, no consistent conclusions have been drawn on the impact of stress on ovarian function based on epidemiological data, clinical trials and experimental studies (2,4,5).
There is a growing body of evidence indicating that psychological stress leads to ovarian dysfunction (2,6,7). Notably, for patients undergoing in vitro fertilization (IVF) treatment, higher anxiety leads to lower clinical pregnancy (8) and fertilization rates (9), decreased embryo quality (10) and fewer oocytes retrieved (11). A prospective study of 274 patients showed that stress reduced the likelihood of conception during the fertile window each day (12) and participation in stress reduction interventions improves IVF outcomes (11). Mental stress can also affect ovarian reserve, leading to decreased levels of antral follicle count (AFC) (13) and anti-Müllerian hormone (AMH) (14). Moreover, mental stress is also involved in an increased risk of premature ovarian insufficiency (POI) (15), functional hypothalamic amenorrhea (FHA) (16) and polycystic ovary syndrome (PCOS) (17). Furthermore, ovarian insufficiency, such as alteration in female sex hormones, also increases the incidence of depression (18). Similarly, ovarian dysfunction is observed in mental stress models; both chronic unpredictable stress (CUS) (19) and chronic restraint stress (CRS) (20) models lead to depression-like behavior and decreased fertility. Stress can also cause general neuroendocrine system changes, which decrease oocyte quality (21). In recent years, there has been a growing recognition that stress is an emerging risk factor for metabolic disorder (22,23). Metabolic disturbances triggered by stress could lead to conditions such as obesity, type 2 diabetes mellitus (T2DM) and ovary damage (2).
The present study aimed to investigate the association between psychological stress and ovarian function based on existing clinical evidence. Research on psychological-associated stress and female reproductive endocrine function from a neuroendocrine-metabolic (NEM) perspective and the underlying molecular mechanisms are summarized. Additionally, strategies to safeguard ovarian function from psychological stress are explored. The present review may provide new perspectives and directions for research and treatment of ovarian dysfunction and infertility.
2. Materials and methods
Search strategy
MEDLINE (clarivate.com.cn/solutions/medline/) was searched for relevant studies published between January 1960 and December 2023 by using title/abstract terms including 'stress', 'psychological stress', 'mental stress', 'anxiety', 'depression', 'distress', 'cortisol', 'alpha amylase', 'primary ovarian insufficiency', 'Functional hypothalamic amenorrhea', 'polycystic ovary syndrome', 'IVF', 'assisted reproductive technology', 'menopause', 'ovarian reserve', 'anti-Müllerian hormone', 'antral follicle count', 'Chronic stress', 'chronic unpredictable stress', 'restraint stress', 'chronic restraint stress', 'cold stress', 'follicles', 'oocytes', 'granulosa cells', 'ovulation', 'estrous cycle', 'ovarian hormone', 'HPA', 'SAM', 'CRH', 'metabolic syndrome', 'insulin resistance', 'diabetes', 'obesity', 'sleep disorders', 'melatonin', 'psychological intervention', 'cognitive behavioral therapy', 'mindfulness-based stress reduction', 'music therapy', 'yoga', 'caloric restriction', 'metformin', 'resveratrol' or in combination, applying AND and OR operators. To obtain more comprehensive retrieval results, the references of relevant papers were also searched.
Inclusion and exclusion criteria
Research and review articles focusing on the role of stress on ovarian function were included. By contrast, research and review articles focusing on the effect of disorders associated with ovarian dysfunction on mental stress, including communications, case reports, guidelines, comments and protocols, were excluded from the present review. Articles written not in English or without available full text were also excluded.
Data extraction
The title and abstract of every article were checked by two authors (YH and WW). Disagreements were resolved by a third author (FF). A total of 7,623 articles were found by the initial search; 7,316 papers were removed after checking the title and abstract. Next, 307 papers were filtered for full text, of which 183 met the inclusion criteria; 36 were population studies on the effect of stress on ovarian function, 71 were animal models and 26 were psychological and 50 were metabolic intervention studies.
3. Stress causes ovarian dysfunction
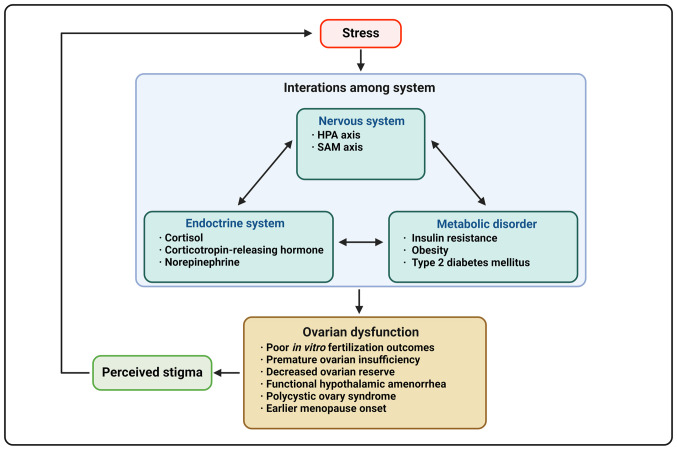
It has been hypothesized that stress may affect ovarian function, however it is unclear whether ovarian dysfunction precedes anxiety or stress precedes ovarian damage (5). Stress is commonly overlooked as a cause of reproductive vulnerability (2). Activation of the stress response system suppresses the hypothalamic-pituitary-ovarian (HPO) axis (24,25). Daily perceived stress can lead to a decrease in estrogen, progesterone and luteinizing hormone (LH), as well as an increase in follicle-stimulating hormone (FSH) in reproductive-aged patients (24). Patients with a history of depression also had higher FSH and LH levels and lower estradiol levels and those having more significant depressive symptoms were twice as likely to experience earlier perimenopause transition than those without such history (25). Additionally, female patients may be more sensitive to lower levels of cortisol than male patients due to increased limbic system activation following repeated stressors (26). The psychological distress caused by decreased ovarian function may further exacerbate the association between psychological stress and impaired ovarian function (Fig. 1; Table I).
Figure 1.
Association between elevated psychological stress and diminished ovarian function. Mental stress triggers the interaction between the nervous and the endocrine system, leading to metabolic disorder. These elements interact with each other, creating a NEM network that results in impaired ovarian function; feelings of shame caused by ovarian insufficiency further exacerbate the psychological burden, ultimately establishing a harmful loop of psychological stress and ovarian dysfunction. Figure generated by using BioRender.com. NEM, neuroendocrine-metabolic; HPA, hypothalamic-pituitary-adrenal; SAM, sympathetic-adrenal-medullary.
Table I.
Impact of psychological stress on ovarian function in population studies.
| A, Assisted reproductive technology
| ||||
|---|---|---|---|---|
| First author (year) | Subjects | Results | Limitations | (Refs.) |
| Chai et al, 2023 | 110 infertile patients; 112 controls | No association between serum cortisol levels and IVF cycles (τ=0.071, P≥0.05) and clinical pregnancy (τ=−0.047, P≥0.05). | Cortisol may be influenced by other factors | (42) |
| Mirzaasgari et al, 2022 | 154 infertile patients | No difference in ISE scale between non-pregnant patients and those with positive pregnancy tests | Large drop-off in subjects; low sample size | (44) |
| Bapayeva et al, 2021 | 142 infertile patients | Higher STAI-S and STAI-T are negatively associated with clinical pregnancy (RR=0.95, 95%CI, 0.91, 0.99, P<0.05; RR=0.92, 95%CI, 0.86, 0.99, P<0.05) | Low response rate; non-response bias | (8) |
| Peng et al, 2021 | 150 patients without clinical pregnancy after first IVF or ICSI; 300 controls | No difference between psychological distress and clinical pregnancy (anxiety, adjusted OR=1.00, 95%CI, 0.97, 1.03; depression, adjusted OR=0.98 95%CI, 0.95, 1.02; perceived stress, adjusted OR=0.98 95%CI, 0.95, 1.01) | Single time point of psychological assessment; small sample size | (45) |
| Sallem et al, 2021 | 79 patients undergoing IVF | BAI score is negatively correlated with embryo segmentation (r=−0.24; P=0.05) and implantation rate (r=0.65; P=0.001).No correlation between serum cortisol levels and IVF outcome | No elimination of some confounding factors | (10) |
| Aimagambetova et al, 2020 | 304 infertile patients | Higher STAI-S and STAI-T are negatively associated with clinical pregnancy outcome (OR=0.94, 95%CI, 0.91, 0.98, P<0.01; OR=0.95, 95%CI, 0.91, 0.99, P=0.02). | Non-response bias; small sample size | (36) |
| Park et al, 2019 | 143 infertile patients | No difference in clinical pregnancy between high or moderate and low stress (FOR=1.11, 95%CI, 0.58, 2.14; FOR=1.37, 95%CI, 0.71, 2.67) | Data of baseline chronic stress, life stressors or stress biomarkers was absent | (46) |
| Cesta et al, 2018 | 485 infertile patients | No difference in clinical pregnancy between lowest and highest categories of perceived stress score (OR=1.04, 95%CI, 0.58, 1.87) | Single time point of psychological assessment; other factors may influence cortisol | (43) |
| Xu et al, 2017 | 842 patients undergoing IVF | Fertilization rate decreases with higher SAS score (91.9%, SAS score, 25-44 vs. 90.4%, SAS score, 45-59 vs. 81.8%, SAS score 60-100], P<0.001) | Non-response bias; single time point of psychological assessment | (9) |
| Terzioglu et al, 2016 | 217 couples undergoing ICSI | Patients with positive pregnancy outcome had lower depression score (P<0.05) | Small sample size | (35) |
| Lynch et al, 2014 | 501 couples | Patients with high SAA levels have a longer time to pregnancy (FOR=0.71, 95%CI, 0.51, 1.00, P<0.05) and double the infertility risk (RR=2.07, 95%CI, 1.04, 4.11) | Single time point of psychological assessment; saliva sample collection | (181) |
| Butts et al, 2014 | 52 patients undergoing IVF | No association between urine cortisol and clinical pregnancy (β=−0.18, 95%CI, -0.04, 0.02, P=0.48). | Small sample size; single time point of uterine sample collection | (50) |
| An et al, 2013 | 264 patients undergoing IVF or ICSI | STAI score is negatively associated with live birth rate (OR=0.855, 95%CI, 0.810, 1.006, P=0.03). | Response bias; small sample size | (34) |
| Turner et al, 2013 | 44 patients undergoing IVF | Patients with positive pregnancy outcome had lower STAI-S score (34.93 vs. 44.35, P=0.05), STAI-T 33.67 vs. 41.30, (P=0.03) and PSS score (11.53 vs. 16.20, P=0.03). | Response bias; small sample size | (37) |
| Quant et al, 2013 | 89 patients undergoing IVF and 77 partners | Partner composite psychological distress score was a predictor of fertilization rate (β=−0.18, SE=0.09, P=0.049). | Single time point of psychological assessment | (38) |
| Pasch et al, 2012 | 202 patients undergoing IVF | STAI (39.96 vs. 41.41, P=0.43) and CES-D score (11.29 vs. 12.39, P=0.48) show no difference between patients with successful and unsuccessful outcomes | Data of psychotherapy treatments was absent; follow-up period was short | (41) |
| Lynch et al, 2012 | 339 patients trying to conceive | No association between baseline psychosocial and pregnancy rate | Response bias; short follow-up period | (47) |
| Li et al, 2011 | 107 infertile patients | Follicular noradrenaline levels are negatively correlated with embryo quality (r=−0.62, P<0.05) and not correlated with pregnancy rate | Small sample size; insufficient sample representativeness | (180) |
| Anderheim et al, 2005 | 166 patients undergoing IVF | No difference in psychological effects between patients with or without adverse pregnancy outcomes | Response bias | (48) |
| Klonoff-Cohen et al, 2001 | 151 patients with primary or secondary infertility | Each PANAS unit increase is linked to 2% fewer oocytes retrieved (log-coefficient, -0.019, 95%CI, -0.03, -0.01, P=0.04). | Small sample size | (11) |
| Csemiczky et al, 2000 | 22 infertile patients | Patients with negative IVF outcome had higher state anxiety level (P<0.06). | Small sample size | (39) |
| Milad et al, 1998 | 40 patients undergoing IVF | No difference in STAI score (42.15 vs. 45.0) and cortisol levels (2.69 vs. 3.12) between patients with or without adverse pregnancy outcomes | High dropout rate | (49) |
|
| ||||
| B, POI | ||||
|
| ||||
| Sun et al, 2022 | 43 patients newly diagnosed with POI | Patients with POI consistently experienced adverse life events associated with work, family stress and sleep issues | Small sample size; lack of psychological assessment | (52) |
| Allshouse et al, 2015 | 160 patients previously diagnosed with POI | Among patients with POI reporting a history of depression, 26% reported that depression occurred >5 years before POI diagnosis and menopausal symptoms do not diminish over time in patients with POI | Certain confounding factors such as treatment of gonadotoxic drugs, treatment of antidepressants, financial status, smoking and alcohol abuse were not eliminated | (15) |
| Schmidt et al, 2011 | 174 patients with spontaneous 46, XX POI; 100 patients with Turner syndrome | Patients with POI have higher lifetime depression rates than patients with Turner syndrome and community-based samples from female subjects (P<0.001) | Confounding factors such as such as treatment of gonadotoxic drugs, financial status, postgraduate alcohol abuse; recall bias | (54) |
|
| ||||
| C, Diminished ovarian reserve | ||||
|
| ||||
| Mínguez-Alarcón et al, 2023 | 520 patients seeking fertility care | Patients in the second (13.3, 95%CI, 12.7, 13.8) and third (13.5, 95%CI, 13.0, 14.1) tertiles of stress have lower AFC compared with patients in the lowest tertile (14.3, 95%CI, 13.8, 14.9, both P<0.05) | Response bias; single time point of psychological assessment | (13) |
| Golenbock et al, 2020 | 332 patients with history of MDD; 644 controls | Depression is negatively associated with AMH levels in patients aged 36-40 years (adjusted PR=0.75, 95%CI, 0.52, 1.09) and nulliparous patients (adjusted PR=0.77, 95%CI, 0.59, 1.00) | Cross-sectional design; unrepresentative population sample | (14) |
| Dong et al, 2017 | 576 infertile patients | SAA is negatively associated with AMH levels (r=−0.336, P<0.0001; adjusted for age, r=−0.336, P<0.0001) | Only one biomarker for stress assessment | (58) |
| Bleil et al, 2012 | 979 patients aged 25-45 years | Higher stress levels are associated with higher AFC (β=0.545, P=0.005) and accelerated follicle loss | Single time point of AFC assessment | (56) |
| Bleil et al, 2012 | 683 patients aged 25-45 years | Psychological stress accelerates AFC decline in patients with low (β=−0.070, P=0.021) but not high positive affect (β=0.018, P=0.54) | Response bias; unrepresentative population sample | (57) |
|
| ||||
| D, FHA | ||||
|
| ||||
| Dundon et al, 2010 | 41 patients with FHA; 39 healthy controls | Patients with FHA have higher depression and anxiety scores in Zung Scale (both P<0.001) | Cross-sectional design; response bias | (63) |
| Lawson et al, 2009 | 13 patients with FHA; 18 amenorrheic patients with anorexia nervosa; 21 healthy controls | Patients with FHA have higher cortisol levels (9.4 vs. 7.6, P=0.04) and HAMD (6.6 vs. 1.1, P<0.001) and HAMA score (7.5 vs. 1.5, P<0.001) compared with healthy controls | Cross-sectional design; response bias | (16) |
| Bomba et al, 2007 | 20 patients with FHA; 20 healthy controls | Both normogonadotropic and hypogonadotropic patients with FHA have higher cortisol levels (16.0 vs. 10.1 and 15.8 vs. 10.1, respectively; both P<0.01) | Small sample size | (61) |
| Marcus et al, 2001 | 28 patients with FHA; 24 patients with organic amenorrhea; 25 healthy controls | Patients with FHA have higher BDI (7.9 vs. 2.9, P=0.02) and HAMD score (6.2 vs. 2.4, P=0.02) | Response bias | (62) |
| Berga et al, 1997 | 19 patients with FHA; 19 patients with other anovulation causes; 19 healthy controls | Patients with FHA have higher cortisol levels than those with other anovulation causes (80.1 vs. 67.7, P<0.05) or healthy controls (80.1 vs. 67.1, P<0.05). | Cortisol may be influenced by other factors | (168) |
IVF, in vitro fertilization; ISE, Infertility Self-Efficacy; STAI, State-trait Anxiety Inventory; RR, relative risk; PR, prevalence ratio; CI, confidence interval; ICSI, intracytoplasmic sperm injection; OR, odds ratio; SAS, Self-Rating Anxiety Scale; SAA, salivary α amylase; PSS, Perceived Stress Scale; CES-D, Center for Epidemiologic Studies Depression; PANAS, Positive and Negative Affective Scale; POI, premature ovarian insufficiency; AMH, anti-Müllerian hormone; AFC, antral follicle count; MDD, major depressive disorder; FHA, functional hypothalamic amenorrhea; HAMD, Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory.
Decreased assisted reproductive technology (ART) outcome
In assisted reproductive medicine, one of the most controversial areas is the underlying influence of psychological factors on pregnancy outcomes. Infertility is a serious health problem around the world and female infertility factors account for at least 35% of all infertility cases (27). The risk of anxiety, depression and distress is generally high in infertile patients (28,29). A 2004 study that interviewed 122 patients before their first infertility clinic visit utilizing a structured psychiatric interview found that 40.2% had a psychiatric disorder, including major depressive, generalized anxiety and dysthymic disorder (30). Volgsten et al (31) reported that, of 413 infertile patients undergoing IVF treatment, 127 (30.8%) were diagnosed with psychiatric disorders, with major depression being the most common. In a study involving 352 female and 274 male patients attending infertility clinics in northern California, 56.5% of patients exhibited notable symptoms of depression and 76% showed notable symptoms of anxiety (32). Infertile patients have higher Beck Depression Inventory (BDI) (14.94±12.90 vs. 8.95±10.49, P<0.0001) and Spielberger Trait Anxiety Inventory (STAI-T) score (48.76±10.96 vs. 41.18±11.26, P<0.0001), indicating a greater experience of anxiety and depression compared with fertile individuals (33).
Numerous studies have explored the link between psychological symptoms before and during ART cycles and subsequent pregnancy rates, yielding conflicting results: Certain studies suggest that higher distress/anxiety/depression levels before and during treatment are associated with lower pregnancy rates (8,9,11,34-39), while others do not (10,40-50). These inconsistencies may be attributed to challenges in accurately assessing stress levels in female patients with infertility and heterogeneity of studies (5,28). Future research should therefore focus on developing more precise psychological assessment methods and establishing consistent time points for data collection to enhance quality of research findings.
POI
POI is characterized by premature loss of ovarian function and permanent cessation of menstruation before the age of 40 years (51). Demographic studies confirm that psychological stress, such as emotional trauma, major life events or chronic stress, may be as a potential trigger for POI in certain cases (52,53). Individuals with POI report continuous exposure to family and work stress and sleep problems, suggesting that adverse life events may contribute to development of POI (52).
Among mental disorders associated with POI, depression is most closely associated to POI. A population study involving 290 patients found that 43% of those with POI had a history of depression, with 26% receiving a depression diagnosis before being diagnosed with POI (15). Depression typically emerges following signs of altered ovarian function but before POI diagnosis (54), possibly due to a more fragile stress system. Female patients are more likely than male patients to develop depression and changes in estrogen levels can increase susceptibility to this condition (18,55). Given the lack of prospective studies on pre-diagnosis stress in patients with POI, more conclusive evidence is needed to determine whether there is an overlapping process or causal association between POI and psychological stress.
Diminished ovarian reserve (DOR)
Various indicators are used to assess ovarian reserve (OR), with AFC and AMH being particularly promising (51). A study on 979 premenopausal patients aged 25-45 years used AFC measured via transvaginal ultrasound to evaluate OR; the finding indicated a positive association between higher stress levels and AFC in younger patients (β=0.545, P=0.005), and stress accelerates age-related AFC decline (β=−0.036, P=0.031), suggesting women with higher stress experienced faster AFC loss (56). Environmental stress might enhance fertility temporarily by increasing the volume of growing follicles, potentially depleting OR in the long-term (56). Additionally, a cross-sectional study suggested that psychological stress accelerates AFC decline (β=−0.070, P=0.021), implying that low positive affectivity may aggravate the adverse impact of stress on AFC decline (57). Consistent findings across populations reveal that higher levels of perceived stress are linked to decreased AFC and AMH levels (13). A cross-sectional study on 576 infertile patients demonstrated a negative association between salivary α amylase (SAA), a stress marker, and AMH levels (r=−0.315, P<0.001; adjusted for age, r=−0.336, P<0.001) (58). Another study noted modest inverse associations between depression and low AMH among patients aged 36-40 years (adjusted P=0.75, 95%CI, 0.52, 1.09) and nulliparous patients (adjusted P=0.77, 95%CI, 0.59, 1.00) (14). Taken together, data from the aforementioned population studies suggested that elevated levels of perceived stress, as well as biomarkers of stress, are associated with reduced OR.
FHA
FHA or anovulation is characterized by the absence of menstruation and ovulation without an identifiable organic cause. The main determinant of the disease is a combination of psychosocial and metabolic stress (59). Predisposing factors include low energy use, nutritional deficiencies, abnormal sleep, emotional and chronic severe stress and overexertion or physical activity (59,60). More severe depressive and anxious symptoms contribute to onset of FHA (16,61-63). Patients with FHA often exhibit dysfunctional attitudes, such as perfectionism, higher need for approval, and struggle to cope with daily stressors (59). Cognitive behavioral therapy accelerates the recovery of ovarian function in patients with FHA, underscoring the role of mental stress in development of this condition (59). As with POI, more prospective studies are needed to explore the effect of psychological stress on FHA.
PCOS
PCOS is a prevalent endocrine disorder in patients of reproductive age, characterized by systemic metabolic issue and disruptions in neuroendocrine-immune function (64). Patients with PCOS are at a higher risk of developing cardiovascular risk factors and associated insulin resistance, dyslipidemia and diabetes (65). Patients with PCOS also face an elevated likelihood of mood and anxiety disorders (17,66,67). There is a notable prevalence of psychiatric issues among patients with PCOS, including depression (26-40%), anxiety (11.6%) and binge-eating (23.3%) (17), indicating a potential role of psychological factors in pathophysiology of PCOS. Psychological aspects have been identified as a notable risk factor for PCOS (68) and emotional distress potentially stems from psychosocial and/or pathophysiological origins (66). Moreover, patients with PCOS may perceive symptom such as hirsutism and acne or potential outcomes such as infertility and obesity as stigmatizing, leading to distress and potentially exacerbating progression of the disease (67). However, as meta-analysis has indicated, few studies have focused on the effect of psychological stress on disease and PCOS (17).
4. Stress models and ovarian dysfunction
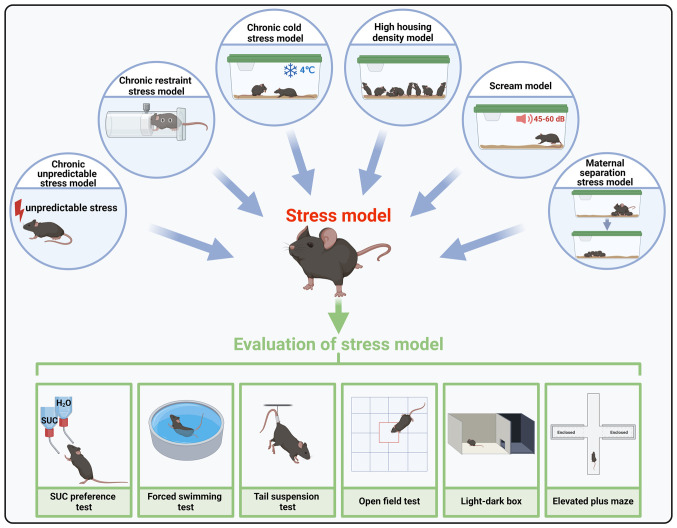
Animal models (69-71), in which the effects of mental stress can be observed, are widely used for studying pathogenesis of stress-related disorders, such as type 2 diabetes, systemic inflammation, obesity and cancer, and their treatment strategies (Fig. 2; Table II).
Figure 2.
Models used in stress-associated ovarian function studies and stress assessment methods. Chronic unpredictable stress and chronic restraint stress models are the most widely used. The cold stress model is primarily applied to examine sympathetic-adrenal-medullary activation effects on the ovary. Additional models, such as high housing density, scream and maternal separation models, are also used to analyze impact of stress on ovarian function. The appropriate modeling method is selected based on experimental requirements and conditions. Following stress modeling, animals displaying anxiety and depression characteristics are assessed using methods such as SUC preference, forced swimming, tail suspension, open field, light-dark box and elevated plus maze tests. The figure was created with BioRender.com. SUC, sucrose.
Table II.
Summary of animal models for ovarian dysfunction induced by psychological stress.
| Animal | Stress | Behavior test | Stress biomarker | Effects on ovary | (Refs.) |
|---|---|---|---|---|---|
| C57BL/6 or BALB/c mice | 8 week CUS | N/A | Increased serum corticosterone levels | Decreased follicle number; irregular estrous cycle; increased serum FSH levels; decreased estradiol, progesterone and AMH levels | (19,85) |
| C57BL/6 mice | 2-8 month CUS | SPT, TST | Increased serum corticosterone levels | Decreased follicle number; increased follicular atresia and ovarian fibrosis; irregular estrous cycle; decreased estradiol and AMH levels and litter size | (77) |
| Kunming mice | 3-4 week CUS | OFT | Increased serum corticosterone and CRH levels | Decreased number of retrieved oocytes and oocyte quality; increased follicular atresia | (83,88) |
| ICR mice | 6 week CUS | SPT, OFT | Decreased serum corticosterone and CRH levels | Decreased oocyte fertilization, cleavage and blastocyst formation rate | (81) |
| Swiss mice | 5 or 30 day CUS | OFT, SPT, FST, EPM | Increased serum corticosterone levels | Inhibition of follicular development; increased follicular atresia rate; irregular estrous cycle | (82,84,87) |
| Sprague-Dawley rats | 4-5 week CUS | SPT, OFT | N/A | Irregular estrous cycle; increased ovarian fibrosis, follicular atresia and FSH levels; decreased estradiol and AMH levels | (76,79,80) |
| C57BL/6, BALB/C or Kunming mice | 4-8 week CRS | N/A | Increased serum corticosterone and CRH levels | Decreased oocyte quality; increased follicular atresia rate; irregular estrous cycle | (20,21,93,98,104) |
| Sprague-Dawley rats | 5 day CRS | N/A | N/A | Decreased progesterone levels; increased fetal resorption | (99) |
| Kunming mice | 24 or 48 h CRS | N/A | Increased serum corticosterone and CRH levels | Decreased oocyte quality; impaired spindle assembly | (97,100,101,103,105) |
| Sprague-Dawley or Wistar rats | 4-12 weeks cold stress | N/A | Increased ovarian NE concentration and serum corticosterone levels | Decreased number of follicles, ovulation and oocyte maturation rate; irregular estrous cycle | (106,107,111,112,114,118) |
| Sprague-Dawley or CIIZ-V rats | 3-8 week cold stress | N/A | N/A | Unchanged estrous cycle and ovarian tissue structure | (117,119) |
| C57BL/6 mice | 10 week cold stress | N/A | Increased serum corticosterone levels | Increased number of atretic follicles; decreased serum AMH levels | (120) |
| Sprague-Dawley rats | 3 week scream sound exposure | SPT, FST | Increased serum corticosterone levels | Decreased AMH and estradiol levels and litter size; increased follicles loss and granulosa cell apoptosis | (121) |
| NMRI mice | 14 day maternal separation stress | N/A | N/A | Impaired follicle development; decreased oocyte maturation rate | (122) |
| Kunming mice | 24 h predatory stress | EPM | Increased serum corticosterone levels | Decreased oocyte quality and embryo development | (126) |
CUS, chronic unpredictable stress; FSH, follicle-stimulating hormone; AMH, anti-Müllerian hormone; SPT, sucrose preference test; OFT, open field test; TST, tail suspension test; CRH, corticotropin-releasing hormone; FST, forced swimming test; CRS, chronic restraint stress; NE, norepinephrine; EPM, elevated plus maze.
Stress models of ovary research
CUS
CUS is a well-established method where animals are subjected to unpredictable stressors over a prolonged period to induce emotional changes, which lead to lasting alterations in animal behavior, gut microbiota and neurological function (72-74). The CUS stressors include restraint stress, tail suspension and swimming, with the intensity of stressors gradually increasing to prevent tolerance (19). Depression-like behaviors induced by CUS are evaluated using tests such as the forced swimming test (FST) and sucrose preference test (SPT) (75). CUS exposure has been shown to disrupt the estrous cycle, decrease the number of primordial and preantral follicles and corpus luteum, elevate serum FSH and cortisol levels and reduce serum estradiol, AMH and gonadotropin-releasing hormone (GnRH) levels, making it an ideal animal model for studying impaired ovarian function (19,76-80).
CUS has been found to negatively impact oocyte developmental potential, with transcriptome analysis revealing disruption in expression of apoptotic genes and cell cycle regulators (81). CUS decreases brain-derived neurotrophic factor (BDNF) expression in antral follicles, resulting in decreased number of retrieved oocytes and rate of blastocyst formation, which could be rescued by exogenous BDNF treatment (82). Another study indicated that CUS significantly decreased the number of retrieved oocytes, fertilization rate and number of embryos (including high-quality embryos and blastocysts), which may be due to different expression of heat shock protein 70 in the embryo after CUS (83). Additionally, supplementation with exogenous growth differentiation factor 9 (GDF9) rescues development of both secondary and antral follicles in stressed mice (84). Granulosa cells in the ovary of depression-like mice induced by CUS exhibit increased apoptosis, possibly due to accumulation of reactive oxygen species (ROS) and activation of the mitogen-activated protein kinase (MAPK) pathway resulting from downregulation of isocitrate dehydrogenase-1 expression (85,86). This suggests CUS can lead to elevated ovarian oxidative stress and mitochondrial dyshomeostasis, supported by increased expression of superoxide dismutase (SOD), mitochondrial fusion protein optic atrophy 1, mitofusin 1 and nucleus-encoded protein succinate dehydrogenase complex A and decreased expression of mitochondrial fission protein 1 in ovary of stressed mice (87,88).
CRS
The CRS model, which is commonly used in studies (89-91) on psychological stress, involves confining animals in a restraint bucket for a designated period each day, inducing anxiety and depression. Typically, animals are restrained for 4 h daily over an 8-week period (20), although the number of restraint days and duration/day can be tailored based on experimental objectives and specific circumstances (92). For example, for studying social interaction, a restraint duration of 5 min is sufficient, however, if the aim is to observe changes in the brain, the restraint time needs to be extended to 6 h (92).
Mice subjected to CRS display elevated serum cortisol and corticotropin-releasing hormone (CRH) levels, indicating activation of the hypothalamic-pituitary-adrenal (HPA) axis (21,93-98). Additionally, CRS results in decreased ovarian weight, disruption in estrous cycles, increased ovarian fibrosis and overactivation of primordial follicles, which may be attributed to the upregulation of Kit expression and activation of the phosphatidylinositol 3-kinase (PI3K)/PTEN/Akt pathway (21). CRS increases oocyte aneuploidy, decreases the percentage of blastocysts and number of cells/blastocyst and impaired corpora lutea function during gestation in mice and rats, leading to decreased pregnancy rates and litter size (99-101). The decline in fertility associated with CRS may be linked to diminished oocyte quality, with germinal vesicle breakdown (GVBD) being a key indicator of oocyte quality (102). Following CRS, female mice exhibit compromised oocyte competence, which was characterized by an increase in spindles defects and chromatin misalignment, decrease in GVBD percentage and prolonged GVBD time, which may be due to abnormalities of the Fas/FasL system or TNF-α signaling in oocytes and granulosa cells (93,97,98,103,104). Moreover, CRS decreases the expression of cyclin B1 (CCNB1), a key regulator of M-phase/mature promoting factor (93). Treatment with MG132, an inhibitor of anaphase-promoting complex/cyclosome, rescues prolonged GVBD time and elevates CCNB1 expression in oocytes of CRS-exposed mice (93). Furthermore, CRS elevates meiotic arrest failure in oocytes by suppressing the cyclic AMP (cAMP) signaling pathway, particularly via the downregulation of G-protein-coupled receptor 3, natriuretic peptide C and natriuretic peptide receptor 2, leading to accelerated oocyte loss and premature ovarian aging (20). Short-time restraint stress accelerates postovulatory oocyte aging due to elevated cytoplasmic calcium levels and increased oxidative stress (105).
Chronic cold stress
Animals exposed to cold stress experience heightened sympathetic activity and elevated levels of norepinephrine in the ovary (106-108), while corticosterone and gonadotropin (Gn) levels are unaffected (109,110). As a result, chronic cold stress is commonly used in the examination of overactivation effects of the sympathetic-adrenal-medullary (SAM) axis. The cold stress regimen typically spans 21-28 days, involving activities such as daily 15-min swims in 15°C water or 3-h exposures to 4°C temperature (111,112).
The majority of studies show that prolonged exposure to cold stress may result in extended estrous cycle, reduced level of estrogen and progesterone, decreased corpus luteum count and increased follicular atresia in rats (111,113-115). The observed hindered follicular development may be attributed to increased nerve growth factor levels in the ovary stemming from sympathetic activation, breakdown of the gap junctions between oocyte and cumulus cells and dysregulation of the NRF2/Connexin-43 (CX43)/steroidogenic acute regulatory (StAR)/progesterone pathway in granulosa cells following cold exposure (106). Cold stress triggers the upregulation of endothelin-1 (ET-1) and ET-receptor type A, alongside downregulation of ET-receptor type B protein, thereby disrupting the ovarian vascular endothelin system, leading to vasoconstriction and microcirculation issues in rats (113). This compromised blood flow may impede ovarian function by decreasing oxygen and nutrient delivery to the ovary (116). However, a previous study indicated that prepubertal rats that experience chronic cold stress do not exhibit altered ovarian architecture that affects the ovulation in adulthood (117). When animals were exposed to both chronic restraint and cold water stress, the results were different (118,119): A study showed that the number of atretic follicles and the percentage of apoptotic granulosa cells are notably increased following exposure to restraint stress, while another study indicated that there were no effects on the ovaries after exposure to cold stress (118,119).
Other stress models
A model of high housing density is conducted by housing eight C57BL/6 mice in a 484 cm2 ventilated cage which results in increased stocking density and elevated stress hormones among the mice (120). Mice housed in more densely packed conditions exhibit higher corticosterone and lower AMH levels, alongside a great number of atretic ovarian follicles (120). The scream model subjects mice to noise levels between 45 and 60 dB for 6 h daily over 3 weeks, leading to decreased levels of AMH and estradiol, loss of primordial and preantral follicles, increased apoptosis in granulosa cell and ultimately decreased litter sizes (121). An additional study indicated that maternal separation stress could simulate early life stress (122), with population studies suggesting that early life stress may be associated with an earlier onset of menarche and menopause (123-125). In a previous study, female litters were separated from their mothers between postnatal days 2 and 16 for 6 h/day, with the home cages of the litters placed on a warming pad set at 33-35°C to prevent undue stress (122). After 14 days, there was a significant decrease in the activity of SOD, glutathione peroxidase and catalase, accompanied by oocyte development disorder (122). A predatory stress model suggests that the rate of blastocyst of formation, and the number of cells/blastocyst significantly decreases in stressed mice (126). Chronic immobilization stress could disrupt the estrous cycle, decrease serum levels of AMH, increase the serum levels of FSH and cortisol and reduce the number of primordial follicles (127). In addition, cortisol injection simulates the impact of stress-induced cortisol elevation on ovarian function (128).
While animal models enhance the current understanding of psychiatric disorders, they have certain limitations; for example, animals are incapable of conveying sadness, guilt or suicidal thoughts, which are symptoms mostly confined to humans (129).
Evaluation of stress models
Following successful modeling, the experimental animals display indications such as anhedonia and melancholy, in addition to other signs of depression such as disturbed sleep, alterations in weight and appetite, anxiousness and isolation from social interaction, mirroring the symptoms observed in humans with major depressive disorder (130).
SPT
SPT is frequently utilized to evaluate anhedonia (131-133). Test subjects are provided with a choice between water sources containing sucrose or plain water; decreased appetite for sucrose is considered to indicate anhedonia (131).
FST
To evaluate behaviors associated with hopelessness, FST is employed. The animal is positioned in a cylinder filled with water; if the subject recognizes that escape is unattainable, it may exhibit depressive-like behavior characterized by limited movement except that necessary to keep the snout above the water level (134).
Tail suspension test (TST)
TST is a common method for assessing depressive-like behavior, with the time spent motionless by the subject over a 6-min period recorded (135).
Open field test (OFT)
OFT detects the locomotor activity and exploratory behavior (136). Upon introduction to a new open field, animals display fear of the unfamiliar surroundings by staying along the outer edges, although curiosity spurs them to venture further into the center (137,138). In general, mice tend to hug the walls due to thigmotaxis, moving primarily along the perimeter (137). This behavior is amplified in mice exhibiting anxiety-like traits; mice with lower anxiety levels are inclined to spend more time in the central, open region of the field (137).
Light-dark box (LDB)
LDB box test exploits instinctual aversion to well-lit areas and spontaneous exploration in response to mild stressors, such as novel environments and light exposure (139). The delay in a mouse entry into the dark section indicates an avoidance of the bright area due to anxiety, while movements between compartments reflect exploratory behavior.
Elevated plus maze (EPM)
EPM is extensively utilized for evaluating anxiety-related behavior in laboratory animals, particularly rodents (140-142). Similar to OFT and LDB, EPM exploits the emotional adaptive responses that animals exhibit when confronted with unavoidable aversive situations (143).
Commonly used models for ovarian function research
Recent studies (75,85,98) exploring the effects of mental stress on ovarian function have predominantly employed CUS and CRS models. Animals exposed to stress in these models exhibit decreased preference for sucrose in the SPT and total distance traveled and locomotor activity duration and increased time spent in the peripheral zones during OFT. On the other hand, the cold stress mode primarily activates the SAM system and does not significantly alter mouse behavior and is more appropriate for studying the effects of stress-induced sympathetic nervous activation on ovarian function. These models not only induce depression-like behaviors but also affect ovarian function, such as irregular estrous cycle, decreased ovarian reserve. Thus, they provide a more comprehensive reflection of the impact of psychological stress on ovarian function.
5. Mechanism of ovarian dysfunction caused by mental stress
During the stress response, the autonomic nervous system reacts instantly by triggering the SAM axis and activating the HPA axis (144). Stimulation of autonomic nerves prompts release of norepinephrine (NE) from neuronal terminals, leading to the activation of the adrenal medulla and subsequent discharge of epinephrine, resulting in activation of the SAM axis (145). The activation of the hypothalamic paraventricular nucleus (PVN) in the brain induces release of CRH and arginine vasopressin (AVP) into the pituitary portal system. CRH and AVP synergistically stimulate secretion of pituitary adrenocorticotropic hormone, which enters the systemic circulation, reaches the adrenal cortex and promotes the production and release of cortisol (146). Additionally, CRH that is released from the PVN area of the inferior colliculus can activate locus coeruleus neurons, increasing NE production (147). The neuroendocrine system serves critical roles in regulating basal homeostasis and responding to threats and it is also involved in the pathogenesis of diseases marked by imbalance or dyshomeostasis (148-150). Prolonged exposure to life stressors in individuals with a susceptible genetic makeup can lead to visceral fat build-up, attributed to persistent hypercortisolism, reactive insulin hypersecretion and reduced growth hormone secretion (68). Furthermore, in adults, stress can induce hypogonadism, and lead to symptoms such as loss of libido and subfertility (68).
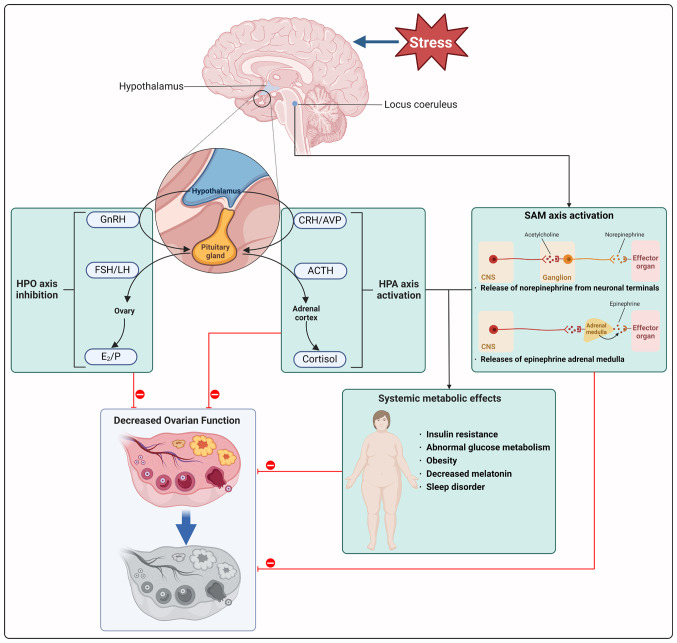
Elevated levels of cortisol have the potential to disturb the equilibrium of hormones key for reproductive function, such as GnRH, LH and FSH, leading to a disruption in ovarian function (151,152). Additionally, stress hormones released by the SAM axis impact the HPO axis by influencing the secretion of GnRH and other regulatory hormones responsible for ovarian function (145). Furthermore, premature cessation of ovulation may be related to elevated serum triglycerides and high-density lipoprotein cholesterol levels (153). Patients with POI exhibit irregularities in lipid and glucose metabolism (154). Fig. 3 demonstrates associations between psychological stress, HPA and HPO axes, sympathetic adrenal system and metabolic disorders influencing ovarian function, highlighting the role of HPA and SAM axis activation along with metabolic disorder (Fig. 4; Tables III and IV).
Figure 3.
Association between psychological stress, HPA, HPO and SAM axes and metabolic disorder in regulating ovarian function. Psychological stress leads to the activation of the HPA and SAM axes, while concurrently suppressing the HPO axes. In response to stress, activation of the paraventricular nucleus region stimulates the secretion of CRH and AVP into the pituitary portal system, stimulating the pituitary gland to release ACTH into the circulation, which triggers cortisol release from the adrenal gland. Simultaneously, the locus coeruleus is activated, resulting in a surge of systemic sympathetic nervous excitement along with increased release of epinephrine from the adrenal medulla. The activation of HPA and SAM axes disrupts neuroendocrine homeostasis, triggering a cascade of metabolic disorders such as glucose metabolism abnormality and obesity. This dysregulation, coupled with the suppression of the HPO axis, impairs ovarian function. Figure created with BioRender.com. HPO, hypothalamic-pituitary-ovarian; HPA, hypothalamic-pituitary-adrenal; SAM, sympathetic-adrenal-medullary; GnRH, gonadotropin-releasing hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; P, Progesterone; CRH, corticotropin-releasing hormone; AVP, arginine vasopressin; CNS, central nervous system.
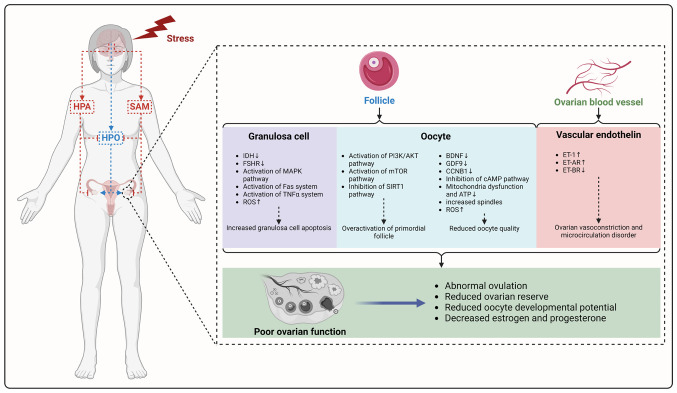
Figure 4.
Mechanisms of ovarian dysfunction caused by stress. Psychological stress affects ovarian function and inhibits the HPO axis by activating the HPA and SAM axes. This causes increased granulosa cell apoptosis, diminished oocyte quality and excessive activation of primordial follicles, culminating in decreased ovarian reserve. Meanwhile, psychological stress leads to disorder of the vascular endothelin system of the ovary, resulting in ovarian vasoconstriction and microcirculation disorder. Figure created with BioRender.com. HPA, hypothalamic-pituitary-adrenal; SAM, sympathetic-adrenal-medullary; HPO, hypothalamic-pituitary-ovarian; IDH, isocitrate dehydrogenase; FSHR, follicle-stimulating hormone; ROS, reactive oxygen species; BDNF, brain derived neurotrophic factor; GDF, growth differentiation factor; CCN, cyclin; ET-AR, ET-receptor types A.
Table III.
Effect of stress on ovary.
| Cell type | Type of stress | Test subject | Mediators | Impact | (Refs.) |
|---|---|---|---|---|---|
| Oocyte | CUS | Mice | HPA axis | Increased follicle atresia with suppressed GDF9 expression | (84) |
| CRS | Mice | HPA axis | Increased abnormal bipolar spindle rates; reduced the expression of CCNB1; increased meiotic arrest failure of oocytes by inhibition of the cAMP pathway; overactivation of primordial follicles due to activation of the PI3K/AKT pathway | (20,21,93) | |
| N/A | Rats | NE | Decreased ovarian sympathetic activity decreases KISS1 levels and increases FSHR expression and follicle growth | (177) | |
| Injection of cortisol | Mice | Cortisol | Impaired oocyte development and mitochondrial membrane potential | (128,161) | |
| Granulosa | CUS | Mice | HPA axis | Increased apoptosis due to the ROS accumulation and MAPK pathway activation | (85) |
| CRS | Mice | HPA axis | Increased apoptosis via activating the Fas/FasL and TNFα system | (97,103)) | |
| N/A | Humans | NE | Induced generation of ROS in granulosa cells | (178) | |
| Injection of cortisol | Mice | Cortisol | Decreased expression of IGF1 and BDNFl increased apoptosis | (128,161) | |
| N/A | Rats | NE | Activation of TGFβ-SMAD7 pathway and apoptosis | (179) |
CUS, chronic unpredictable stress; HPA, hypothalamic-pituitary-adrenal; GDF9, growth differentiation factor 9; CRS, chronic restraint stress; CCNB1, cyclin B1; cAMP, cyclic AMP; KISS, kisspeptin; FSHR, follicle-stimulating hormone receptor; ROS, reactive oxygen species; NE, norepinephrine; IGF1, insulin-like growth factor 1; BDNF, brain-derived neurotrophic factor; TGFβ, transforming growth factor β; ET-AR, endothelin-receptor type A.
Table IV.
Effect of metabolic disorder on the ovary.
| First author (year) | Subjects | Effect on ovarian function | Limitations | (Refs.) |
|---|---|---|---|---|
| Qin et al, 2023 | 265 infertile patients | T2DM decreases clinical pregnancy rate (adjusted OR, 0.458, 95%CI, 0.235, 0.891, P=0.022) and live birth rate (adjusted OR, 0.227, 95%CI, 0.101, 0.513, P<0.001) | Incomplete medical records; lack of detailed population characteristics | (207) |
| Sekhar et al, 2015 | 600 patients (300 with T2DM and 300 controls) | Patients with T2DM have earlier menopause age (44.65 vs. 48.2 years, P<0.01) | Assessment of menopause age susceptible to recall bias | (206) |
| Khalil et al, 2011 | 2,171 patients (117 with and 2054 without diabetes mellitus) | Patients with diabetes have earlier menopause age (48.9 vs. 51.7 years, P=0.005) | No differentiation between T1DM and T2DM | (205) |
| Bernardi et al, 2017 | 1,654 female subjects | BMI was negatively associated with AMH (β=−0.015, 95%CI, −0.021, −0.009, P<0.0001) | Recall bias; association between obesity and AMH lacks generalizability to other racial/ethnic groups | (225) |
| Machtinger et al, 2012 | Oocytes from 276 patients (105 from severely obese and 171 from normal BMI patients) | Oocytes from severely obese patients have higher incidence of double spindles (58.9 vs. 35.1%, P=0.003) and prevalence of disarranged spindles (28.6 vs. 8.6%, P=0.04) | Oocytes had been discarded and failed to fertilize, which was unclear if the results are generalizable to fertilized oocytes from the same cohort | (236) |
| Freeman et al, 2007 | 122 female subjects | Obese patients have lower AMH levels (geometric mean, 0.016 vs. 0.046; geometric mean ratio, 0.35, 95%CI, 0.13, 0.92, P=0.034) | AMH of postmenopausal patients was below assay threshold | (227) |
| Douchi et al, 2002 | 83 female patients with obesity | Patients with menstrual disorder have higher trunk-to-leg fat ratio (1.48 vs. 11.25, P<0.01) | Lack of control group of non-obese patients | (229) |
| Stock et al, 2019 | 80,840 female subjects | Subjects working rotating night shifts have an increased risk of earlier menopause (multivariable-adjusted HR, 1.09, 95%CI, 1.02, 1.16) | Work schedule information was not detailed | (245) |
T2DM, type 2 diabetes mellitus; AMH, anti-Müllerian hormone; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
HPA axis
The HPA axis serves a key role in the functioning of the neuroendocrine system, overseeing responses to stress and managing bodily functions such as digestion, immunity, mood, sexual behavior and energy regulation (68). Activation of the HPA axis impacts ovarian function by boosting the levels of cortisol and CRH. Additionally, it can suppress release of gonadotropins, such as FSH and LH, thereby affecting synthesis of steroid hormones, ovulation and menstrual cycle (155). Previous research has indicated that the surge in cortisol and CRH resulting from HPA axis activation, along with the inhibition of the HPO axis, are key factors in impairing ovarian function (156).
In a CRS model, activation of the HPA axis results in impaired oocyte developmental potential and decreased fertility (21,93-95). Previous studies indicated that patients undergoing IVF experienced higher levels of state anxiety and serum cortisol (39,157). Additionally, patients with high anticipatory state anxiety and cortisol concentrations have lower pregnancy rates (158), supporting the link between HPA axis activation and decreased fertility. However, a cross-sectional study of 89 infertile patients revealed that increased perceived mental stress is an independent predictor of increasing FSH levels (β=0.22, P=0.045) and likelihood for DOR (OR=9.97, 95%CI, 1.66, 55.99, P=0.012), but there was no significant difference in serum cortisol levels between patients with DOR and normal OR (r=−0.176, P=0.287), which suggests that serum cortisol levels, representing current stress, may not significantly be associated with ovarian reserve or infertility (159). In direct exposure studies, mouse oocytes exposed to physiological or stress-induced levels of cortisol during in vitro maturation did not show any impact on nuclear maturation or embryo development (94,160). Conversely, injection of cortisol into female mice resulted in impaired oocyte development, decreased mitochondrial membrane potential and increased oxidative stress and granulosa cell apoptosis through activating the TNF-α system (128). These effects may be linked to decreased expression of insulin-like growth factor 1 (IGF-1) and BDNF in granulosa cells and decreased FasL/Fas secretion in ovaries and oocytes (161). The differing impacts of cortisol in vivo and in vitro may be attributed to the indirect influence of cortisol on oocytes via the NEM network. In rats, exposure to CRS leads to increased expression of hypothalamic RFamide-related peptide-3 (RFRP3) and levels of circulating corticosterone, resulting in decreased HPG axis function (95). However, following a complete estrous cycle, female rats exhibit decreased inclination towards mating; the stress-induced reproductive dysfunction in female rats is alleviated by suppressing RFRP3 expression using a cytomegalovirus promoter-driven lentivirus vector. This indicates that elevated levels of RFRP3 under stress may have enduring detrimental impacts on fertility (95).
During stress, the hypothalamus produces a neuropeptide known as CRH, which serves a vital role in the regulation of ovarian functions, as its receptors have been identified in reproductive organs, including ovary, uterus and placenta (162). Previous research has shown that CRH is responsible for activation of primordial follicles in cultured newborn mouse ovaries in vitro (21). Furthermore, CRS could result in a decrease in oocyte competence and an increase in both CRH and CRH receptor (CRH-R) expression in the ovary (97). Co-culturing mouse granulosa cells with CRH activates the Fas/FasL and TNF-α systems, leading to heightened apoptosis of granulosa cells and oocytes, as well as impaired early embryonic development (97,103). CRH-R antagonists such as antalarmin are effective in inhibiting these damaging effects (97). In another study, injection of CRH in female rats for 12 days post-mating resulted in a 40% disruption in pregnancy (163). These findings suggest that CRH negatively impacts reproductive function.
Stress-induced activation of the HPA axis results in increased levels of glucocorticoids in the bloodstream, along with disrupted functioning of the HPO axis and ovarian cycle. In female mice, chronic stress reduces the synthesis and release of LH mediated by GnRH (164). One explanation for decreased LH secretion may be the elevated levels of cortisol due to stress. A previous study on mice exposed to long-term corticosterone demonstrated a notable decrease in LH levels, accompanied by alteration in neuronal activity in the pituitary gonadotroph cells (165). CRS in rats inhibits the preovulatory LH surge (166), while CUS has been shown to raise cortisol levels and SOD activity (87), both of which impact ovulation. Moreover, patients diagnosed with FHA exhibit high activation of the HPA axis, indicated by increased levels of cortisol in the blood (167). Furthermore, patients with FHA who experience spontaneous recovery of ovarian function show lower serum cortisol levels post-recovery compared with those who do not recover from FHA (168).
SAM axis
Ovarian function is regulated by the autonomic nervous system, which cooperates with the HPO axis (169). Sympathetic fibers from the celiac ganglia extend to ovarian follicles, mainly targeting the theca layer, where NE, the principal neurotransmitter, interacts with β-adrenergic receptors situated in the theca and granulosa cells (170-172). Neural and hormonal signals integrate to control steroid production and development of ovarian follicles (173). Notably, during stressful conditions, excessive activation of the SAM axis disrupts this equilibrium, leading to compromised ovarian function (174).
Previous research on rats has shown that normal aging process is associated with elevated NE levels and increased sympathetic activity within the ovary (175), implying that SAM axis activation promotes ovarian aging. Chronic exposure to cold stress activates the sympathetic nervous system, causing prolonged estrous cycle, decreased levels of estrogen and progesterone and corpus luteum count and heightened follicular atresia in rats (114,115,117,176).
Surgical denervation of the ovary and blocking the β-adrenergic receptor using propranolol decrease ovarian sympathetic activity, leading to a decrease in ovarian kisspeptin (KISS1) and an increase in FSH receptor (FSHR) expression. This may enhance follicle growth, suggesting activation of the SAM axis could hinder ovarian function by elevating ovarian KISS1 levels (177). NE is detected in human follicular fluid (178). However, physiological levels of NE are unable to trigger NE receptor activation but can induce the generation of ROS in granulosa cells (178). In vitro, rat granulosa cells display increased apoptosis upon co-culture with NE (179). Furthermore, following recovery from cold stress model, rats exhibited sustained elevated levels of ovarian NE, disrupted estrous cycle and reduced fertility, indicating that SAM axis activation may have lasting impacts on ovarian function in rats (111).
Data from population studies also corroborate the harm caused by SAM axis activation on ovarian function (12,58,180,181). A previous population-based study including 274 patients showed significant decreases in the likelihood of conceiving during the fertile window in the first cycle of attempting pregnancy for patients with high SAA levels compared with those with low levels (fecundability odds ratio, FOR=0.85, 95%CI, 0.67, 1.09) (12). Although SAA is a marker for stress and SAM activity, these findings support the idea that stress impacts female fertility through the SAM pathway (12). Similarly, a study on 401 patients found that those in the top tertile for SAA levels had a 29% decrease in fecundity (longer time to pregnancy) compared with those in the bottom tertile (FOR=0.71, 95%CI, 1.04, 4.11), and this decrease in fecundity leads to an increased risk of infertility (Relative Risk, RR=2.07, 95%CI=1.04, 4.11) (181). Aside from its potential impact on fertility rates, SAM axis activation may also influence OR. A study on 576 infertile patients found that higher levels of SAA were linked to lower serum AMH (r=−0.315; adjusted r=−0.336, both P<0.0001) (58). Another population study involving 107 patients revealed that follicular NE levels had a negative association with proportion of good quality embryos (r=−0.62, P<0.05) but not with clinical pregnancy rates (180).
Metabolic disorder
The activation of stress-associated neuroendocrine systems plays a key role in maintaining body equilibrium, yet excessive stress can disrupt homeostasis (148). Previous studies indicate that life stress poses a risk and has prognostic implications for metabolic conditions such as obesity, T2DM and metabolic syndrome (3,182). Moreover, involvement of the HPA and SAM axes due to mental stress is linked to increased metabolic disorder (183,184). Notably, patients with metabolic syndrome or T2DM exhibit lower OR compared with healthy counterparts (185,186), highlighting the connection between stress, metabolic disorder and ovarian function.
Abnormal glucose metabolism and insulin resistance
Previous research suggests that stressors such as stressful life events, emotional distress, anger, poor sleep and work stress disrupt glucose balance and promote insulin resistance (187). As a result, these stressors are considered independent risk factors for T2DM (188,189) and individuals experiencing chronic stress are at a higher risk of developing T2DM compared with non-stressed individuals (190). T2DM is a chronic metabolic disorder characterized by hyperglycemia and insulin resistance, both of which may contribute to ovarian dysfunction (191,192).
As reviewed by Dri et al (193), granulosa, theca and stromal compartments of the ovary express insulin receptors, which bind to insulin and IGF-1, dysregulating dynamics of OR and/or impairing the survival and competence of the oocytes. Previous studies in vitro have demonstrated that insulin can stimulate these receptors in granulosa and theca cells, resulting in elevated levels of androgen, estrogen and progesterone (194-196). Prolonged stress and insulin resistance can lead to reduced β-cell function, necessitating exogenous insulin administration (197). Chronic exposure to high systemic insulin levels stimulates ovarian insulin and IGF-1 receptors, resulting in follicle stimulation and increased production of ovarian androgens (198). Persistent hyperglycemia can negatively impact folliculogenesis and ovarian function, potentially via advanced glycation end-products (AGEs) and their receptors (191,199). AGEs decrease oocyte developmental competence and vascularization, induce hypoxia and decrease glucose uptake (200). Insulin resistance can also disrupt ovulation by impeding recruitment of dominant follicles, leading to anovulatory states and menstrual irregularity (201). Moreover, in T1DM, folliculogenesis, oogenesis and embryo development are impaired in mice (202), but the underlying mechanism needs to be further investigated.
Previous population studies have indicated an association between abnormal glucose metabolism, insulin resistance and ovarian function (173-180). The incidence of oligomenorrhea is notably higher in patients with T2DM compared with those with normal blood glucose levels (203,204). Additionally, Study of Women's Health Across the Nation Bone, a longitudinal and ethnically diverse research project (n=2,171), observed that patients with diabetes at the start of the study reached menopause ~3 years sooner than non-diabetic counterparts (P=0.002) (205). Similarly, Sekhar et al (206) reported that the mean age of menopause is lower in patients with T2DM (44.65 years) compared with those without T2DM (48.2 years; P<0.01). A retrospective cohort study by Qin et al (207) found that patients with T2DM had significantly decreased AMH levels (2.43±2.31 vs. 3.58±2.58 μg/l, P<0.001), and T2DM was an independent risk factor for clinical pregnancy rate (adjusted OR=0.458, adjusted 95% CI, 0.235, 0.891, P=0.022) and live birth rate (adjusted OR=0.227, adjusted 95%CI, 0.101, 0.513, P<0.001). Furthermore, early menopause (onset at <45 years of age) was found to be more prevalent among patients with T2DM compared with those without T2DM (206). Decreased estrogen secretion post-menopause can increase visceral fat deposition and insulin resistance, significantly elevating risk of T2DM (208), and hormone replacement therapy has been shown to mitigate this risk (209,210). Similarly, decreased insulin sensitivity and impaired glucose metabolism are observed in animal models that underwent ovariectomy, but these effects are reversed by chronic estrogen administration (211,212). These findings highlight the key role of estrogens in maintaining glucose homeostasis. Consequently, stress-induced impaired glucose metabolism may lead to ovarian dysfunction, which can cause further abnormal glucose metabolism.
In summary, stress-induced abnormal glucose metabolism has detrimental effects on ovarian function throughout the reproductive lifecycle. However, more animal studies and cohort studies are required to elucidate the interplay between these factors.
Obesity
Obesity has emerged as a growing health crisis with notable repercussions for public health. In recent years, accumulating evidence indicated that stress, notably increased levels of cortisol, plays a crucial role in the onset of obesity (213-216). Chronic stress activates the HPA axis, which results in elevated circulating cortisol levels. This causes the redistribution of white adipose tissue towards the abdominal area, and also increases appetite, particularly for calorie-dense foods (217). Notably, the obesity epidemic coincides with factors such as elevated cortisol production, prolonged stress, consumption of high glycemic index foods and poor sleep quality (3). The Prospective Urban and Rural Epidemiological study involving 120,000 individuals found that participants with higher life stress scores (encompassing work, family and financial stress and major life events) exhibited a higher prevalence of abdominal obesity compared with those with lower life stress (218). Moreover, in elderly female patients, central obesity becomes common due to redistribution of adipose tissue associated with aging (219). Similar patterns observed in fat redistribution due to chronic stress and aging in female patients suggest a potential connection between these factors (220). Recent studies increasingly highlight that obesity is associated not only with stress but also with diminished ovarian function (221-223).
Population-based studies have demonstrated an inverse association between obesity and OR (186-192). A cross-sectional study on 36 healthy patients aged 40-52 years showed that AMH and inhibin levels of obese patients were reduced by 77 (0.06, 95%CI, 0.12, 0.67 vs. 0.02 ng/ml, 95%CI, 0.12, 0.67, P=0.02) and 24% (12.5, 95%CI, 10.1, 15.4 ng/ml vs. 9.5 ng/ml, 95% CI, 8.0, 11.3, P=0.08), respectively, compared with normal-weight patients after adjusting for age, body mass index (BMI), race, smoking status and alcohol use (224). In a previous study involving 1,654 African American patients aged 23-35 years, it was observed that obese participants exhibited 23.7% lower AMH concentration (2.9 vs. 3.8 ng/ml) compared with those with a BMI ≤25 kg/m2 (225). This finding has been corroborated by several other research studies (226-228). Additionally, obesity is linked to anovulatory cycles, irregular menstrual period and, diminished implantation and pregnancy rate and it may also be associated with PCOS (229,230). Research on the effects of a high-fat diet (HFD) on ovarian follicular development has indicated a decrease in number of primordial follicles (231). Exposure to HFD results in follicular atresia, and accelerated follicle loss in rats via the activation of mTOR and the inhibition of sirtuin 1 (SIRT1) signaling, leading to premature ovarian failure (POF) (232). Previous studies on diet-induced obesity in mice reveal increased oocyte apoptosis (233), the presence of multiple spindles (double spindles) (234) and oocyte mitochondrial dysfunction such as decreased mitochondrial membrane potential, mitochondrial distribution and ATP levels (235). These findings are supported by population studies: For example, Machtinger et al (236) found that severe obesity is associated with a higher incidence of spindle anomalies in failed fertilized oocytes, with observations of double spindles (OR=2.68, 95%CI, 1.39-5.15, P=0.003) and disorganized single spindles (OR=4.58, 95%CI, 1.05-19.86, P=0.04). A recent study reported that an increase in BMI was negatively associated with OR measured by AMH levels in patients with PCOS (β=−0.03, 95%CI, −0.06-0.00, P=0.034) (237). Although direct evidence linking stress-induced obesity with impaired ovarian function is lacking, the aforementioned indirect evidence supports this connection.
Sleep disorder and decreased melatonin
Sleep disorder caused by mental stress are widely hypothesized to be associated with infertility and ovarian dysfunction (238-240). Previous studies on animals have indicated that sleep deprivation (SD) disrupts the regularity of the estrous cycle and results in anovulation, potentially leading to reproductive dysfunction (241,242). Negative effects of SD may impede follicular development during ovarian hyperstimulation due to an increase in corticosterone levels in rats (243). SD has been shown to significantly elevate serotonin levels, which decreases estradiol production induced by FSH and FSH-driven expression of StAR protein in follicles, resulting in decreased estrogen levels in the bloodstream (244). This is also supported by population-based research: A large-scale prospective cohort study that monitored 80,840 patients from the Nurses' Health Study between 1991 and 2013 discovered that working rotating night shifts for 2 or >10 years is associated with earlier onset of menopause (245).
Melatonin secretion decreases with age, which serves a crucial role in regulating sleep and metabolic balance, with its lipophilic nature giving it potent antioxidant properties (246). Melatonin is a key player in combating aging by protecting cells from oxidative damage, a process exacerbated by the natural decline in melatonin levels over time (247,248). Previous findings linked CUS to increased ovarian ROS levels, with ROS-induced oxidative stress being a notable contributor to ovarian damage and aging (85). Previous studies have demonstrated that melatonin safeguards mouse ovarian granulosa cells by mitigating oxidative stress-induced DNA damage, mitochondrial dysfunction, lipid peroxidation and apoptosis (249), which are associated with the SIRT1/FOXO1 and FOS pathways (249-252). Moreover, melatonin improves oocyte quality by neutralizing ROS generated during follicle maturation and ovulation and fertilization via NADPH, glutathione (GSH), bone Morphogenetic Protein 15 (BMP15) and SIRT1/SOD2-associated mechanisms (253-256). On the other hand, melatonin supplements also significantly alleviated spindle/chromosome disorganization, which may involve the SIRT2-dependent H4K16 deacetylation pathway (257,258). Clinical trials have indicated that melatonin treatment in infertile patients can elevate follicular melatonin levels, reduce oxidative damage and enhance fertilization and pregnancy rates (253,257,259). Importantly, decreased melatonin secretion has been linked to a higher risk of diabetes (260), underscoring the association between melatonin levels, metabolic health and ovarian function.
6. Intervention strategies for ovarian dysfunction
Psychological intervention strategies for ovarian dysfunction
The likelihood of depression, anxiety and distress is notably high among patients experiencing infertility and psychological symptoms adversely affect fertility (261). Additionally, onset of depression often occurs before the diagnosis of POI, implying that depression and/or its treatment may contribute to development of POI (15).
The effectiveness of psychological interventions in decreasing psychological distress and association with significant increases in pregnancy rates has been shown (5). After receiving antidepressant medication and psychotherapy, infertile patients with depression have higher pregnancy rates (262). Patients with POF/POI may benefit from psychological interventions, as emotional and other menopausal symptoms may be prolonged and not always alleviated by hormone therapy (15). Stress management and psychological therapy have been linked to spontaneous pregnancy in certain infertile couples. Multiple studies have documented cases of infertile couples conceiving naturally after adopting a child (263,264). The aforementioned data suggests that psychological interventions may be a promising approach for these individuals to enhance quality of life.
Cognitive behavioral therapy (CBT)
CBT can help patients alleviate stress and achieve relaxation by identifying and analyzing problems, recognizing unproductive thoughts and behaviors, creating and implementing replacement thoughts and behaviors, regular review and adjustment of strategies, and applying learned skills to daily life (265). CBT has been demonstrated to be an effective approach in treating depressive disorder, with meta-analyses of randomized controlled trials corroborating its efficacy for both anxiety (266-268) and depression (269,270).
A randomized controlled trial involving 56 pregnant patients with a history of primary infertility indicated that CBT counseling alleviates perceived stress, and anxiety, as well as enhancing the quality of life score (271). Domar et al (272) showed that infertile patients assigned to either structured CBT or standard support groups had a higher pregnancy rate (52 vs. 20%, P=0.05), compared with those who did not participate in these programs during their second IVF cycle; however, P-value decreased slightly to 0.038 after adjusting for intracytoplasmic sperm injection (ICSI) through logistic regression. Mean stress scores after CBT treatment in infertile patients were significantly decreased compared with before (2.7±0.62 vs. 3.5±0.62; P<0.05) (273), which was confirmed by a previous web-based CBT study (274). Researchers have identified CBT as an effective treatment for enhancing the quality of life (vasomotor symptoms, physical, psychosocial, sexual and total domain) of perimenopausal patients (275-280). A randomized controlled trial conducted with 50 patients with POI revealed mean scores of stress [adjusted mean difference (AMD), −10.97; 95%CI, −11.64, −10.29; P<0.001], state anxiety (AMD, −14.76; 95%CI, −15.77, −13.74; P<0.001), trait anxiety (AMD, −14.41; 95%CI, −15.47, −13.74; P<0.001) and depression (AMD, −7.44; 95%CI, −8.41, −6.46; P<0.001) were significantly lower in the CBT group compared with control group receiving routine care (281), suggesting healthcare providers should employ this method to enhance mental health in these patients.
A study allocated 16 patients with FHA equally to CBT group or an observation group for 20 weeks; 6/8 patients in the CBT group experienced recovery of ovarian activity compared with 2/88 in the observation group (87.5 vs. 25.0%, χ2=7.14) (282). Besides promoting ovulation, CBT has also been shown to lower cortisol levels in patients with FHA (283). CBT not only reinstates ovarian function but also impacts metabolic processes (284). A pilot study on 12 adolescents with PCOS demonstrated that CBT notably decreases weight (104±26 vs. 93±18 kg, P<0.05) and improves depression score (17±3 vs. 9.6±2, P<0.01) (285). Another study involving 15 overweight/obese adolescents with PCOS revealed that weekly CBT + lifestyle modification sessions (LS) for 8 weeks significantly led to weight loss, enhanced quality of life and reduced depressive symptoms compared with those only receiving LS intervention (286).
Mindfulness-based stress reduction (MBSR)
MBSR, a strategy of lessening stress and anxiety through meditation, breathwork and body awareness, is a methodical mindfulness meditation plan that is progressively accessible in medical and healthcare environments to boost mental health and general wellbeing (287). MBSR has been demonstrated to decrease mental suffering and enhance life quality in patients with cancer, heart disease and depressions (288,289).
Individuals diagnosed with POF or POI often experience prolonged mood disturbance and other menopausal symptoms that may not be effectively alleviated by hormone therapy (15), indicating that MBSR may offer potential benefits. A randomized controlled trial including 197 symptomatic peri- and postmenopausal patients found that both MBSR and menopause education control (MEC) decreases total Greene Climacteric Scale score and symptom score reduction of anxiety and depression is significant in the MBSR compared with the MEC group (anxiety, 5.97±2.49 vs. 6.97±2.57, P=0.07; depression, 4.78±2.60 vs. 5.39±2.41, P=0.031) (290). In patients with POI, mindfulness practices have been shown to decrease scores of menopause-specific quality of life (compared with baseline, 48.32±4.96 vs. 95.6±9.77, P<0.0001; compared with control, 48.32±4.96 vs. 102.6±14.9, P<0.0001) and the frequency of hot flushes (6.74±6.34 vs. 23.4±13.9, P<0.0001) (291). MBSR improves emotional wellbeing and biological outcomes in infertile patients (292-294).
Music therapy
Previous research has shown that music therapy has the potential to decrease stress, improve quality of life and alleviate depression and anxiety (295). In a randomized-controlled study of 48 postmenopausal patients, music therapy significantly decreased BDI score (11.81±8.62 vs. 16.44±6.09, P=0.031) compared with control and the Menopause Rating Scale (MRS) total and sub-scale scores were significantly decreased when comparing before and after intervention (296).
In a study of 40 patients with perimenopause syndrome, music therapy was more likely to improve psychological and emotional symptoms compared with CBT (MRS total, 9.2 vs. 3.5, P=0.008; MRS-psychological, 6.5 vs. 0.9, P=0.004; Patient Health Questionnaire 9 score, 4.3 vs. 1.6, P=0.009) (297). A comprehensive meta-analysis indicated that music therapy decreases the anxiety (MD=−3.09, 95%CI, −5.57, −0.61, P=0.01) and pain score (MD=−2.93, 95%CI, −3.86, −2.00, P<0.0001) and increased the satisfaction score (MD=1.51, 95%CI, 0.40, 2.61, P=0.008) in infertile patients undergoing ART (298). While music therapy increases the clinical pregnancy rates, the difference is not statistically significant (RR=1.08, 95%CI, 0.94, 1.26, P=0.28) (298,299).
Other methods to manage stress
Alongside seeking advice from professional psychologists, individuals may opt for self-care. An evaluation of 166 patients undergoing first-time IVF through a randomized controlled prospective study, examining the effectiveness of a cognitive coping and relaxation intervention (CCRI) that could be self-administered indicated that patients who engaged in the CCRI demonstrated enhanced positive reappraisal coping, better Fertility Quality of Life score and reduced levels of anxiety (300). Another randomized controlled prospective pilot study explored the impact of an online version of the mind/body program (301), where patients assigned to the intervention group showed significant decreases in anxiety and depression, along with an increased likelihood of pregnancy.
Yoga has been shown to be a beneficial intervention for anxiety and depression, stress reduction and enhancement of the overall wellbeing among the general public (302). Furthermore, yoga can alleviate anxiety and depression in individuals undergoing IVF treatment and increase the ART success rate (303). Yoga can enhance the quality of life in relation to infertility, with a specific focus on diminishing adverse emotions and cognition linked to infertility (304). A separate study investigating yoga as a complementary approach for patients receiving IVF treatment yielded comparable results (305). Yoga alleviates the menopausal symptoms, anxiety and depression of menopausal patients and decreases the levels of FSH and LH (306,307).
Frederiksen et al (308) assessed advantages of expressive writing for individuals with from infertility; male and female patients exhibited a noticeable decrease in depressive symptoms. In addition, adhering to a balanced diet, leading a healthy lifestyle and managing work-life balance are potential strategies to alleviate stress and safeguard ovarian function (309).
Metabolic interventions for ovarian dysfunction
As aforementioned, the activation of stress-associated neuroendocrine systems causes metabolic imbalance in the body, and metabolic disorders lead to ovary dysfunction.
Caloric restriction (CR)
CR refers to a reduction in calorie consumption without eliminating essential nutrients (310). CR is hypothesized to extend the lifespan of rodents, yeast, worms, flies and primates by decreasing body weight and slowing aging process (311). CR has been shown to mitigate age-associated diseases such as neurodegeneration, hepatic steatosis and T2DM (312).
Notably, recent studies have shown that CR can protect ovarian function. CR decreases aneuploidy, chromosomal misalignment and meiotic spindle abnormality in oocytes in aged mice (313-315). CR can attenuate aging-associated decrease in chromosomal cohesion and protect the normal progress of meiosis (315). CR enhances ovarian estrogen sensitivity and inhibits ovulation, thereby extending reproductive lifespan (316,317). CR can also reduce primordial follicle activation and lead to increased ovarian Forkhead box O3 (Foxo3a) expression, activation of the SIRT1 signaling pathway and suppression of the mTOR signaling pathway, which may increase preservation of the ovarian primordial follicular reserve and delay menopause onset (318-322).
Obesity impairs ovarian function and obese patients are often encouraged to lose weight before conception to increase the chances of a successful pregnancy (323). However, the protective effect of CR on ovarian function lacks large-scale multicenter clinical trials and CR is a long-term dietary adjustment process that is difficult to maintain (324). Recently, there has been considerable interest in identifying drugs that can mimic the effects of CR. CR mimetics (CRMs) promote lifespan and sustain the health benefits of CR without requiring dietary restriction (325-329). CRMs have good clinical prospects because they do not require long-term dietary restriction and have better patient compliance.
Metformin
Metformin is a widely prescribed medication for management of T2DM (330) that reduces blood glucose by decreasing glucose absorption, enhancing peripheral glucose uptake and increasing insulin sensitivity (331). Metformin can decrease the risk of all-cause mortality and cardiovascular death in patients with T2DM (332). Moreover, metformin is also known for anti-aging (333) and anticancer (334) effects.
Metformin has been demonstrated to increase OR and protect ovarian function through regulatory mechanisms such as inhibiting the mTOR pathway, enhancing SIRT1 expression and decreasing oxidative stress damage (335,336). Furthermore, in aged mice, metformin decreases mitochondrial ROS via SIRT3-mediated acetylation of SOD2K68 in oocytes (337). For chemotherapy-induced ovarian damage, metformin may attenuate carboplatin-induced ovarian damage, potentially through its antioxidative effects (338). In addition, metformin decreases aging-related ovarian fibrosis. Metformin-responsive macrophage subpopulations appear in aged mice treated with metformin, which may decrease ovarian fibrosis by clearing fibroblasts that produce the senescence-associated secretory phenotype in aged ovaries (339). Therefore, metformin may serve as a promising drug to protect reproductive function.
Resveratrol
Resveratrol is a derivative of stilbene produced by >70 species of plants (340) in response to external adverse stimuli. Resveratrol has multiple protective effects in mammal models of T2DM, metabolic syndrome, cancer and neurodegenerative and cardiovascular diseases (341). For example, resveratrol increases SIRT1 levels and improves insulin resistance in a HFD rat model, indicating resveratrol has the potential to combat insulin resistance and maintain glucose metabolism homeostasis (342). Furthermore, resveratrol induces a CR-like transcriptional signature in mice and mimics metabolic changes of human CR (343).
In humans, resveratrol can improve the follicular microenvironment to improve IVF outcomes (344). Intraperitoneal injection of resveratrol during superovulation improves oocyte quality in both young and old mice, and increased the number of oocytes retrieved from old mice (345). Resveratrol improves fertility of reproductive age female mice, which may be achieved by activating SIRT1 and increasing the expression of Foxo3a (346-349). In vitro, resveratrol increased first polar body emission rates and improves oocyte mitochondrial function (350-352). In vitro, resveratrol protects granulosa cells by activating autophagy to resist oxidative stress (353,354). In conclusion, resveratrol is beneficial to ovarian function and protects oocytes.
7. Conclusion
Psychological stress is associated with poor health and impaired ovarian function (2,4). The present review summarizes the adverse effects of mental stress on ovarian function, which are associated with decreased IVF success rates and OR and the occurrence of POI, FHA and PCOS. Mental stress is involved in the pathogenesis of POI, FHA and POCS (15,17,61). However, more prospective studies are needed to explore the effect of psychological stress in initiation and progression of these diseases. Elevated levels of perceived stress, as well as SAA, could reduce OR (13,56,58). However, salivary cortisol, another biomarker used to measure psychological stress, has no association with fecundability (181). Cortisol exhibits a distinct circadian rhythm, with peak levels occurring in the morning and decreasing throughout the day; thus, it may be important to obtain multiple samples/day from a patient in order to investigate its effects on human health (355). Mental stress has an adverse effect on the outcome of IVF or ICSI, although some studies suggest that there is no association between them (48). To the best of our knowledge, no studies have reported that psychological stress has a positive effect on pregnancy outcomes. Anderheim et al (48) found no evidence that mental stress impairs IVF outcomes. However, Aimagambetova et al (36) found that higher STAI-S and STAI-T are negatively associated with clinical pregnancy outcomes. A potential reason for this discrepancy is that, when filling out psychological questionnaires, patients may not provide accurate assessments of distress. Different inclusion criteria, assessment tools for psychological status and study design may be additional reasons for the inconsistent results. The influence of stress on ART outcomes remains controversial, but, for infertile patients, psychological intervention can alleviate anxiety and depression, potentially resulting in notably improved pregnancy rates. Multi-center clinical trials with large sample sizes are necessary in future research. Under ideal conditions, prospective studies through puberty to perimenopause should regularly collect psychological questionnaires/scales, metabolic and ovarian function indexes to demonstrate causal mechanisms linking stress and ovarian dysfunction.
The present study summarizes animal models used to study the impact of mental stress on ovarian function, such as CUS, CRS and chronic cold stress, as well as evaluation methods. Mental stress affects the follicle and ovarian vascular function via the HPA, SAM and HPO axis and neurometabolic network, in which stress induces granulosa cell apoptosis by activating the MAPK, FAS and TNF-α pathway and increasing ROS, leading to overactivation of primordial follicles and decreasing the number of follicles by activating the PI3K/AKT pathway, as well as oocyte quality by downregulating BDNF, GDF9 and CCNB1, inhibiting the cAMP pathway, mitochondrial energy metabolism imbalance and inducing ROS accumulation. In addition, stress can lead to disorder of ovarian vascular structure and microenvironment by upregulating expression of ET-1 and ET-AR and downregulating expression of ET-BR, leading to a decline in ovarian function (Fig. 4).
Multiple studies have examined the impact of chronic stress on the neuroendocrine system over extended periods (156,356). In the existing studies, traditional biological stress markers (cortisol and α-amylase) have been widely used to assess stress levels of patients and experimental animals (10,58,85,181). However, due to the differences in susceptibility between individuals and evaluation methods, previous results are inconsistent. Obayashi (357) summarized advantages and weaknesses of different salivary stress markers in the assessment of stress, such as cortisol, α-amylase, chromogranin A and IgA and proposed optimization of the method of saliva collection in order to improve the consistency of stress levels. As Shah et al (358) noted, when exploring the association between psychological stress and ovarian function, a comprehensive assessment of stress levels should include physiological markers (such as heart rate variability, blood pressure and cytokine levels) and biochemical indicators (cortisol, catecholamines, copeptin, SAA, IL-6 and C-reactive protein levels), as well as individual subjective assessments (self-reported stress levels, perceived stress scale or psychometric evaluations). Neuroendocrine changes caused by mental stress lead to systemic inflammation, leading to an increase in systemic inflammatory markers and health issues such as cardiovascular disease, cognitive dysfunction and accelerated aging (359). Therefore, inflammatory biomarkers such as TNF-α, HIF-1α and asymmetric dimethylarginine (ADMA) may serve as biological indicators to measure mental stress and its impact on physical health. Combinations of different markers and approaches such as wearable device-based stress detection (360) may establish effective stress markers. However, its effects need more clinical research for verification.
Existing studies focus on the direct role of psychological stress on the onset of POI, FHA and PCOS (15,17,61). For example, most studies have explored the impact of PCOS on the mental stress or psychological-associated disease (361-363). Large-scale prospective studies with lager samples are needed to support whether mental stress causes PCOS. Furthermore, the existing evidence that mental stress impairs ovarian function through metabolic disturbances is indirect (207,236). cohort studies and animal experiments are required to determine whether mental stress directly affects ovarian function through metabolism-related diseases.
It is key to elucidate the specific molecular mechanism underlying the effect of on the ovary and provide psychological intervention strategies for patients with ovarian dysfunction, especially for those in need of ART intervention. Hence, psychological interventions (CBT, MBSR and music therapy) may be beneficial to reduce adverse influence of stress and improve ovarian function and reproductive outcomes. The present review may provide an alternative intervention strategy for improvement of ovarian dysfunction and infertility.
Acknowledgements
Not applicable.
Abbreviations
- IVF
in vitro fertilization
- AFC
antral follicle count
- AMH
anti-Müllerian hormone
- POI
premature ovarian insufficiency
- FHA
functional hypothalamic amenorrhea
- PCOS
polycystic ovary syndrome
- CUS
chronic unpredictable stress
- CRS
chronic restraint stress
- T2DM
type 2 diabetes mellitus
- NEM
neuroendocrine-metabolic
- HPO
hypothalamic-pituitary-ovarian
- LH
luteinizing hormone
- FSHR
follicle-stimulating hormone receptor
- ART
assisted reproductive technology
- BDI
Beck Depression Inventory
- STAI-T
Spielberger Trait Anxiety Inventory
- DOR
diminished ovarian reserve
- SAA
salivary α amylase
- FST
forced swimming test
- SPT
sucrose preference test
- GnRH
gonadotropin-releasing hormone
- BDNF
brain-derived neurotrophic factor
- GDF
growth differentiation factor
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- CRH-R
corticotropin-releasing hormone receptor
- HPA
hypothalamic-pituitary-adrenal
- GVBD
germinal vesicle breakdown
- CCNB1
cyclin B1
- SAM
sympathetic-adrenal-medullary
- StAR
steroidogenic acute regulatory
- ET-1
endothelin-1
- TST
tail suspension test
- OFT
open field test
- LDB
light-dark box
- EPM
elevated plus maze
- PVN
paraventricular nucleus
- AVP
arginine vasopressin
- IGF
insulin-like growth factor
- RFRP3
RFamide-related peptide-3
- KISS
kisspeptin
- AGE
advanced glycation end-product
- BMI
body mass index
- HFD
high-fat diet
- SIRT1
sirtuin 1
- POF
premature ovarian failure
- CBT
cognitive behavioral therapy
- AMD
adjusted mean difference
- MBSR
mindfulness-based stress reduction
- MEC
menopause education control
- MRS
Menopause Rating Scale
- CCRI
cognitive coping and relaxation intervention
- CRM
caloric restriction mimetic
Funding Statement
The present study was supported by the National Key Research and Development Program of China (grant nos. 2022YFC2704100 and 2021YFC2700402-02) and National Natural Science Foundation of China (grant no. 81902669).
Availability of data and materials
Not applicable.
Authors' contributions
YL and FF conceived and designed the study. YH and FF wrote the manuscript and constructed figures and tables. WuW, WM, WeW, WR and SW revised the manuscript. All the authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang S, Quan L, Chavarro JE, Slopen N, Kubzansky LD, Koenen KC, Kang JH, Weisskopf MG, Branch-Elliman W, Roberts AL. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post-COVID-19 conditions. JAMA Psychiatry. 2022;79:1081–1091. doi: 10.1001/jamapsychiatry.2022.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valsamakis G, Chrousos G, Mastorakos G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology. 2019;100:48–57. doi: 10.1016/j.psyneuen.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 3.Kivimäki M, Bartolomucci A, Kawachi I. The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. 2023;19:10–27. doi: 10.1038/s41574-022-00746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bala R, Singh V, Rajender S, Singh K. Environment, lifestyle, and female infertility. Reprod Sci. 2021;28:617–638. doi: 10.1007/s43032-020-00279-3. [DOI] [PubMed] [Google Scholar]
- 5.Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci. 2018;20:41–47. doi: 10.31887/DCNS.2018.20.1/klrooney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda E, Nomura K, Hiraike O, Sugimori H, Kinoshita A, Osuga Y. Domestic work stress and self-rated psychological health among women: A cross-sectional study in Japan. Environ Health Prev Med. 2019;24:75. doi: 10.1186/s12199-019-0833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Aungsuroch Y. Work stress, perceived social support, self-efficacy and burnout among Chinese registered nurses. J Nurs Manag. 2019;27:1445–1453. doi: 10.1111/jonm.12828. [DOI] [PubMed] [Google Scholar]
- 8.Bapayeva G, Aimagambetova G, Issanov A, Terzic S, Ukybassova T, Aldiyarova A, Utepova G, Daribay Z, Bekbossinova G, Balykov A, et al. The effect of stress, anxiety and depression on in vitro fertilization outcome in kazakhstani public clinical setting: A cross-sectional study. J Clin Med. 2021;10:937. doi: 10.3390/jcm10050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Ouyang N, Li R, Tuo P, Mai M, Wang W. The effects of anxiety and depression on in vitro fertilisation outcomes of infertile Chinese women. Psychol Health Med. 2017;22:37–43. doi: 10.1080/13548506.2016.1218031. [DOI] [PubMed] [Google Scholar]
- 10.Sallem A, Essoussi H, Mustapha HB, Zaouali M, Ajina M. Impact of psychological stress on the outcomes of assisted reproduction in Tunisian infertile women. Pan Afr Med J. 2021;40:250. doi: 10.11604/pamj.2021.40.250.32207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian transfer. Fertil Steril. 2001;76:675–687. doi: 10.1016/S0015-0282(01)02008-8. [DOI] [PubMed] [Google Scholar]
- 12.Buck Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, Schisterman EF, Pyper C. Stress reduces conception probabilities across the fertile window: Evidence in support of relaxation. Fertil Steril. 2011;95:2184–2189. doi: 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mínguez-Alarcón L, Williams PL, Souter I, Ford JB, Hauser R, Chavarro JE, Earth Study Team Perceived stress and markers of ovarian reserve among subfertile women. Reprod Biomed Online. 2023;46:956–964. doi: 10.1016/j.rbmo.2023.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golenbock SW, Wise LA, Lambert-Messerlian GM, Eklund EE, Harlow BL. Association between a history of depression and anti-müllerian hormone among late-reproductive aged women: The Harvard study of moods and cycles. Womens Midlife Health. 2020;6:9. doi: 10.1186/s40695-020-00056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allshouse AA, Semple AL, Santoro NF. Evidence for prolonged and unique amenorrhea-related symptoms in women with premature ovarian failure/primary ovarian insufficiency. Menopause. 2015;22:166–174. doi: 10.1097/GME.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 16.Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, Wexler T, Herzog DB, Klibanski A. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. J Clin Endocrinol Metab. 2009;94:4710–4716. doi: 10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 18.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Zhao F, Zhang Y, Wang W, Cao Q. Diminished ovarian reserve induced by chronic unpredictable stress in C57BL/6 mice. Gynecol Endocrinol. 2020;36:49–54. doi: 10.1080/09513590.2019.1631274. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Y, Xu J, Tao C, Lin Y, Lin X, Li K, Liu Q, Saiyin H, Hu S, Yao G, et al. Chronic stress induces meiotic arrest failure and ovarian reserve decline via the cAMP signaling pathway. Front Endocrinol (Lausanne) 2023;14:1177061. doi: 10.3389/fendo.2023.1177061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu M, Sun J, Wang Q, Zhang Q, Wei C, Lai D. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS One. 2018;13:e0194894. doi: 10.1371/journal.pone.0194894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole L, Hackett RA. Diabetes distress: The psychological burden of living with diabetes. Lancet Diabetes Endocrinol. 2024;12:439–441. doi: 10.1016/S2213-8587(24)00126-8. [DOI] [PubMed] [Google Scholar]
- 23.Bartoskova Polcrova A, Dalecka A, Szabo D, Gonzalez Rivas JP, Bobak M, Pikhart H. Social and environmental stressors of cardiometabolic health. Sci Rep. 2024;14:14179. doi: 10.1038/s41598-024-64847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schliep KC, Mumford SL, Vladutiu CJ, Ahrens KA, Perkins NJ, Sjaarda LA, Kissell KA, Prasad A, Wactawski-Wende J, Schisterman EF. Perceived stress, reproductive hormones, and ovulatory function: A prospective cohort study. Epidemiology. 2015;26:177–184. doi: 10.1097/EDE.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: The Harvard study of moods and cycles. Arch Gen Psychiatry. 2003;60:29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Albert KM, Newhouse PA. Estrogen, stress, and depression: cognitive and biological interactions. Annu Rev Clin Psychol. 2019;15:399–423. doi: 10.1146/annurev-clinpsy-050718-095557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yatsenko SA, Rajkovic A. Genetics of human female infertility. Biol Reprod. 2019;101:549–566. doi: 10.1093/biolre/ioz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthiesen SMS, Frederiksen Y, Ingerslev HJ, Zachariae R. Stress, distress and outcome of assisted reproductive technology (ART): A meta-analysis. Hum Reprod. 2011;26:2763–2776. doi: 10.1093/humrep/der246. [DOI] [PubMed] [Google Scholar]
- 29.Massey AJ, Campbell B, Raine-Fenning N, Aujla N, Vedhara K. The association of physiological cortisol and IVF treatment outcomes: A systematic review. Reprod Med Biol. 2014;13:161–176. doi: 10.1007/s12522-014-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen TH, Chang SP, Tsai CF, Juang KD. Prevalence of depressive and anxiety disorders in an assisted reproductive technique clinic. Hum Reprod. 2004;19:2313–2318. doi: 10.1093/humrep/deh414. [DOI] [PubMed] [Google Scholar]
- 31.Volgsten H, Skoog Svanberg A, Ekselius L, Lundkvist O, Sundström Poromaa I. Prevalence of psychiatric disorders in infertile women and men undergoing in vitro fertilization treatment. Hum Reprod. 2008;23:2056–2063. doi: 10.1093/humrep/den154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasch LA, Holley SR, Bleil ME, Shehab D, Katz PP, Adler NE. Addressing the needs of fertility treatment patients and their partners: Are they informed of and do they receive mental health services? Fertil Steril. 2016;106:209–215.e2. doi: 10.1016/j.fertnstert.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Lakatos E, Szigeti JF, Ujma PP, Sexty R, Balog P. Anxiety and depression among infertile women: A cross-sectional survey from Hungary. BMC Womens Health. 2017;17:48. doi: 10.1186/s12905-017-0410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment: Psychological and neurohormonal assessment. J Assist Reprod Genet. 2013;30:35–41. doi: 10.1007/s10815-012-9904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terzioglu F, Turk R, Yucel C, Dilbaz S, Cinar O, Karahalil B. The effect of anxiety and depression scores of couples who underwent assisted reproductive techniques on the pregnancy outcomes. Afr Health Sci. 2016;16:441–450. doi: 10.4314/ahs.v16i2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aimagambetova G, Issanov A, Terzic S, Bapayeva G, Ukybassova T, Baikoshkarova S, Aldiyarova A, Shauyen F, Terzic M. The effect of psychological distress on IVF outcomes: Reality or speculations? PLoS One. 2020;15:e0242024. doi: 10.1371/journal.pone.0242024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner K, Reynolds-May MF, Zitek EM, Tisdale RL, Carlisle AB, Westphal LM. Stress and anxiety scores in first and repeat IVF cycles: A pilot study. PLoS One. 2013;8:e63743. doi: 10.1371/journal.pone.0063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quant HS, Zapantis A, Nihsen M, Bevilacqua K, Jindal S, Pal L. Reproductive implications of psychological distress for couples undergoing IVF. J Assist Reprod Genet. 2013;30:1451–1458. doi: 10.1007/s10815-013-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csemiczky G, Landgren BM, Collins A. The influence of stress and state anxiety on the outcome of IVF-treatment: Psychological and endocrinological assessment of Swedish women entering IVF-treatment: Stress and state anxiety on outcome of IVF-treatment. Acta Obstet Gynecol Scand. 2000;79:113–118. doi: 10.1034/j.1600-0412.2000.079002113.x. [DOI] [PubMed] [Google Scholar]
- 40.Boivin J, Griffiths E, Venetis CA. Emotional distress in infertile women and failure of assisted reproductive technologies: Meta-analysis of prospective psychosocial studies. BMJ. 2011;342:d223. doi: 10.1136/bmj.d223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasch LA, Gregorich SE, Katz PK, Millstein SG, Nachtigall RD, Bleil ME, Adler NE. Psychological distress and in vitro fertilization outcome. Fertil Steril. 2012;98:459–464. doi: 10.1016/j.fertnstert.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chai Y, Li Q, Wang Y, Niu B, Chen H, Fan T, Ke X, Zou H. Cortisol dysregulation in anxiety infertile women and the influence on IVF treatment outcome. Front Endocrinol (Lausanne) 2023;14:1107765. doi: 10.3389/fendo.2023.1107765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cesta CE, Johansson ALV, Hreinsson J, Rodriguez-Wallberg KA, Olofsson JI, Holte J, Wramsby H, Wramsby M, Cnattingius S, Skalkidou A, Nyman Iliadou A. A prospective investigation of perceived stress infertility-related stress and cortisol levels in women undergoing in vitro fertilization: Influence on embryo quality and clinical pregnancy rate. Acta Obstet Gynecol Scand. 2018;97:258–268. doi: 10.1111/aogs.13280. [DOI] [PubMed] [Google Scholar]
- 44.Mirzaasgari H, Momeni F, Pourshahbaz A, Keshavarzi F, Hatami M. The relationship between coping strategies and infertility self-efficacy with pregnancy outcomes of women undergoing in vitro fertilization: A prospective cohort study. Int J Reprod Biomed. 2022;20:539–548. doi: 10.18502/ijrm.v20i7.11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng M, Wen M, Jiang T, Jiang Y, Lv H, Chen T, Ling X, Li H, Meng Q, Huang B, et al. Stress, anxiety, and depression in infertile couples are not associated with a first IVF or ICSI treatment outcome. BMC Pregnancy Childbirth. 2021;21:725. doi: 10.1186/s12884-021-04202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, Stanford JB, Porucznik CA, Christensen K, Schliep KC. Daily perceived stress and time to pregnancy: A prospective cohort study of women trying to conceive. Psychoneuroendocrinology. 2019;110:104446. doi: 10.1016/j.psyneuen.2019.104446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lynch CD, Sundaram R, Buck Louis GM, Lum KJ, Pyper C. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil Steril. 2012;98:453–458. doi: 10.1016/j.fertnstert.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderheim L, Holter H, Bergh C, Möller A. Does psychological stress affect the outcome of in vitro fertilization? Hum Reprod. 2005;20:2969–2975. doi: 10.1093/humrep/dei219. [DOI] [PubMed] [Google Scholar]
- 49.Milad MP, Klock SC, Moses S, Chatterton R. Stress and anxiety do not result in pregnancy wastage. Hum Reprod. 1998;13:2296–2300. doi: 10.1093/humrep/13.8.2296. [DOI] [PubMed] [Google Scholar]
- 50.Butts CD, Bloom MS, Frye CA, Walf AA, Parsons PJ, Steuerwald AJ, Ilonze C, Fujimoto VY. Urine cortisol concentration as a biomarker of stress is unrelated to IVF outcomes in women and men. J Assist Reprod Genet. 2014;31:1647–1653. doi: 10.1007/s10815-014-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuenkel CA, Gompel A. Primary ovarian insufficiency. N Engl J Med. 2023;388:154–163. doi: 10.1056/NEJMcp2116488. [DOI] [PubMed] [Google Scholar]
- 52.Sun J, Fan Y, Guo Y, Pan H, Zhang C, Mao G, Huang Y, Li B, Gu T, Wang L, et al. Chronic and cumulative adverse life events in women with primary ovarian insufficiency: An exploratory qualitative study. Front Endocrinol (Lausanne) 2022;13:856044. doi: 10.3389/fendo.2022.856044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu YP, Fu JC, Hong ZL, Zeng DF, Guo CQ, Li P, Wu JX. Psychological stressors involved in the pathogenesis of premature ovarian insufficiency and potential intervention measures. Gynecol Endocrinol. 2024;40:2360085. doi: 10.1080/09513590.2024.2360085. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt PJ, Luff JA, Haq NA, Vanderhoof VH, Koziol DE, Calis KA, Rubinow DR, Nelson LM. Depression in women with spontaneous 46, XX primary ovarian insufficiency. J Clin Endocrinol Metab. 2011;96:E278–E287. doi: 10.1210/jc.2010-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in womenin transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 56.Bleil ME, Adler NE, Pasch LA, Sternfeld B, Gregorich SE, Rosen MP, Cedars MI. Psychological stress and reproductive aging among pre-menopausal women. Hum Reprod. 2012;27:2720–2728. doi: 10.1093/humrep/des214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bleil ME, Adler NE, Pasch LA, Sternfeld B, Gregorich SE, Rosen MP, Cedars MI. Depressive symptomatology, psychological stress, and ovarian reserve: A role for psychological factors in ovarian aging? Menopause. 2012;19:1176–1185. doi: 10.1097/gme.0b013e31825540d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong YZ, Zhou FJ, Sun YP. Psychological stress is related to a decrease of serum anti-müllerian hormone level in infertile women. Reprod Biol Endocrinol. 2017;15:51. doi: 10.1186/s12958-017-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lania A, Gianotti L, Gagliardi I, Bondanelli M, Vena W, Ambrosio MR. Functional hypothalamic and drug-induced amenorrhea: An overview. J Endocrinol Invest. 2019;42:1001–1010. doi: 10.1007/s40618-019-01013-w. [DOI] [PubMed] [Google Scholar]
- 60.Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, Murad MH, Santoro NF, Warren MP. Functional hypothalamic amenorrhea: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:1413–1439. doi: 10.1210/jc.2017-00131. [DOI] [PubMed] [Google Scholar]
- 61.Bomba M, Gambera A, Bonini L, Peroni M, Neri F, Scagliola P, Nacinovich R. Endocrine profiles and neuropsychologic correlates of functional hypothalamic amenorrhea in adolescents. Fertil Steril. 2007;87:876–885. doi: 10.1016/j.fertnstert.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–316. doi: 10.1016/S0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 63.Dundon CM, Rellini AH, Tonani S, Santamaria V, Nappi R. Mood disorders and sexual functioning in women with functional hypothalamic amenorrhea. Fertil Steril. 2010;94:2239–2243. doi: 10.1016/j.fertnstert.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 64.Cai J, Zhang Y, Wang Y, Li S, Wang L, Zheng J, Jiang Y, Dong Y, Zhou H, Hu Y, et al. High thyroid stimulating hormone level is associated with hyperandrogenism in euthyroid polycystic ovary syndrome (PCOS) women, independent of age, BMI, and thyroid autoimmunity: A cross-sectional analysis. Front Endocrinol (Lausanne) 2019;10:222. doi: 10.3389/fendo.2019.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang AY, Lalia AZ, Jenkins GD, Dutta T, Carter RE, Singh RJ, Nair KS. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. 2017;71:52–63. doi: 10.1016/j.metabol.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farrell K, Antoni MH. Insulin resistance, obesity, inflammation, and depression in polycystic ovary syndrome: Biobehavioral mechanisms and interventions. Fertil Steril. 2010;94:1565–1574. doi: 10.1016/j.fertnstert.2010.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hillman SC, Bryce C, Caleyachetty R, Dale J. Women's experiences of diagnosis and management of polycystic ovary syndrome: A mixed-methods study in general practice. Br J Gen Pract. 2020;70:e322–e329. doi: 10.3399/bjgp20X708881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 69.Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schürmann A, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol. 2018;14:140–162. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 70.Seemann S, Zohles F, Lupp A. Comprehensive comparison of three different animal models for systemic inflammation. J Biomed Sci. 2017;24:60. doi: 10.1186/s12929-017-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheon DJ, Orsulic S. Mouse models of cancer. Annu Rev Pathol. 2011;6:95–119. doi: 10.1146/annurev.pathol.3.121806.154244. [DOI] [PubMed] [Google Scholar]
- 72.Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–116. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 73.Du Preez A, Onorato D, Eiben I, Musaelyan K, Egeland M, Zunszain PA, Fernandes C, Thuret S, Pariante CM. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav Immun. 2021;91:24–47. doi: 10.1016/j.bbi.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Khan AR, Geiger L, Wiborg O, Czéh B. Stress-induced morphological, cellular and molecular changes in the brain-lessons learned from the chronic mild stress model of depression. Cells. 2020;9:1026. doi: 10.3390/cells9041026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiang Y, Jiang L, Gou J, Sun Y, Zhang D, Xin X, Song Z, Huang J. Chronic unpredictable mild stress-induced mouse ovarian insufficiency by interrupting lipid homeostasis in the ovary. Front Cell Dev Biol. 2022;10:933674. doi: 10.3389/fcell.2022.933674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu XY, Chen HH, Zhang N, Ding MX, Qiu YE, Pan XM, Fang YS, Lin YP, Zheng Q, Wang WQ. Effects of chronic unpredictable mild stress on ovarian reserve in female rats: Feasibility analysis of a rat model of premature ovarian failure. Mol Med Rep. 2018;18:532–540. doi: 10.3892/mmr.2018.8989. [DOI] [PubMed] [Google Scholar]
- 77.Ma J, Wang L, Yang D, Luo J, Gao J, Wang J, Guo H, Li J, Wang F, Wu J, Hu R. Chronic stress causes ovarian fibrosis to impair female fertility in mice. Cell Signal. 2024;122:111334. doi: 10.1016/j.cellsig.2024.111334. [DOI] [PubMed] [Google Scholar]
- 78.Ding SM, Shi LG, Xing F, Cui SS, Cheng HR, Liu Y, Ji DM, Liang D, Cao YX, Liu YJ. Melatonin protects against mitochondrial dyshomeostasis and ovarian damage caused by chronic unpredictable mild stress through the eIF2α-AFT4 signaling pathway in mice. Reprod Sci. 2024;31:3191–3201. doi: 10.1007/s43032-024-01647-z. [DOI] [PubMed] [Google Scholar]
- 79.Fu X, Zheng Q, Zhang N, Ding M, Pan X, Wang W, Chen H. CUMS promotes the development of premature ovarian insufficiency mediated by nerve growth factor and its receptor in rats. BioMed Res Int. 2020;2020:1946853. doi: 10.1155/2020/1946853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu HX, Lin SX, Gong Y, Huo ZX, Zhao CY, Zhu HM, Xi SY. Chaiyu-dixian formula exerts protective effects on ovarian follicular abnormal development in chronic unpredictable mild stress (CUMS) rat model. Front Pharmacol. 2020;11:245. doi: 10.3389/fphar.2020.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao X, Ma R, Zhang X, Cheng R, Jiang N, Guo M, Rong B, Liu Y, Chen M, Feng W, Xia T. Reduced growth capacity of preimplantation mouse embryos in chronic unpredictable stress model. Mol Reprod Dev. 2021;88:80–95. doi: 10.1002/mrd.23439. [DOI] [PubMed] [Google Scholar]
- 82.Wu LM, Hu MH, Tong XH, Han H, Shen N, Jin RT, Wang W, Zhou GX, He GP, Liu YS. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries: relationship to oocytes developmental potential. PLoS One. 2012;7:e52331. doi: 10.1371/journal.pone.0052331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li XH, Pang HQ, Qin L, Jin S, Zeng X, Bai Y, Li SW. HSP70 overexpression may play a protective role in the mouse embryos stimulated by CUMS. Reprod Biol Endocrinol. 2015;13:125. doi: 10.1186/s12958-015-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu LM, Liu YS, Tong XH, Shen N, Jin RT, Han H, Hu MH, Wang W, Zhou GX. Inhibition of follicular development induced by chronic unpredictable stress is associated with growth and differentiation factor 9 and gonadotropin in mice. Biol Reprod. 2012;86:121. doi: 10.1095/biolreprod.111.093468. [DOI] [PubMed] [Google Scholar]
- 85.Sun J, Guo Y, Fan Y, Wang Q, Zhang Q, Lai D. Decreased expression of IDH1 by chronic unpredictable stress suppresses proliferation and accelerates senescence of granulosa cells through ROS activated MAPK signaling pathways. Free Radic Biol Med. 2021;169:122–136. doi: 10.1016/j.freeradbiomed.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 86.Du Y, Ma J, Wu T, Li F, Pan J, Du L, Zhang M, Diao X, Wu R. Downgrading breast imaging reporting and data system categories in ultrasound using strain elastography and computer-aided diagnosis system: A multicenter, prospective study. Br J Radiol. 2024;97:1653–1660. doi: 10.1093/bjr/tqae136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kala M, Nivsarkar M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen Comp Endocrinol. 2016;225:117–124. doi: 10.1016/j.ygcen.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 88.Gao Y, Chen F, Kong QQ, Ning SF, Yuan HJ, Lian HY, Luo MJ, Tan JH. Stresses on female mice impair oocyte developmental potential: Effects of stress severity and duration on oocytes at the growing follicle stage. Reprod Sci. 2016;23:1148–1157. doi: 10.1177/1933719116630416. [DOI] [PubMed] [Google Scholar]
- 89.Scott K, Phan TT, Boukelmoune N, Heijnen CJ, Dantzer R. Chronic restraint stress impairs voluntary wheel running but has no effect on food-motivated behavior in mice. Brain Behav Immun. 2023;107:319–329. doi: 10.1016/j.bbi.2022.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen H, Fu S, Li X, Shi M, Qian J, Zhao S, Yuan P, Ding L, Xia X, Zheng JC. Microglial glutaminase 1 mediates chronic restraint stress-induced depression-like behaviors and synaptic damages. Signal Transduct Target Ther. 2023;8:452. doi: 10.1038/s41392-023-01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 92.Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Sun J, Guo Y, Zhang Q, Bu S, Li B, Wang Q, Lai D. Chronic restraint stress disturbs meiotic resumption through APC/C-mediated cyclin B1 excessive degradation in mouse oocytes. Cell Cycle. 2018;17:1591–1601. doi: 10.1080/15384101.2018.1471316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang SY, Wang JZ, Li JJ, Wei DL, Sui HS, Zhang ZH, Zhou P, Tan JH. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod. 2011;84:672–681. doi: 10.1095/biolreprod.110.087890. [DOI] [PubMed] [Google Scholar]
- 95.Geraghty AC, Muroy SE, Zhao S, Bentley GE, Kriegsfeld LJ, Kaufer D. Knockdown of hypothalamic RFRP3 prevents chronic stress-induced infertility and embryo resorption. Life. 2015;4:e04316. doi: 10.7554/eLife.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liang B, Wei DL, Cheng YN, Yuan HJ, Lin J, Cui XZ, Luo MJ, Tan JH. Restraint stress impairs oocyte developmental potential in mice: Role of CRH-induced apoptosis of ovarian cells. Biol Reprod. 2013;89:64. doi: 10.1095/biolreprod.113.110619. [DOI] [PubMed] [Google Scholar]
- 97.Li CY, Li ZB, Kong QQ, Han X, Xiao B, Li X, Chang ZL, Tan JH. Restraint-induced corticotrophin-releasing hormone elevation triggers apoptosis of ovarian cells and impairs oocyte competence via activation of the Fas/FasL system. Biol Reprod. 2018;99:828–837. doi: 10.1093/biolre/ioy091. [DOI] [PubMed] [Google Scholar]
- 98.Huang Y, Liu Q, Huang G, Wen J, Chen G. Hypothalamic kisspeptin neurons regulates energy metabolism and reproduction under chronic stress. Front Endocrinol (Lausanne) 2022;13:844397. doi: 10.3389/fendo.2022.844397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sugino N, Nakamura Y, Okuno N, Shimamura K, Teyama T, Ishimatsu M, Kato H. Effects of restraint stress on luteal function in rats during mid-pregnancy. J Reprod Fertil. 1994;101:23–26. doi: 10.1530/jrf.0.1010023. [DOI] [PubMed] [Google Scholar]
- 100.Zhou P, Lian HY, Cui W, Wei DL, Li Q, Liu YX, Liu XY, Tan JH. Maternal-restraint stress increases oocyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly checkpoint proteins in mice. Biol Reprod. 2012;86:83. doi: 10.1095/biolreprod.111.095281. [DOI] [PubMed] [Google Scholar]
- 101.Lian HY, Gao Y, Jiao GZ, Sun MJ, Wu XF, Wang TY, Li H, Tan JH. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction. 2013;146:559–568. doi: 10.1530/REP-13-0268. [DOI] [PubMed] [Google Scholar]
- 102.Higaki S, Kishi M, Koyama K, Nagano M, Katagiri S, Takada T, Takahashi Y. Early germinal vesicle breakdown is a predictor of high preimplantation developmental competent oocytes in mice. Zygote. 2017;25:41–48. doi: 10.1017/S0967199416000290. [DOI] [PubMed] [Google Scholar]
- 103.Zhao XY, Li ZB, Yuan HJ, Han X, Wu JS, Feng XY, Zhang M, Tan JH. Restraint stress and elevation of corticotrophin-releasing hormone in female mice impair oocyte competence through activation of the tumour necrosis factor α (TNF-α) system. Reprod Fertil Dev. 2020;32:862–872. doi: 10.1071/RD20002. [DOI] [PubMed] [Google Scholar]
- 104.Guo Y, Sun J, Bu S, Li B, Zhang Q, Wang Q, Lai D. Melatonin protects against chronic stress-induced oxidative meiotic defects in mice MII oocytes by regulating SIRT1. Cell Cycle. 2020;19:1677–1695. doi: 10.1080/15384101.2020.1767403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen RR, Wang J, Zhang M, Kong QQ, Sun GY, Jin CH, Tan JH. Restraint stress of female mice during oocyte development facilitates oocyte postovulatory aging. Aging (Albany NY) 2022;14:9186–9199. doi: 10.18632/aging.204400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dorfman M, Arancibia S, Fiedler JL, Lara HE. Chronic intermittent cold stress activates ovarian sympathetic nerves and modifies ovarian follicular development in the rat. Biol Reprod. 2003;68:2038–2043. doi: 10.1095/biolreprod.102.008318. [DOI] [PubMed] [Google Scholar]
- 107.Bernuci MP, Szawka RE, Helena CVV, Leite CM, Lara HE, Anselmo-Franci JA. Locus coeruleus mediates cold stress-induced polycystic ovary in rats. Endocrinology. 2008;149:2907–2916. doi: 10.1210/en.2007-1254. [DOI] [PubMed] [Google Scholar]
- 108.Barra R, Cruz G, Mayerhofer A, Paredes A, Lara HE. Maternal sympathetic stress impairs follicular development and puberty of the offspring. Reproduction. 2014;148:137–145. doi: 10.1530/REP-14-0150. [DOI] [PubMed] [Google Scholar]
- 109.Bernuci MP, Leite CM, Barros P, Kalil B, Leoni GB, Del Bianco-Borges B, Franci CR, Szawka RE, Lara HE, Anselmo-Franci JA. Transitory activation of the central and ovarian norepinephrine systems during cold stress-induced polycystic ovary in rats. J Neuroendocrinol. 2013;25:23–33. doi: 10.1111/j.1365-2826.2012.02373.x. [DOI] [PubMed] [Google Scholar]
- 110.Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: A test of Selye's doctrine of nonspecificity. Am J Physiol. 1998;275:R1247–R1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- 111.Riquelme R, Ruz F, Mayerhofer A, Lara HE. Role of ovarian sympathetic nerves and cholinergic local system during cold stress. J Endocrinol. 2019;242:115–124. doi: 10.1530/JOE-19-0125. [DOI] [PubMed] [Google Scholar]
- 112.Casillas F, Betancourt M, Juárez-Rojas L, Ducolomb Y, López A, Ávila-Quintero A, Zamora J, Ommati MM, Retana-Márquez S. Chronic stress detrimentally affects in vivo maturation in rat oocytes and oocyte viability at all phases of the estrous cycle. Animals (Basel) 2021;11:2478. doi: 10.3390/ani11092478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang D, Cheng X, Fang H, Ren Y, Li X, Ren W, Xue B, Yang C. Effect of cold stress on ovarian & uterine microcirculation in rats and the role of endothelin system. Reprod Biol Endocrinol. 2020;18:29. doi: 10.1186/s12958-020-00584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Retana-Márquez S, Juárez-Rojas L, Ávila-Quintero A, Rojas-Maya S, Perera G, Casillas F, Betancourt M, Gómez-Quiroz L. Neuroendocrine disruption is associated to infertility in chronically stressed female rats. Reprod Biol. 2020;20:474–483. doi: 10.1016/j.repbio.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 115.Keremu A, Yaoliwasi A, Tuerhong M, Kadeer N, Heyi, Yiming A, Yilike X. Research on the establishment of chronic stress-induced premature ovarian failure the rat model and effects of Chinese medicine Muniziqi treatment. Mol Reprod Dev. 2019;86:175–186. doi: 10.1002/mrd.23092. [DOI] [PubMed] [Google Scholar]
- 116.Ernsberger U, Deller T, Rohrer H. The sympathies of the body: Functional organization and neuronal differentiation in the peripheral sympathetic nervous system. Cell Tissue Res. 2021;386:455–475. doi: 10.1007/s00441-021-03548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Espinoza JA, Navarrete MI, Linares R, Chaparro-Ortega A, Ramírez DA, Rosas G, Vieyra E, Domínguez R, Morales-Ledesma L. Effects of chronic exposure to cold stress on ovarian functions in prepubertal rats. Reprod Biol. 2023;23:100756. doi: 10.1016/j.repbio.2023.100756. [DOI] [PubMed] [Google Scholar]
- 118.Divyashree S, Yajurvedi HN. Long-term chronic stress exposure induces PCO phenotype in rat. Reproduction. 2016;152:765–774. doi: 10.1530/REP-16-0404. [DOI] [PubMed] [Google Scholar]
- 119.Bunsueb S, Lapyuneyong N, Tongpan S, Arun S, Iamsaard S. Chronic stress increases the tyrosine phosphorylation in female reproductive organs: An experimental study. Int J Reprod Biomed. 2021;19:87–96. doi: 10.18502/ijrm.v19i1.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim J, You S. High housing density-induced chronic stress diminishes ovarian reserve via granulosa cell apoptosis by angiotensin II overexpression in mice. Int J Mol Sci. 2022;23:8614. doi: 10.3390/ijms23158614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xi W, Mao H, Cui Z, Yao H, Shi R, Gao Y. Scream sound-induced chronic psychological stress results in diminished ovarian reserve in adult female rat. Endocrinology. 2022;163:bqac042. doi: 10.1210/endocr/bqac042. [DOI] [PubMed] [Google Scholar]
- 122.Ghatebi M, Zavareh S, Lashkarbolouki T, Elahdadi Salmani M. Implications from early life stress on the development of mouse ovarian follicles: Focus on oxidative stress. J Obstet Gynaecol Res. 2019;45:1506–1514. doi: 10.1111/jog.14007. [DOI] [PubMed] [Google Scholar]
- 123.Yuan J, Yu Y, Liu D, Sun Y. Associations between distinct dimensions of early life adversity and accelerated reproductive strategy among middle-aged women in China. Am J Obstet Gynecol. 2022;226:104.e1–104.e14. doi: 10.1016/j.ajog.2021.07.033. [DOI] [PubMed] [Google Scholar]
- 124.Mishra GD, Cooper R, Tom SE, Kuh D. Early life circumstances and their impact on menarche and menopause. Womens Health (Lond) 2009;5:175–190. doi: 10.2217/17455057.5.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang N, Huang Y, Wen J, Su Q, Huang Y, Cai L, Lin W, Zong L, Huang H, Qian X, et al. Early life exposure to famine and reproductive aging among Chinese women. Menopause. 2019;26:463–468. doi: 10.1097/GME.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 126.Liu YX, Cheng YN, Miao YL, Wei DL, Zhao LH, Luo MJ, Tan JH. Psychological stress on female mice diminishes the developmental potential of oocytes: A study using the predatory stress model. PLoS One. 2012;7:e48083. doi: 10.1371/journal.pone.0048083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Onalan E, Erbay B, Buran İK, Erol D, Tektemur A, Kuloglu T, Ozercan IH. Effects and mechanism of AP39 on ovarian functions in rats exposed to cisplatin and chronic immobilization stress. J Menopausal Med. 2024;30:104–119. doi: 10.6118/jmm.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan HJ, Li ZB, Zhao XY, Sun GY, Wang GL, Zhao YQ, Zhang M, Tan JH. Glucocorticoids impair oocyte competence and trigger apoptosis of ovarian cells via activating the TNF-α system. Reproduction. 2020;160:129–140. doi: 10.1530/REP-20-0025. [DOI] [PubMed] [Google Scholar]
- 129.Becker M, Pinhasov A, Ornoy A. Animal models of depression: What can they teach Us about the human disease? Diagnostics (Basel) 2021;11:123. doi: 10.3390/diagnostics11010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kendler KS. The genealogy of major depression: Symptoms and signs of melancholia from 1880 to 1900. Mol Psychiatry. 2017;22:1539–1553. doi: 10.1038/mp.2017.148. [DOI] [PubMed] [Google Scholar]
- 131.Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 132.Primo MJ, Fonseca-Rodrigues D, Almeida A, Teixeira PM, Pinto-Ribeiro F. Sucrose preference test: A systematic review of protocols for the assessment of anhedonia in rodents. Eur Neuropsychopharmacol. 2023;77:80–92. doi: 10.1016/j.euroneuro.2023.08.496. [DOI] [PubMed] [Google Scholar]
- 133.Verharen JPH, de Jong JW, Zhu Y, Lammel S. A computational analysis of mouse behavior in the sucrose preference test. Nat Commun. 2023;14:2419. doi: 10.1038/s41467-023-38028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 135.Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 136.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- 137.Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103. doi: 10.1007/978-1-4939-8994-2_9. [DOI] [PubMed] [Google Scholar]
- 138.Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015:e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/S0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 140.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kraeuter AK, Guest PC, Sarnyai Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol Biol. 2019;1916:69–74. doi: 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- 142.Schrader AJ, Taylor RM, Lowery-Gionta EG, Moore NLT. Repeated elevated plus maze trials as a measure for tracking within-subjects behavioral performance in rats (Rattus norvegicus) PLoS One. 2018;13:e0207804. doi: 10.1371/journal.pone.0207804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pietropaolo S. Mood and anxiety-related phenotypes in mice: Characterization using behavioral tests-Edited by T. D. Gould. Genes Brain Behav. 2010;9:544–544. doi: 10.1111/j.1601-183X.2010.00592.x. [DOI] [Google Scholar]
- 144.Bae J, Lynch CD, Kim S, Sundaram R, Sapra KJ, Buck Louis GM. Preconception stress and the secondary sex ratio in a population-based preconception cohort. Fertil Steril. 2017;107:714–722. doi: 10.1016/j.fertnstert.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34:271–292. vii. doi: 10.1016/j.ecl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 147.McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron. 2015;87:605–620. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agorastos A, Chrousos GP. The neuroendocrinology of stress: The stress-related continuum of chronic disease development. Mol Psychiatry. 2022;27:502–513. doi: 10.1038/s41380-021-01224-9. [DOI] [PubMed] [Google Scholar]
- 149.Yoo ES, Yu J, Sohn JW. Neuroendocrine control of appetite and metabolism. Exp Mol Med. 2021;53:505–516. doi: 10.1038/s12276-021-00597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Di Comite G, Grazia Sabbadini M, Corti A, Rovere-Querini P, Manfredi AA. Conversation galante: How the immune and the neuroendocrine systems talk to each other. Autoimmun Rev. 2007;7:23–29. doi: 10.1016/j.autrev.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 151.Smeenk JMJ, Verhaak CM, Vingerhoets AJJM, Sweep CGJ, Merkus JMWM, Willemsen SJ, van Minnen A, Straatman H, Braat DD. Stress and outcome success in IVF: The role of self-reports and endocrine variables. Hum Reprod. 2005;20:991–996. doi: 10.1093/humrep/deh739. [DOI] [PubMed] [Google Scholar]
- 152.Hjollund NHI, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, Ernst E, Giwercman A, Skakkebaek NE, Olsen J. Distress and reduced fertility: A follow-up study of first-pregnancy planners. Fertil Steril. 1999;72:47–53. doi: 10.1016/S0015-0282(99)00186-7. [DOI] [PubMed] [Google Scholar]
- 153.Ates S, Yesil G, Sevket O, Molla T, Yildiz S. Comparison of metabolic profile and abdominal fat distribution between karyotypically normal women with premature ovarian insufficiency and age matched controls. Maturitas. 2014;79:306–310. doi: 10.1016/j.maturitas.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 154.Huang Y, Lv Y, Qi T, Luo Z, Meng X, Ying Q, Li D, Li C, Lan Y, Chu K, et al. Metabolic profile of women with premature ovarian insufficiency compared with that of age-matched healthy controls. Maturitas. 2021;148:33–39. doi: 10.1016/j.maturitas.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 155.Ferin M. Clinical review 105: Stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- 156.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Ann Intern Med. 1998;129:229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 157.Harlow CR, Fahy UM, Talbot WM, Wardle PG, Hull MG. Stress and stress-related hormones during in-vitro fertilization treatment. Hum Reprod. 1996;11:274–279. doi: 10.1093/HUMREP/11.2.274. [DOI] [PubMed] [Google Scholar]
- 158.Demyttenaere K, Nijs P, Evers-Kiebooms G, Koninckx PR. Coping and the ineffectiveness of coping influence the outcome of in vitro fertilization through stress responses. Psychoneuroendocrinology. 1992;17:655–665. doi: 10.1016/0306-4530(92)90024-2. [DOI] [PubMed] [Google Scholar]
- 159.Pal L, Bevilacqua K, Santoro NF. Chronic psychosocial stressors are detrimental to ovarian reserve: A study of infertile women. J Psychosom Obstet Gynecol. 2010;31:130–139. doi: 10.3109/0167482X.2010.485258. [DOI] [PubMed] [Google Scholar]
- 160.Andersen CY. Effect of glucocorticoids on spontaneous and follicle-stimulating hormone induced oocyte maturation in mouse oocytes during culture. J Steroid Biochem Mol Biol. 2003;85:423–427. doi: 10.1016/S0960-0760(03)00190-0. [DOI] [PubMed] [Google Scholar]
- 161.Yuan HJ, Han X, He N, Wang GL, Gong S, Lin J, Gao M, Tan JH. Glucocorticoids impair oocyte developmental potential by triggering apoptosis of ovarian cells via activating the Fas system. Sci Rep. 2016;6:24036. doi: 10.1038/srep24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: An update. J Reprod Immunol. 2010;85:33–39. doi: 10.1016/j.jri.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 163.Rivier C, Vale W. Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology. 1984;114:914–921. doi: 10.1210/endo-114-3-914. [DOI] [PubMed] [Google Scholar]
- 164.Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHβ-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26:1716–1731. doi: 10.1210/me.2011-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Luo E, Stephens SBZ, Chaing S, Munaganuru N, Kauffman AS, Breen KM. Corticosterone blocks ovarian cyclicity and the LH surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157:1187–1199. doi: 10.1210/en.2015-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Roozendaal MM, Swarts JJM, Van Maanen JC, Wiegant VM, Mattheij JA. Inhibition of the LH surge in cyclic rats by stress is not mediated by opioids. Life Sci. 1997;60:735–742. doi: 10.1016/S0024-3205(96)00652-2. [DOI] [PubMed] [Google Scholar]
- 167.Horowitz MA, Zunszain PA. Neuroimmune and neuroendocrine abnormalities in depression: Two sides of the same coin. Ann N Y Acad Sci. 2015;1351:68–79. doi: 10.1111/nyas.12781. [DOI] [PubMed] [Google Scholar]
- 168.Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–1030. doi: 10.1016/S0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- 169.Deady LD, Sun J. A follicle rupture assay reveals an essential role for follicular adrenergic signaling in drosophila ovulation. PLoS Genet. 2015;11:e1005604. doi: 10.1371/journal.pgen.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aguado LI. Role of the central and peripheral nervous system in the ovarian function. Microsc Res Tech. 2002;59:462–473. doi: 10.1002/jemt.10232. [DOI] [PubMed] [Google Scholar]
- 171.Lara HE, Dorfman M, Venegas M, Luza SM, Luna SL, Mayerhofer A, Guimaraes MA, Rosa E Silva AA, Ramírez VD. Changes in sympathetic nerve activity of the mammalian ovary during a normal estrous cycle and in polycystic ovary syndrome: Studies on norepinephrine release. Microsc Res Tech. 2002;59:495–502. doi: 10.1002/jemt.10229. [DOI] [PubMed] [Google Scholar]
- 172.Morales-Ledesma L, Linares R, Rosas G, Morán C, Chavira R, Cárdenas M, Domínguez R. Unilateral sectioning of the superior ovarian nerve of rats with polycystic ovarian syndrome restores ovulation in the innervated ovary. Reprod Biol Endocrinol. 2010;8:99. doi: 10.1186/1477-7827-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dissen GA, Romero C, Paredes A, Ojeda SR. Neurotrophic control of ovarian development. Microsc Res Tech. 2002;59:509–515. doi: 10.1002/jemt.10227. [DOI] [PubMed] [Google Scholar]
- 174.Uchida S, Kagitani F. Autonomic nervous regulation of ovarian function by noxious somatic afferent stimulation. J Physiol Sci. 2015;65:1–9. doi: 10.1007/s12576-014-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Acuña E, Fornes R, Fernandois D, Garrido MP, Greiner M, Lara HE, Paredes AH. Increases in norepinephrine release and ovarian cyst formation during ageing in the rat. Reprod Biol Endocrinol. 2009;7:64. doi: 10.1186/1477-7827-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Casillas F, Flores-González A, Juárez-Rojas L, López A, Betancourt M, Casas E, Bahena I, Bonilla E, Retana-Márquez S. Chronic stress decreases fertility parameters in female rats. Syst Biol Reprod Med. 2023;69:234–244. doi: 10.1080/19396368.2023.2171822. [DOI] [PubMed] [Google Scholar]
- 177.Fernandois D, Cruz G, Na EK, Lara HE, Paredes AH. Kisspeptin level in the aging ovary is regulated by the sympathetic nervous system. J Endocrinol. 2017;232:97–105. doi: 10.1530/JOE-16-0181. [DOI] [PubMed] [Google Scholar]
- 178.Saller S, Merz-Lange J, Raffael S, Hecht S, Pavlik R, Thaler C, Berg D, Berg U, Kunz L, Mayerhofer A. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology. 2012;153:1472–1483. doi: 10.1210/en.2011-1769. [DOI] [PubMed] [Google Scholar]
- 179.Zhang L, Gao J, Cui S. miR-21 is involved in norepinephrine-mediated rat granulosa cell apoptosis by targeting SMAD7. J Mol Endocrinol. 2017;58:199–210. doi: 10.1530/JME-16-0248. [DOI] [PubMed] [Google Scholar]
- 180.Li XH, Ma YG, Geng LH, Qin L, Hu H, Li SW. Baseline psychological stress and ovarian norepinephrine levels negatively affect the outcome of in vitro fertilisation. Gynecol Endocrinol. 2011;27:139–143. doi: 10.3109/09513590.2010.501871. [DOI] [PubMed] [Google Scholar]
- 181.Lynch CD, Sundaram R, Maisog JM, Sweeney AM, Louis GMB. Preconception stress increases the risk of infertility: Results from a couple-based prospective cohort study-the LIFE study. Hum Reprod. 2014;29:1067–1075. doi: 10.1093/humrep/deu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hackett RA, Steptoe A. Type 2 diabetes mellitus and psychological stress-a modifiable risk factor. Nat Rev Endocrinol. 2017;13:547–560. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 183.Afrisham R, Paknejad M, Soliemanifar O, Sadegh-Nejadi S, Meshkani R, Ashtary-Larky D. The influence of psychological stress on the initiation and progression of diabetes and cancer. Int J Endocrinol Metab. 2019;17:e67400. doi: 10.5812/ijem.67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Joseph JJ, Golden SH. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017;1391:20–34. doi: 10.1111/nyas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Balkan F, Cetin N, Usluogullari CA, Unal OK, Usluogullari B. Evaluation of the ovarian reserve function in patients with metabolic syndrome in relation to healthy controls and different age groups. J Ovarian Res. 2014;7:63. doi: 10.1186/1757-2215-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Isik S, Ozcan HN, Ozuguz U, Tutuncu YA, Berker D, Alimli AG, Akbaba G, Karademir MA, Guler S. Evaluation of ovarian reserve based on hormonal parameters, ovarian volume, and antral follicle count in women with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97:261–269. doi: 10.1210/jc.2011-1923. [DOI] [PubMed] [Google Scholar]
- 187.Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 188.Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid Med Cell Longev. 2020;2020:8609213. doi: 10.1155/2020/8609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: A systematic review. J Psychosom Res. 2002;53:1053–1060. doi: 10.1016/S0022-3999(02)00417-8. [DOI] [PubMed] [Google Scholar]
- 190.Razzoli M, Pearson C, Crow S, Bartolomucci A. Stress, overeating, and obesity: Insights from human studies and preclinical models. Neurosci Biobehav Rev. 2017;76:154–162. doi: 10.1016/j.neubiorev.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Codner E, Merino PM, Tena-Sempere M. Female reproduction and type 1 diabetes: From mechanisms to clinical findings. Hum Reprod Update. 2012;18:568–585. doi: 10.1093/humupd/dms024. [DOI] [PubMed] [Google Scholar]
- 192.Nandi A, Poretsky L. Diabetes and the female reproductive system. Endocrinol Metab Clin North Am. 2013;42:915–946. doi: 10.1016/j.ecl.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 193.Dri M, Klinger FG, De Felici M. The ovarian reserve as target of insulin/IGF and ROS in metabolic disorder-dependent ovarian dysfunctions. Reprod Fertil. 2021;2:R103–R112. doi: 10.1530/RAF-21-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Barbieri RL, Makris A, Ryan KJ. Effects of insulin on steroidogenesis in cultured porcine ovarian theca. Fertil Steril. 1983;40:237–241. doi: 10.1016/S0015-0282(16)47243-2. [DOI] [PubMed] [Google Scholar]
- 195.Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62:904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- 196.Cara JF, Rosenfield RL. Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–739. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 197.Hudish LI, Reusch JE, Sussel L. β Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest. 2019;129:4001–4008. doi: 10.1172/JCI129188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Escobar-Morreale HF, Roldán B, Barrio R, Alonso M, Sancho J, De La Calle H, García-Robles R. High prevalence of the polycystic ovary syndrome and hirsutism in women with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2000;85:4182–4187. doi: 10.1210/jcem.85.11.6931. [DOI] [PubMed] [Google Scholar]
- 199.Diamanti-Kandarakis E, Piperi C, Patsouris E, Korkolopoulou P, Panidis D, Pawelczyk L, Papavassiliou AG, Duleba AJ. Immunohistochemical localization of advanced glycation end-products (AGEs) and their receptor (RAGE) in polycystic and normal ovaries. Histochem Cell Biol. 2007;127:581–589. doi: 10.1007/s00418-006-0265-3. [DOI] [PubMed] [Google Scholar]
- 200.Secomandi L, Borghesan M, Velarde M, Demaria M. The role of cellular senescence in female reproductive aging and the potential for senotherapeutic interventions. Hum Reprod Update. 2022;28:172–189. doi: 10.1093/humupd/dmab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: Relevance to mechanism of anovulation. J Clin Endocrinol Metab. 1998;83:3984–3991. doi: 10.1210/jcem.83.11.5232. [DOI] [PubMed] [Google Scholar]
- 202.Lee J, Lee HC, Kim SY, Cho GJ, Woodruff TK. Poorly-controlled type 1 diabetes mellitus impairs LH-LHCGR signaling in the ovaries and decreases female fertility in mice. Yonsei Med J. 2019;60:667–678. doi: 10.3349/ymj.2019.60.7.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zargar AH, Gupta VK, Wani AI, Masoodi SR, Bashir MI, Laway BA, Ganie MA, Salahuddin M. Prevalence of ultrasonography proved polycystic ovaries in North Indian women with type 2 diabetes mellitus. Reprod Biol Endocrinol. 2005;3:35. doi: 10.1186/1477-7827-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Peppard HR, Marfori J, Iuorno MJ, Nestler JE. Prevalence of polycystic ovary syndrome among premenopausal women with type 2 diabetes. Diabetes Care. 2001;24:1050–1052. doi: 10.2337/diacare.24.6.1050. [DOI] [PubMed] [Google Scholar]
- 205.Khalil N, Sutton-Tyrrell K, Strotmeyer ES, Greendale GA, Vuga M, Selzer F, Crandall CJ, Cauley JA. Menopausal bone changes and incident fractures in diabetic women: A cohort study. Osteoporos Int. 2011;22:1367–1376. doi: 10.1007/s00198-010-1357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sekhar TVDS, Medarametla S, Rahman A, Adapa SS. Early menopause in type 2 diabetes-A study from a South Indian tertiary care centre. J Clin Diagn Res. 2015;9:OC08–OC10. doi: 10.7860/JCDR/2015/14181.6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qin X, Du J, He R, Li Y, Zhu Q, Li Y, Li H, Liang X. Adverse effects of type 2 diabetes mellitus on ovarian reserve and pregnancy outcomes during the assisted reproductive technology process. Front Endocrinol (Lausanne) 2023;14:1274327. doi: 10.3389/fendo.2023.1274327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 209.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E, Heart and Estrogen/progestin Replacement Study Glycemic effects of postmenopausal hormone therapy: The heart and estrogen/progestin replacement study. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1–9. doi: 10.7326/0003-4819-138-1-200301070-00005. [DOI] [PubMed] [Google Scholar]
- 210.Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV, Women's Health Initiative Investigators Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the women's health initiative hormone trial. Diabetologia. 2004;47:1175–1187. doi: 10.1007/s00125-004-1448-x. [DOI] [PubMed] [Google Scholar]
- 211.Wagner JD, Thomas MJ, Williams JK, Zhang L, Greaves KA, Cefalu WT. Insulin sensitivity and cardiovascular risk factors in ovariectomized monkeys with estradiol alone or combined with nomegestrol acetate. J Clin Endocrinol Metab. 1998;83:896–901. doi: 10.1210/jcem.83.3.4628. [DOI] [PubMed] [Google Scholar]
- 212.Kumagai S, Holmäng A, Björntorp P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol Scand. 1993;149:91–97. doi: 10.1111/j.1748-1716.1993.tb09596.x. [DOI] [PubMed] [Google Scholar]
- 213.Cantave CY, Ruttle PL, Coté SM, Lupien SJ, Geoffroy MC, Vitaro F, Brendgen M, Tremblay R, Boivin M, Ouellet-Morin I. Body mass index across development and adolescent hair cortisol: The role of persistence, variability, and timing of exposure. Int J Obes (Lond) 2024 Oct 4; doi: 10.1038/s41366-024-01640-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 214.Christakoudi S, Asimakopoulos AG, Riboli E, Tsilidis KK. Links between the genetic determinants of morning plasma cortisol and body shape: A two-sample Mendelian randomisation study. Sci Rep. 2024;14:3230. doi: 10.1038/s41598-024-53727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kühnel A, Hagenberg J, Knauer-Arloth J, Ködel M, Czisch M, Sämann PG, BeCOME working group. Binder EB, Kroemer NB. Stress-induced brain responses are associated with BMI in women. Commun Biol. 2023;6:1031. doi: 10.1038/s42003-023-05396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lin YC, Lin CY, Saffari M, Tsai MC, Chang YH, Strong C, Chen JK, Hsieh YP, Yang YN, Latner JD. Weight stigma is associated with body mass index among college students in Taiwan: The mediated role of internalized weight stigma. BMC Psychol. 2023;11:365. doi: 10.1186/s40359-023-01414-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Fardet L, Fève B. Systemic glucocorticoid therapy: A review of its metabolic and cardiovascular adverse events. Drugs. 2014;74:1731–1745. doi: 10.1007/s40265-014-0282-9. [DOI] [PubMed] [Google Scholar]
- 218.Santosa A, Rosengren A, Ramasundarahettige C, Rangarajan S, Gulec S, Chifamba J, Lear SA, Poirier P, Yeates KE, Yusuf R, et al. Psychosocial risk factors and cardiovascular disease and death in a population-based cohort from 21 low-, middle-, and high-income countries. JAMA Netw Open. 2021;4:e2138920. doi: 10.1001/jamanetworkopen.2021.38920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ponti F, Santoro A, Mercatelli D, Gasperini C, Conte M, Martucci M, Sangiorgi L, Franceschi C, Bazzocchi A. Aging and imaging assessment of body composition: From fat to facts. Front Endocrinol (Lausanne) 2020;10:861. doi: 10.3389/fendo.2019.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van Der Valk ES, Savas M, Van Rossum EFC. Stress and obesity: Are there more susceptible individuals? Curr Obes Rep. 2018;7:193–203. doi: 10.1007/s13679-018-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen X, Xiao Z, Cai Y, Huang L, Chen C. Hypothalamic mechanisms of obesity-associated disturbance of hypothalamic-pituitary-ovarian axis. Trends Endocrinol Metab. 2022;33:206–217. doi: 10.1016/j.tem.2021.12.004. [DOI] [PubMed] [Google Scholar]
- 222.Morimoto A, Rose RD, Smith KM, Dinh DT, Umehara T, Winstanley YE, Shibahara H, Russell DL, Robker RL. Granulosa cell metabolism at ovulation correlates with oocyte competence and is disrupted by obesity and aging. Hum Reprod. 2024;39:2053–2066. doi: 10.1093/humrep/deae154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Schon SB, Cabre HE, Redman LM. The impact of obesity on reproductive health and metabolism in reproductive-age females. Fertil Steril. 2024;122:194–203. doi: 10.1016/j.fertnstert.2024.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Martinuzzi K, Ryan S, Luna M, Copperman AB. Elevated body mass index (BMI) does not adversely affect in vitro fertilization outcome in young women. J Assist Reprod Genet. 2008;25:169–175. doi: 10.1007/s10815-008-9213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bernardi LA, Carnethon MR, de Chavez PJ, Ikhena DE, Neff LM, Baird DD, Marsh EE. Relationship between obesity and anti-Müllerian hormone in reproductive-aged African American women. Obesity (Silver Spring) 2017;25:229–235. doi: 10.1002/oby.21681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Moy V, Jindal S, Lieman H, Buyuk E. Obesity adversely affects serum anti-müllerian hormone (AMH) levels in Caucasian women. J Assist Reprod Genet. 2015;32:1305–1311. doi: 10.1007/s10815-015-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LCL, Strauss JF., III Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87:101–106. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 228.Wang Y, Wu L, Yang Z, Xu R, Duan Y, Lin J, Cui X, Fan C, Zhou Y, Bao W, et al. Association of body mass index with serum anti-Müllerian hormone and inhibin B levels among 8323 women attending a reproductive medical center: A cross-sectional study. Endocrine. 2022;75:284–292. doi: 10.1007/s12020-021-02839-2. [DOI] [PubMed] [Google Scholar]
- 229.Douchi T, Kuwahata R, Yamamoto S, Oki T, Yamasaki H, Nagata Y. Relationship of upper body obesity to menstrual disorders. Acta Obstet Gynecol Scand. 2002;81:147–150. doi: 10.1034/j.1600-0412.2002.810210.x. [DOI] [PubMed] [Google Scholar]
- 230.Rittmaster RS, Deshwal N, Lehman L. The role of adrenal hyperandrogenism, insulin resistance, and obesity in the pathogenesis of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1993;76:1295–1300. doi: 10.1210/jcem.76.5.8388405. [DOI] [PubMed] [Google Scholar]
- 231.Di Berardino C, Peserico A, Capacchietti G, Zappacosta A, Bernabò N, Russo V, Mauro A, El Khatib M, Gonnella F, Konstantinidou F, et al. High-fat diet and female fertility across lifespan: A comparative lesson from mammal models. Nutrients. 2022;14:4341. doi: 10.3390/nu14204341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang N, Luo LL, Xu JJ, Xu MY, Zhang XM, Zhou XL, Liu WJ, Fu YC. Obesity accelerates ovarian follicle development and follicle loss in rats. Metabolism. 2014;63:94–103. doi: 10.1016/j.metabol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 233.Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. doi: 10.1210/en.2010-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, Schedl T, Moley KH. High fat diet induced developmental defects in the mouse: Oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7:e49217. doi: 10.1371/journal.pone.0049217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Reynolds KA, Boudoures AL, Chi MMY, Wang Q, Moley KH. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27:716–724. doi: 10.1071/RD14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Machtinger R, Combelles CMH, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod. 2012;27:3198–3207. doi: 10.1093/humrep/des308. [DOI] [PubMed] [Google Scholar]
- 237.Kloos J, Perez J, Weinerman R. Increased body mass index is negatively associated with ovarian reserve as measured by anti-Müllerian hormone in patients with polycystic ovarian syndrome. Clin Obes. 2024;29:e12638. doi: 10.1111/cob.12638. [DOI] [PubMed] [Google Scholar]
- 238.Beroukhim G, Esencan E, Seifer DB. Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: A comprehensive review. Reprod Biol Endocrinol. 2022;20:16. doi: 10.1186/s12958-022-00889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yan J, Zhang X, Zhu K, Yu M, Liu Q, De Felici M, Zhang T, Wang J, Shen W. Sleep deprivation causes gut dysbiosis impacting on systemic metabolomics leading to premature ovarian insufficiency in adolescent mice. Theranostics. 2024;14:3760–3776. doi: 10.7150/thno.95197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Weng L, Hong H, Zhang Q, Xiao C, Zhang Q, Wang Q, Huang J, Lai D. Sleep deprivation triggers the excessive activation of ovarian primordial follicles via β2 adrenergic receptor signaling. Adv Sci (Weinh) 2024;11:e2402393. doi: 10.1002/advs.202402393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Andersen ML, Alvarenga TAF, Guindalini C, Perry JC, Silva A, Zager A, Tufik S. Paradoxical sleep deprivation influences sexual behavior in female rats. J Sex Med. 2009;6:2162–2172. doi: 10.1111/j.1743-6109.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- 242.Koehl M, Battle S, Meerlo P. Sex differences in sleep: The response to sleep deprivation and restraint stress in mice. Sleep. 2006;29:1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- 243.Yang CK, Tsai HD, Wu CH, Cho CL. The role of glucocorticoids in ovarian development of sleep deprived rats. Taiwan J Obstet Gynecol. 2019;58:122–127. doi: 10.1016/j.tjog.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 244.Yang CK, Wu RSC, Wu CH, Lin TRY, Tsai HD. Sleep deprivation enhances peripheral serotonin secretion to regulate the large follicle steroidogenesis of rats. Taiwan J Obstet Gynecol. 2015;54:260–265. doi: 10.1016/j.tjog.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 245.Stock D, Knight JA, Raboud J, Cotterchio M, Strohmaier S, Willett W, Eliassen AH, Rosner B, Hankinson SE, Schernhammer E. Rotating night shift work and menopausal age. Hum Reprod. 2019;34:539–548. doi: 10.1093/humrep/dey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: Under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 247.Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin as a free radical scavenger in the ovarian follicle. Endocr J. 2013;60:1–13. doi: 10.1507/endocrj.EJ12-0263. [DOI] [PubMed] [Google Scholar]
- 248.Lahiri DK, Ge Y, Sharman EH, Bondy SC. Age-related changes in serum melatonin in mice: Higher levels of combined melatonin and 6-hydroxymelatonin sulfate in the cerebral cortex than serum, heart, liver and kidney tissues. J Pineal Res. 2004;36:217–223. doi: 10.1111/j.1600-079X.2004.00120.x. [DOI] [PubMed] [Google Scholar]
- 249.Tanabe M, Tamura H, Taketani T, Okada M, Lee L, Tamura I, Maekawa R, Asada H, Yamagata Y, Sugino N. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J Reprod Dev. 2015;61:35–41. doi: 10.1262/jrd.2014-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shen M, Cao Y, Jiang Y, Wei Y, Liu H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: Implication of an antioxidation-independent mechanism. Redox Biol. 2018;18:138–157. doi: 10.1016/j.redox.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Zhai B, Li X, Zhao Z, Cao Y, Liu X, Liu Z, Ma H, Lu W. Melatonin protects the apoptosis of sheep granulosa cells by suppressing oxidative stress via MAP3K8 and FOS pathway. Genes (Basel) 2023;14:1067. doi: 10.3390/genes14051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xu G, Dong Y, Wang Z, Ding H, Wang J, Zhao J, Liu H, Lv W. Melatonin attenuates oxidative stress-induced apoptosis of bovine ovarian granulosa cells by promoting mitophagy via SIRT1/FoxO1 signaling pathway. Int J Mol Sci. 2023;24:12854. doi: 10.3390/ijms241612854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res. 2008;44:280–287. doi: 10.1111/j.1600-079X.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 254.Zhang H, Li C, Wen D, Li R, Lu S, Xu R, Tang Y, Sun Y, Zhao X, Pan M, Ma B. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022;49:102215. doi: 10.1016/j.redox.2021.102215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lord T, Nixon B, Jones KT, Aitken RJ. Melatonin prevents postovulatory oocyte aging in the mouse and extends the window for optimal fertilization in vitro. Biol Reprod. 2013;88:67. doi: 10.1095/biolreprod.112.106450. [DOI] [PubMed] [Google Scholar]
- 256.Zhang M, Lu Y, Chen Y, Zhang Y, Xiong B. Insufficiency of melatonin in follicular fluid is a reversible cause for advanced maternal age-related aneuploidy in oocytes. Redox Biol. 2020;28:101327. doi: 10.1016/j.redox.2019.101327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yang D, Mu Y, Wang J, Zou W, Zou H, Yang H, Zhang C, Fan Y, Zhang H, Zhang H, et al. Melatonin enhances the developmental potential of immature oocytes from older reproductive-aged women by improving mitochondrial function. Heliyon. 2023;9:e19366. doi: 10.1016/j.heliyon.2023.e19366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Li C, He X, Huang Z, Han L, Wu X, Li L, Xin Y, Ge J, Sha J, Yin Z, Wang Q. Melatonin ameliorates the advanced maternal age-associated meiotic defects in oocytes through the SIRT2-dependent H4K16 deacetylation pathway. Aging (Albany NY) 2020;12:1610–1623. doi: 10.18632/aging.102703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Takasaki A, Nakamura Y, Tamura H, Shimamura K, Morioka H. Melatonin as a new drug for improving oocyte quality. Reprod Med Biol. 2004;2:139–144. doi: 10.1111/j.1447-0578.2003.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Baden MY, Hu FB, Vetter C, Schernhammer E, Redline S, Huang T. Sleep duration patterns in early to middle adulthood and subsequent risk of type 2 diabetes in women. Diabetes Care. 2020;43:1219–1226. doi: 10.2337/dc19-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Rooney KL, Domar AD. The impact of stress on fertility treatment. Curr Opin Obstet Gynecol. 2016;28:198–201. doi: 10.1097/GCO.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 262.Ramezanzadeh F, Noorbala AA, Abedinia N, Rahimi Forooshani A, Naghizadeh MM. Psychiatric intervention improved pregnancy rates in infertile couples. Malays J Med Sci. 2011;18:16–24. [PMC free article] [PubMed] [Google Scholar]
- 263.Rock J, Tietze C, McLaughlin HB. Effect of adoption on infertility. Fertil Steril. 1965;16:305–312. doi: 10.1016/S0015-0282(16)35583-2. [DOI] [PubMed] [Google Scholar]
- 264.Weir WC, Weir DR. Adoption and subsequent conceptions. Fertil Steril. 1966;17:283–288. doi: 10.1016/S0015-0282(16)35897-6. [DOI] [PubMed] [Google Scholar]
- 265.Nakao M, Shirotsuki K, Sugaya N. Cognitive-behavioral therapy for management of mental health and stress-related disorders: Recent advances in techniques and technologies. Biopsychosoc Med. 2021;15:16. doi: 10.1186/s13030-021-00219-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hoffman SG, Smits JAJ. Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–632. doi: 10.4088/JCP.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 268.Olatunji BO, Davis ML, Powers MB, Smits JAJ. Cognitive-behavioral therapy for obsessive-compulsive disorder: A meta-analysis of treatment outcome and moderators. J Psychiatr Res. 2013;47:33–41. doi: 10.1016/j.jpsychires.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 269.Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- 270.Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: A review of meta-analyses. Cogn Ther Res. 2012;36:427–440. doi: 10.1007/s10608-012-9476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Golshani F, Hasanpour S, Mirghafourvand M, Esmaeilpour K. Effect of cognitive behavioral therapy-based counseling on perceived stress in pregnant women with history of primary infertility: A controlled randomized clinical trial. BMC Psychiatry. 2021;21:278. doi: 10.1186/s12888-021-03283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Domar AD, Rooney KL, Wiegand B, Orav EJ, Alper MM, Berger BM, Nikolovski J. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertil Steril. 2011;95:2269–2273. doi: 10.1016/j.fertnstert.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 273.Faramarzi M, Pasha H, Esmailzadeh S, Kheirkhah F, Heidary S, Afshar Z. The effect of the cognitive behavioral therapy and pharmacotherapy on infertility stress: A randomized controlled trial. Int J Fertil Steril. 2013;7:199–206. [PMC free article] [PubMed] [Google Scholar]
- 274.Sexton MB, Byrd MR, O'Donohue WT, Jacobs NN. Web-based treatment for infertility-related psychological distress. Arch Womens Ment Health. 2010;13:347–358. doi: 10.1007/s00737-009-0142-x. [DOI] [PubMed] [Google Scholar]
- 275.John JB, Chellaiyan DVG, Gupta S, Nithyanandham R. How effective the mindfulness-based cognitive behavioral therapy on quality of life in women with menopause. J Midlife Health. 2022;13:169–174. doi: 10.4103/jmh.jmh_178_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Green SM, Furtado M. Cognitive behavioral therapy for sexual concerns during perimenopause: A four session study protocol. Front Glob Womens Health. 2021;2:744748. doi: 10.3389/fgwh.2021.744748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Green SM, Donegan E, Frey BN, Fedorkow DM, Key BL, Streiner DL, McCabe RE. Cognitive behavior therapy for menopausal symptoms (CBT-Meno): A randomized controlled trial. Menopause. 2019;26:972–980. doi: 10.1097/GME.0000000000001363. [DOI] [PubMed] [Google Scholar]
- 278.Green SM, Haber E, McCabe RE, Soares CN. Cognitive-behavioral group treatment for menopausal symptoms: A pilot study. Arch Womens Ment Health. 2013;16:325–332. doi: 10.1007/s00737-013-0339-x. [DOI] [PubMed] [Google Scholar]
- 279.Reddy NV, Omkarappa DB. Cognitive-behavioral therapy for depression among menopausal woman: A randomized controlled trial. J Fam Med Prim Care. 2019;8:1002–1006. doi: 10.4103/jfmpc.jfmpc_396_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Donegan E, Frey BN, McCabe RE, Streiner DL, Fedorkow DM, Furtado M, Green SM. Impact of the CBT-Meno protocol on menopause-specific beliefs, dysfunctional attitudes, and coping behaviors. Menopause. 2022;29:963–972. doi: 10.1097/GME.0000000000002003. [DOI] [PubMed] [Google Scholar]
- 281.Shabani F, Farvareshi M, Hamdi K, Sadeghzadeh Oskouei B, Montazeri M, Mirghafourvand M. The effect of cognitive-behavioral therapy on stress and anxiety of women with premature ovarian insufficiency: A randomized controlled trial. Post Reprod Health. 2022;28:211–221. doi: 10.1177/20533691221136309. [DOI] [PubMed] [Google Scholar]
- 282.Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–981. doi: 10.1016/S0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- 283.Michopoulos V, Mancini F, Loucks TL, Berga SL. Neuroendocrine recovery initiated by cognitive behavioral therapy in women with functional hypothalamic amenorrhea: A randomized, controlled trial. Fertil Steril. 2013;99:2084–2091.e1. doi: 10.1016/j.fertnstert.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sockalingam S, Leung SE, Ma C, Tomlinson G, Hawa R, Wnuk S, Jackson T, Urbach D, Okrainec A, Brown J, et al. Efficacy of telephone-based cognitive behavioral therapy for weight loss, disordered eating, and psychological distress after bariatric surgery: A randomized clinical trial. JAMA Netw Open. 2023;6:e2327099. doi: 10.1001/jamanetworkopen.2023.27099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rofey DL, Szigethy EM, Noll RB, Dahl RE, Lobst E, Arslanian SA. Cognitive-behavioral therapy for physical and emotional disturbances in adolescents with polycystic ovary syndrome: A pilot study. J Pediatr Psychol. 2009;34:156–163. doi: 10.1093/jpepsy/jsn057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Cooney LG, Milman LW, Hantsoo L, Kornfield S, Sammel MD, Allison KC, Epperson CN, Dokras A. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: A pilot randomized clinical trial. Fertil Steril. 2018;110:161–171.e1. doi: 10.1016/j.fertnstert.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300:1350–1352. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- 288.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57:35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 289.Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: Variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68:29–36. doi: 10.1016/j.jpsychores.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 290.Wong C, Yip BHK, Gao T, Lam KYY, Woo DMS, Yip ALK, Chin CY, Tang WPY, Choy MMT, Tsang KWK, et al. Mindfulness-based stress reduction (MBSR) or psychoeducation for the reduction of menopausal symptoms: A randomized, controlled clinical trial. Sci Rep. 2018;8:6609. doi: 10.1038/s41598-018-24945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Pyri F, Abedi P, Maraghi E, Jefreh M. The effect of mindfulness on quality of life among women with premature ovarian insufficiency: A randomized clinical trial. J Midlife Health. 2021;12:116–121. doi: 10.4103/jmh.JMH_66_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Majeed MH, Ali AA, Sudak DM. Mindfulness-based interventions for chronic pain: Evidence and applications. Asian J Psychiatry. 2018;32:79–83. doi: 10.1016/j.ajp.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 293.Kalhori F, Masoumi SZ, Shamsaei F, Mohammadi Y, Yavangi M. Effect of mindfulness-based group counseling on depression in infertile women: Randomized clinical trial study. Int J Fertil Steril. 2020;14:10–6. doi: 10.22074/ijfs.2020.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Galhardo A, Cunha M, Pinto-Gouveia J. A 7-year follow-up study of the mindfulness-based program for infertility: Are there long-term effects? Clin Psychol Psychother. 2019;26:409–417. doi: 10.1002/cpp.2362. [DOI] [PubMed] [Google Scholar]
- 295.Aalbers S, Fusar-Poli L, Freeman RE, Spreen M, Ket JC, Vink AC, Maratos A, Crawford M, Chen XJ, Gold C. Music therapy for depression. Cochrane Database Syst Rev. 2017;11:CD004517. doi: 10.1002/14651858.CD004517.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Koçak DY, Varişoğlu Y. The effect of music therapy on menopausal symptoms and depression: A randomized-controlled study. Menopause. 2022;29:545–552. doi: 10.1097/GME.0000000000001941. [DOI] [PubMed] [Google Scholar]
- 297.Kim S, Kim SM, Hwang H, Kim MK, Kim HJ, Park S, Han DH. The effects of music therapy on the psychological status of women with perimenopause syndrome. Menopause. 2023;30:1045–1052. doi: 10.1097/GME.0000000000002241. [DOI] [PubMed] [Google Scholar]
- 298.Mahmoud MY, Labib K, Sileem SA, Mustafa FA, Hamed WM, Abd Elhamid A, Saleh DM, Alanwar A, Riad AAM, Abdelhakim AM, et al. The impact of music therapy on anxiety and pregnancy rate among infertile women undergoing assisted reproductive technologies: A systematic review and meta-analysis. J Psychosom Obstet Gynecol. 2022;43:205–213. doi: 10.1080/0167482X.2021.1977277. [DOI] [PubMed] [Google Scholar]
- 299.Aba YA, Avci D, Guzel Y, Ozcelik SK, Gurtekin B. Effect of music therapy on the anxiety levels and pregnancy rate of women undergoing in vitro fertilization-embryo transfer: A randomized controlled trial. Appl Nurs Res. 2017;36:19–24. doi: 10.1016/j.apnr.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 300.Domar AD, Gross J, Rooney K, Boivin J. Exploratory randomized trial on the effect of a brief psychological intervention on emotions, quality of life, discontinuation, and pregnancy rates in in vitro fertilization patients. Fertil Steril. 2015;104:440–451.e7. doi: 10.1016/j.fertnstert.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 301.Clifton J, Parent J, Seehuus M, Worrall G, Forehand R, Domar A. An internet-based mind/body intervention to mitigate distress in women experiencing infertility: A randomized pilot trial. PLoS One. 2020;15:e0229379. doi: 10.1371/journal.pone.0229379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Brinsley J, Schuch F, Lederman O, Girard D, Smout M, Immink MA, Stubbs B, Firth J, Davison K, Rosenbaum S. Effects of yoga on depressive symptoms in people with mental disorders: A systematic review and meta-analysis. Br J Sports Med. 2021;55:992–1000. doi: 10.1136/bjsports-2019-101242. [DOI] [PubMed] [Google Scholar]
- 303.Darbandi S, Darbandi M, Khorram Khorshid HR, Sadeghi MR. Yoga can improve assisted reproduction technology outcomes in couples with infertility. Altern Ther Health Med. 2018;24:50–55. [PubMed] [Google Scholar]
- 304.Oron G, Allnutt E, Lackman T, Sokal-Arnon T, Holzer H, Takefman J. A prospective study using hatha yoga for stress reduction among women waiting for IVF treatment. Reprod Biomed Online. 2015;30:542–548. doi: 10.1016/j.rbmo.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 305.Valoriani V, Lotti F, Vanni C, Noci MC, Fontanarosa N, Ferrari G, Cozzi C, Noci I. Hatha-yoga as a psychological adjuvant for women undergoing IVF: A pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;176:158–162. doi: 10.1016/j.ejogrb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 306.Lu X, Liu L, Yuan R. Effect of the information support method combined with yoga exercise on the depression, anxiety, and sleep quality of menopausal women. Psychiatr Danub. 2020;32:380–388. doi: 10.24869/psyd.2020.380. [DOI] [PubMed] [Google Scholar]
- 307.Jorge MP, Santaella DF, Pontes IMO, Shiramizu VKM, Nascimento EB, Cabral A, Lemos TM, Silva RH, Ribeiro AM. Hatha yoga practice decreases menopause symptoms and improves quality of life: A randomized controlled trial. Complement Ther Med. 2016;26:128–135. doi: 10.1016/j.ctim.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 308.Frederiksen Y, O'Toole MS, Mehlsen MY, Hauge B, Elbaek HO, Zachariae R, Ingerslev HJ. The effect of expressive writing intervention for infertile couples: a randomized controlled trial. Hum Reprod. 2017;32:391–402. doi: 10.1093/humrep/dew320. [DOI] [PubMed] [Google Scholar]
- 309.Hart RJ. Physiological aspects of female fertility: Role of the environment, modern lifestyle, and genetics. Physiol Rev. 2016;96:873–909. doi: 10.1152/physrev.00023.2015. [DOI] [PubMed] [Google Scholar]
- 310.Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, Hochhauser E. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving 'SIRT1 and PGC-1α'. Cardiovasc Diabetol. 2018;17:111. doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 312.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL. Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA. 2011;108:12319–12324. doi: 10.1073/pnas.1018793108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Smits A, Marei WFA, Moorkens K, Bols PEJ, De Neubourg D, Leroy JLMR. Obese outbred mice only partially benefit from diet normalization or calorie restriction as preconception care interventions to improve metabolic health and oocyte quality. Hum Reprod. 2022;37:2867–2884. doi: 10.1093/humrep/deac226. [DOI] [PubMed] [Google Scholar]
- 315.Mishina T, Tabata N, Hayashi T, Yoshimura M, Umeda M, Mori M, Ikawa Y, Hamada H, Nikaido I, Kitajima TS. Single-oocyte transcriptome analysis reveals aging-associated effects influenced by life stage and calorie restriction. Aging Cell. 2021;20:e13428. doi: 10.1111/acel.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Słuczanowska-Głąbowska S, Laszczyńska M, Piotrowska K, Grabowska M, Grymuła K, Ratajczak MZ. Caloric restriction increases ratio of estrogen to androgen receptors expression in murine ovaries-potential therapeutic implications. J Ovarian Res. 2015;8:57. doi: 10.1186/s13048-015-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB, Longo DL, Schlessinger D, Ko MSh. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biol. 2008;6:24. doi: 10.1186/1741-7007-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Garcia DN, Saccon TD, Pradiee J, Rincón JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, et al. Effect of caloric restriction and rapamycin on ovarian aging in mice. Geroscience. 2019;41:395–408. doi: 10.1007/s11357-019-00087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Isola JVV, Zanini BM, Hense JD, Alvarado-Rincón JA, Garcia DN, Pereira GC, Vieira AD, Oliveira TL, Collares T, Gasperin BG, et al. Mild calorie restriction, but not 17α-estradiol, extends ovarian reserve and fertility in female mice. Exp Gerontol. 2022;159:111669. doi: 10.1016/j.exger.2021.111669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Liu WJ, Zhang XM, Wang N, Zhou XL, Fu YC, Luo LL. Calorie restriction inhibits ovarian follicle development and follicle loss through activating SIRT1 signaling in mice. Eur J Med Res. 2015;20:22. doi: 10.1186/s40001-015-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Zhou XL, Xu JJ, Ni YH, Chen XC, Zhang HX, Zhang XM, Liu WJ, Luo LL, Fu YC. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res. 2014;7:97. doi: 10.1186/s13048-014-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Li L, Fu YC, Xu JJ, Lin XH, Chen XC, Zhang XM, Luo LL. Caloric restriction promotes the reserve of follicle pool in adult female rats by inhibiting the activation of mammalian target of rapamycin signaling. Reprod Sci. 2015;22:60–67. doi: 10.1177/1933719114542016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Smits A, Marei WFA, De Neubourg D, Leroy JLMR. Diet normalization or caloric restriction as a preconception care strategy to improve metabolic health and oocyte quality in obese outbred mice. Reprod Biol Endocrinol. 2021;19:166. doi: 10.1186/s12958-021-00848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Chen G, Xie W, Nah J, Sauvat A, Liu P, Pietrocola F, Sica V, Carmona-Gutierrez D, Zimmermann A, Pendl T, et al. 3,4-Dimethoxychalcone induces autophagy through activation of the transcription factors TFE3 and TFEB. EMBO Mol Med. 2019;11:e10469. doi: 10.15252/emmm.201910469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Kepp O, Chen G, Carmona-Gutierrez D, Madeo F, Kroemer G. A discovery platform for the identification of caloric restriction mimetics with broad health-improving effects. Autophagy. 2020;16:188–189. doi: 10.1080/15548627.2019.1688984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Zhang H, Ni W, Yu G, Geng Y, Lou J, Qi J, Chen Y, Li F, Ye H, Ma H, et al. 3,4-Dimethoxychalcone, a caloric restriction mimetic, enhances TFEB-mediated autophagy and alleviates pyroptosis and necroptosis after spinal cord injury. Theranostics. 2023;13:810–832. doi: 10.7150/thno.78370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Jafari M, Macho-González A, Diaz A, Lindenau K, Santiago-Fernández O, Zeng M, Massey AC, de Cabo R, Kaushik S, Cuervo AM. Calorie restriction and calorie-restriction mimetics activate chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2024;121:e2317945121. doi: 10.1073/pnas.2317945121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Sciarretta S, Forte M, Castoldi F, Frati G, Versaci F, Sadoshima J, Kroemer G, Maiuri MC. Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc Res. 2021;117:1434–1449. doi: 10.1093/cvr/cvaa297. [DOI] [PubMed] [Google Scholar]
- 329.Pallauf K, Günther I, Kühn G, Chin D, de Pascual-Teresa S, Rimbach G. The potential of resveratrol to act as a caloric restriction mimetic appears to be limited: Insights from studies in mice. Adv Nutr. 2021;12:995–1005. doi: 10.1093/advances/nmaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 331.Viollet B, Guigas B, Garcia NS, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: An overview. Clin Sci (Lond) 2012;122:253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Masoudi FA, Wang Y, Inzucchi SE, Setaro JF, Havranek EP, Foody JM, Krumholz HM. Metformin and thiazolidinedione use in medicare patients with heart failure. JAMA. 2003;290:81–85. doi: 10.1001/jama.290.1.81. [DOI] [PubMed] [Google Scholar]
- 333.Valencia WM, Palacio A, Tamariz L, Florez H. Metformin and ageing: Improving ageing outcomes beyond glycaemic control. Diabetologia. 2017;60:1630–1638. doi: 10.1007/s00125-017-4349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Morales DR, Morris AD. Metformin in cancer treatment and prevention. Annu Rev Med. 2015;66:17–29. doi: 10.1146/annurev-med-062613-093128. [DOI] [PubMed] [Google Scholar]
- 335.Qin X, Du D, Chen Q, Wu M, Wu T, Wen J, Jin Y, Zhang J, Wang S. Metformin prevents murine ovarian aging. Aging (Albany NY) 2019;11:3785–3794. doi: 10.18632/aging.102016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Huang CC, Chou CH, Yang YS, Ho HN, Shun CT, Wen WF, Chen SU, Chen MJ. Metformin: A novel promising option for fertility preservation during cyclophosphamide-based chemotherapy. Mol Hum Reprod. 2021;27:gaaa084. doi: 10.1093/molehr/gaaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Cao Y, Wang Z, Zhang C, Bian Y, Zhang X, Liu X, Chen W, Zhao Y. Metformin promotes in vitro maturation of oocytes from aged mice by attenuating mitochondrial oxidative stress via SIRT3-dependent SOD2ac. Front Cell Dev Biol. 2022;10:1028510. doi: 10.3389/fcell.2022.1028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ayhan S, Hancerliogullari N, Guney G, Gozukucuk M, Caydere M, Guney SS, Tokmak A, Ustun Y. Does the addition of metformin to carboplatin treatment decreases ovarian reserve damage associated with carboplatin usage? J Ovarian Res. 2023;16:184. doi: 10.1186/s13048-023-01259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Landry DA, Yakubovich E, Cook DP, Fasih S, Upham J, Vanderhyden BC. Metformin prevents age-associated ovarian fibrosis by modulating the immune landscape in female mice. Sci Adv. 2022;8:eabq1475. doi: 10.1126/sciadv.abq1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Gambini J, Inglés M, Olaso G, Lopez-Grueso R, Bonet-Costa V, Gimeno-Mallench L, Mas-Bargues C, Abdelaziz KM, Gomez-Cabrera MC, Vina J, Borras C. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev. 2015;2015:837042. doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Rajman L, Chwalek K, Sinclair DA. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metabv. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Côté CD, Rasmussen BA, Duca FA, Zadeh-Tahmasebi M, Baur JA, Daljeet M, Breen DM, Filippi BM, Lam TK. Resveratrol activates duodenal Sirt1 to reverse insulin resistance in rats through a neuronal network. Nat Med. 2015;21:498–505. doi: 10.1038/nm.3821. [DOI] [PubMed] [Google Scholar]
- 343.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14:612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Battaglia R, Caponnetto A, Caringella AM, Cortone A, Ferrara C, Smirni S, Iannitti R, Purrello M, D'Amato G, Fioretti B, Di Pietro C. Resveratrol treatment induces mito-miRNome modification in follicular fluid from aged women with a poor prognosis for in vitro fertilization cycles. Antioxidants (Basel) 2022;11:1019. doi: 10.3390/antiox11051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Li R, Li E, Kamili G, Ou S, Yang D. Effect of resveratrol on superovulation in mice. Biomed Pharmacother. 2022;146:112565. doi: 10.1016/j.biopha.2021.112565. [DOI] [PubMed] [Google Scholar]
- 346.Gou M, Li J, Yi L, Li H, Ye X, Wang H, Liu L, Sun B, Zhang S, Zhu Z, et al. Reprogramming of ovarian aging epigenome by resveratrol. PNAS Nexus. 2022;2:pgac310. doi: 10.1093/pnasnexus/pgac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Wu H, Xue J, Di H, Lv C, Hao Y, Nie Z. Resveratrol improves ovarian function in aged rat by inhibiting oxidative stress and activating the Sirt1. Gen Physiol Biophys. 2022;41:53–61. doi: 10.4149/gpb_2021040. [DOI] [PubMed] [Google Scholar]
- 348.Zhou J, Xue Z, He HN, Liu X, Yin SY, Wu DY, Zhang X, Schatten H, Miao YL. Resveratrol delays postovulatory aging of mouse oocytes through activating mitophagy. Aging (Albany NY) 2019;11:11504–11519. doi: 10.18632/aging.102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Liang QX, Lin YH, Zhang CH, Sun HM, Zhou L, Schatten H, Sun QY, Qian WP. Resveratrol increases resistance of mouse oocytes to postovulatory aging in vivo. Aging (Albany NY) 2018;10:1586–1596. doi: 10.18632/aging.101494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Liu MJ, Sun AG, Zhao SG, Liu H, Ma SY, Li M, Huai YX, Zhao H, Liu HB. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil Steril. 2018;109:900–907. doi: 10.1016/j.fertnstert.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 351.Okamoto N, Sato Y, Kawagoe Y, Shimizu T, Kawamura K. Short-term resveratrol treatment restored the quality of oocytes in aging mice. Aging (Albany NY) 2022;14:5628–5640. doi: 10.18632/aging.204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sun YL, Tang SB, Shen W, Yin S, Sun QY. Roles of resveratrol in improving the quality of postovulatory aging oocytes in vitro. Cells. 2019;8:1132. doi: 10.3390/cells8101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cai M, Sun H, Huang Y, Yao H, Zhao C, Wang J, Zhu H. Resveratrol protects rat ovarian luteinized granulosa cells from H2O2 -induced dysfunction by activating autophagy. Int J Mol Sci. 2023;24:10914. doi: 10.3390/ijms241310914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Moreira-Pinto B, Costa L, Felgueira E, Fonseca BM, Rebelo I. Low doses of resveratrol protect human granulosa cells from induced-oxidative stress. Antioxidants (Basel) 2021;10:561. doi: 10.3390/antiox10040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.PSY.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- 356.Campagne DM. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21:1651–1658. doi: 10.1093/humrep/del078. [DOI] [PubMed] [Google Scholar]
- 357.Obayashi K. Salivary mental stress proteins. Clin Chim Acta. 2013;425:196–201. doi: 10.1016/j.cca.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 358.Shah K, Kumari R, Jain M. Unveiling stress markers: A systematic review investigating psychological stress biomarkers. Dev Psychobiol. 2024;66:e22490. doi: 10.1002/dev.22490. [DOI] [PubMed] [Google Scholar]
- 359.Lavalle S, Masiello E, Iannella G, Magliulo G, Pace A, Lechien JR, Calvo-Henriquez C, Cocuzza S, Parisi FM, Favier V, et al. Unraveling the complexities of oxidative stress and inflammation biomarkers in obstructive sleep apnea syndrome: A comprehensive review. Life (Basel) 2024;14:425. doi: 10.3390/life14040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Ok J, Park S, Jung YH, Kim T. Wearable and implantable cortisol-sensing electronics for stress monitoring. Adv Mater. 2024;36:2211595. doi: 10.1002/adma.202211595. [DOI] [PubMed] [Google Scholar]
- 361.Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ. Depression, anxiety and perceived stress in women with and without PCOS: A community-based study. Psychol Med. 2019;49:1510–1520. doi: 10.1017/S0033291718002076. [DOI] [PubMed] [Google Scholar]
- 362.Tay CT, Teede HJ, Hill B, Loxton D, Joham AE. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: A community-based cohort study. Fertil Steril. 2019;112:353–361. doi: 10.1016/j.fertnstert.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 363.Rowlands IJ, Teede H, Lucke J, Dobson AJ, Mishra GD. Young women's psychological distress after a diagnosis of polycystic ovary syndrome or endometriosis. Hum Reprod. 2016;31:2072–2081. doi: 10.1093/humrep/dew174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.