Abstract
Myocarditis is an inflammatory disease of the myocardium with heterogeneous etiology, clinical presentation, and prognosis; when it is associated with myocardial dysfunction, this identifies the entity of inflammatory cardiomyopathy. In the last few decades, the relevance of the immune system in myocarditis onset and progression has become evident, thus having crucial clinical relevance in terms of treatment and prognostic stratification. In fact, the advances in cardiac immunology have led to a better characterization of the cellular subtypes involved in the pathogenesis of inflammatory cardiomyopathy, whether the etiology is infectious or autoimmune/immune-mediated. The difference in the clinical course between spontaneous recovery to acute, subacute, or chronic progression to end-stage heart failure may be explained not only by classical prognostic markers but also through immune-pathological mechanisms at a cellular level. Nevertheless, much still needs to be clarified in terms of immune characterization and molecular mechanisms especially in biopsy-proven myocarditis. The aims of this review are to (1) describe inflammatory cardiomyopathy etiology, especially immune-mediated/autoimmune forms, (2) analyze recent findings on the role of different immune cells subtypes in myocarditis, (3) illustrate the potential clinical relevance of such findings, and (4) highlight the need of further studies in pivotal areas of myocarditis cellular immunology.
Keywords: myocarditis, immune system, immunosuppressive therapy, autoimmune disease, systemic immune-mediated disease
1. Introduction
Myocarditis is an inflammatory disease of the myocardium, diagnosed through well-established histological, immunological, and immunohistochemical criteria. It presents with a broad spectrum of etiologies, clinical manifestations, and outcomes [1,2]. Acute myocyte damage may trigger the activation of both innate and adaptive immune responses, leading to an inflammatory response. In most patients, the immune reaction is eventually downregulated, and the myocardium recovers. In a sizable portion of cases, however, persistent inflammation leads to ongoing myocyte damage, resulting in ventricular dilation, reduced contractility, and end-stage heart failure [3,4]. When associated with myocardial dysfunction, biopsy-proven myocarditis is termed inflammatory cardiomyopathy [1], a complex and clinically relevant disorder.
In recent years, extensive research efforts have been made to elucidate the underlying immunopathogenesis of myocarditis, employing a variety of methodologies, including animal models [5,6] and human studies on patients and healthy controls [7,8,9]. These investigations aimed to provide a comprehensive understanding of how both innate immune cells (e.g., macrophages and dendritic cells) and adaptive immune cells (e.g., T cells and B cells) initiate and amplify the immune response [10,11,12]. However, despite these advances, many aspects, particularly the immune characterization and molecular mechanisms underlying biopsy-proven autoimmune myocarditis and inflammatory cardiomyopathy, remain poorly understood.
The aims of this review are to (1) describe inflammatory cardiomyopathy etiology, with a focus on immune-mediated/autoimmune forms, (2) analyze recent findings on the role of different immune cells subtypes in myocarditis, (3) illustrate the potential clinical relevance of these findings, and (4) highlight the need for further studies in pivotal areas of myocarditis cellular immunology.
2. Clinical Presentation of Myocarditis and Inflammatory Cardiomyopathies
Myocarditis encompasses a heterogeneous spectrum of clinical manifestations, ranging from mild to severe forms. This variability complicates diagnosis, risk assessment, and therapeutic management [2]. The majority of the cases of clinically suspected infarct-like cases occur in men (60–80%), with a median age of presentation between 30 and 45 years [13,14]. However, the incidence of myocarditis, especially with elusive clinical presentations such as arrhythmias or chronic heart failure, is difficult to assess due to the inconsistent use of the diagnostic gold standard, endomyocardial biopsy (EMB).
In fact, myocarditis may even be paucisymptomatic, with a slow and insidious course, thereby often leading to delayed diagnosis. Conversely, it may present with a sudden onset of unexplained cardiac signs and symptoms or progress rapidly to a fulminant form [2,15]. Commonly reported symptoms include chest pain (82% to 95% of cases), fever (58–65% of cases), dyspnea (19–49% of cases), and syncope (5–7% of cases) [16,17]. In approximately 7% to 12% of acute myocarditis cases, the onset is fulminant, characterized by cardiogenic shock (CS) or acute heart failure (HF) and left ventricular (LV) dysfunction, with or without malignant ventricular arrhythmias and/or conduction abnormalities [18,19].
Clinical manifestations may also mimic those of other cardiomyopathies, including Takotsubo syndrome (TS) [20,21] and arrhythmogenic right ventricular cardiomyopathy (ARVC). In a non-negligible proportion of cases, ARVC patients may present with acute chest pain episodes and elevated myocardial enzyme levels, frequently with preserved biventricular systolic function, during so-called ‘hot phases’, which have been observed in 5% of a previously reported ARVC cohort [22,23]. This is even more relevant considering the newly identified phenotypes of arrhythmogenic cardiomyopathy (ACM), including the “left-dominant” and “biventricular” disease subtypes [24], in which a phenotypical and clinical overlap with inflammatory cardiomyopathy should be carefully investigated, in order to promptly define a correct diagnostic and therapeutic patient work-up. Since histological evidence of inflammatory infiltrates in ARVC patients has been provided since the 1990s, multi-parametric assessment of myocarditis in the context of ACM, especially during the so-called “hot phases”, is encouraged [25].
3. Challenges in Diagnosis: The Role of Endomyocardial Biopsy and Imaging Techniques
EMB is the diagnostic gold standard for myocarditis. According to the 2013 position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases, EMB should not be restricted to hemodynamically or electrically unstable patients but should instead be considered for any clinically suspected myocarditis case where a definitive etiological diagnosis could impact the outcome. This has been reinforced by the latest consensus statement by the three leading international HF societies [19]. EMB should be performed following the exclusion of other potential cardiac or extracardiac conditions that could explain the symptoms and imaging findings, particularly coronary artery disease, which can be ruled out through invasive coronary angiography or coronary CT, according to the patients’ pretest probability of relevant coronary atherosclerosis [2,26,27]. The EMB examination is based on conventional histopathological analysis according to the Dallas criteria [28,29], supplemented by immunohistochemical characterization of the inflammatory infiltrate and polymerase chain reaction (PCR) detection of infectious agents. The type of inflammatory infiltrate—whether eosinophilic, polymorphous, lymphocytic, or granulomatous—plays a critical role in both prognostic stratification and therapeutic decision-making [2,3,26].
When EMB is not initially performed during clinical evaluation—particularly in cases where any markers of severe prognosis are present and specific etiological treatment is not required—the 2013 ESC criteria allow for a diagnosis of clinically suspected myocarditis [2,19]. This diagnosis is largely based on ruling out coronary artery disease through coronary angiography and identifying myocarditis typical findings on CMR imaging.
Over the past two decades, CMR has emerged as a reliable and accurate non-invasive diagnostic technique to support clinical suspicions of myocarditis, due to its ability to provide volume quantification, contractility assessment, and myocardial tissue characterization [30,31]. In 2009, the original Lake Louise criteria (LLC) were established to enhance CMR diagnostic accuracy for suspected myocarditis through uniform protocols. These criteria included (1) global or regional myocardial systolic dysfunction, (2) myocardial edema, and (3) myocardial hyperemia or increased vascular permeability, as indicated by early (EGE) or late gadolinium enhancement (LGE) on CMR [32]. The diagnosis of myocarditis required at least two of the three aforementioned criteria, with one being either myocardial edema or myocardial LGE. To improve diagnostic accuracy, the latest revisions to the LLC have integrated new mapping techniques and now require both myocardial edema (one T2-based criterion) and non-ischemic myocardial injury (one T1-based criterion) to be present in order to raise suspicion of myocardial inflammation [33,34] (Figure 1).
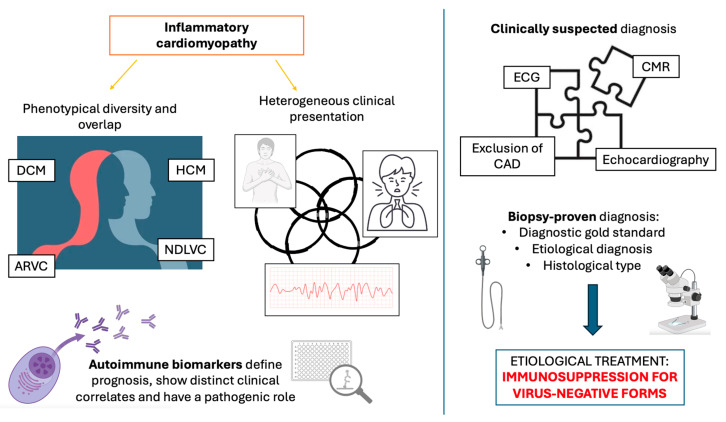
Figure 1.
Overview of a guideline-based clinical and diagnostic approach to inflammatory cardiomyopathy. Inflammatory cardiomyopathy presents a high grade of clinical heterogeneity (clinical presentation may range from chronic heart failure to abrupt onset of life-threatening ventricular arrhythmias) and phenotypical diversity (non-invasive findings may mimic other cardiomyopathies such as ARVC, DCM, etc.). Autoimmune biomarkers may suggest an immune-mediated etiology and identify patients with worse phenotype and follow-up [35,36]. A diagnosis of clinically suspected myocarditis is mostly based on CMR findings, but only EMB can achieve a definitive and etiological diagnosis, possibly identifying candidates for tailored immunosuppression in virus-negative cases. ARVC: left ventricular cardiomyopathy; CAD: coronary artery disease; DCM: dilated cardiomyopathy; ECG: electrocardiogram; HCM: hypertrophic cardiomyopathy. Created with Biorender.
While CMR is valuable in various clinical scenarios, especially in distinguishing between coronary-ischemic and inflammatory myocardial damage, it does not provide information about the underlying etiology or the histological subtype of myocarditis [15].
Furthermore, the prognostic role of LGE in myocarditis is still open to debate. While studies on non-ischemic cardiomyopathy found LGE to be quantitively associated with worse outcomes [37], a recent single-center study on 207 clinically suspected or biopsy-proven myocarditis patients showed that higher biventricular systolic function and a greater extent of LGE on CMR at diagnosis were associated with better outcomes when assessed at any follow-up point. Conversely, larger biventricular volumes, CMR-based dilated cardiomyopathy (DCM) features, and the presence of an ischemic LGE pattern at diagnosis were predictors of worse outcomes [38].
Regarding nuclear imaging techniques, positron emission tomography with 2-deoxy-2-fluoro-D-glucose (FDG-PET) can be used to evaluate the inflammatory activity of the heart and monitor treatment responses specific conditions, such as cardiac sarcoidosis [39,40,41]. However, data regarding the use of FDG-PET in myocarditis evaluation remain limited. Only a few case reports and series have documented FDG findings in viral myocarditis [42,43,44,45,46], eosinophilic myocarditis [43], and GCM [44,45].
4. Etiology of Myocarditis: Viral and Toxic Causes
The etiopathogenesis of myocarditis is complex, primarily categorized as either viral or autoimmune/immune-mediated [2]. Myocarditis can result from various infectious agents, with viruses being the most common culprits, though bacteria and parasites may also play a role. While viral infections dominate in Western countries, Central and South America present a higher incidence of Chagas disease, which is caused by the protozoan Trypanosoma Cruzi [15]. Notably, around 27% of patients may present with multiple viral infections affecting the myocardium [4].
Viral myocarditis can be categorized based on the viral tropism. Viruses that are primarily cardiotropic, such as adenoviruses and enteroviruses, directly target the myocardium, while others like parvovirus B19 (PVB19) are vasculotropic and may persist lifelong. Lymphotropic viruses, including cytomegalovirus (CMV) and Epstein–Barr virus (EBV), target lymphatic tissues, and certain strains like Influenza A and B can exert cardiotoxic effects on the myocardium [4]. Accurate diagnosis of viral myocarditis is essential and should be based on PCR testing from myocardial tissue rather than serological markers, which may not reliably indicate current infection [4,47]. Given the complexity of viral pathogens, treatment strategies should be multidisciplinary, with close collaboration with infectious disease specialists to ensure tailored antiviral therapy, especially in cases of chronic or recurrent infections [19,48].
Toxic causes of myocarditis are a minority, occurring either as hypersensitivity myocarditis, which is unrelated to drug dosage, or as dose-dependent direct cardiac toxicity. Among these less common causes is mesalazine, an established first-line treatment for inflammatory bowel disease (IBD) and mainstay therapy for mild-to-moderate ulcerative colitis (UC) [49]. While mesalazine has been associated with myocarditis, with a reported incidence as high as 0.3% and potentially fatal outcomes [50], data from a single-center experience and a literature review suggest that, in the absence of EMB-based confirmation, the true incidence may be overestimated [51].
An immune-mediated pathological reaction may arise from clear toxic myocardial damage. A clear example of a toxic myocarditis with an underlying autoimmune mechanism is myocarditis induced by immune checkpoint inhibitors (ICIs), which warrants particular attention due to the relevant morbidity and mortality [52]. Over the past decade, ICIs have significantly improved outcomes for cancer patients. However, ICIs can also trigger a wide range of potentially life-threatening immune-related adverse events (irAEs) including fulminant myocarditis [53].
5. Focus on Immune-Mediated Myocarditis and Inflammatory Cardiomyopathy
The role of autoimmunity in myocarditis is well established, occurring either as post-infectious immune-mediated myocardial damage or as a primary organ-specific autoimmune disease [2]. Additionally, non-infectious autoimmune myocarditis may occur in various systemic immune-mediated diseases (SIDs) [54], which include autoimmune and autoinflammatory diseases affecting at least two organ systems [55], such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), systemic sclerosis (SSc), and mixed connective tissue disease (MCTD). Myocarditis is a hallmark of SIDs, often associated with worse prognoses and necessitating an intensified immunosuppressive regimen [39,56,57].
In both organ-specific myocarditis and systemic immune-mediated myocardial damage, the role of humoral immunity is well established. Research dating back to the late 1980s and early 1990s reported the presence of anti-heart autoantibodies (AHAs) [58,59,60,61] in cases of acute and chronic myocarditis or DCM [62,63,64]. These biomarkers are present in 60–80% of patients with biopsy-proven organ-specific autoimmune myocarditis/inflammatory cardiomyopathy across its full spectrum of clinical presentations, including fulminant, acute, subacute, chronic heart failure, pseudo-infarction, and arrhythmic presentation [3,62,65], and their presence is correlated with poor prognosis [66]. Furthermore, their detection in asymptomatic relatives of patients with idiopathic DCM may serve as a predictive marker for disease development [65,67]. AHA antigens are the α and β isoforms of the cardiac myosin heavy chain and are therefore considered cardiac-specific autoantigens [59].
AHAs and anti-intercalated disc autoantibodies (AIDAs) serve as disease-specific markers of immune-mediated myocardial damage also in the context of SIDs, such as systemic sclerosis [68] and sarcoidosis with cardiac involvement. [69]. Future studies are warranted to clarify whether or not AHAs and AIDAs play a direct pathogenic role in systemic immune-mediated myocardial damage, as suggested in organ-specific myocarditis [70]. Other autoimmune biomarkers, such as anti-Desmoglein-2 antibodies, have been identified in myocarditis and various other cardiac and non-cardiac immune-mediated diseases. These biomarkers not only correlate with specific disease features and prognostic markers but also suggest a potentially pivotal role in disease pathogenesis [71,72,73].
6. Interplay of Genetic Predisposition and Autoimmunity
Autoimmune diseases typically arise from the interaction between genetic predisposition and environmental triggers. This interplay results in immune dysregulation and a failure to recognize self-antigens, ultimately leading to a loss of tolerance. Similarly, in autoimmune myocarditis, numerous studies have sought to clarify the relationship between environmental factors, genetic background, and disease development [74,75,76]. Genetic factors may significantly influence both susceptibility to myocarditis and its clinical evolution, particularly in patients with severe left ventricular (LV) dysfunction who may progress to DCM [77].
Genetic polymorphisms in the major histocompatibility complex (MHC) genes can affect antigen binding affinities, with specific MHC genes linked to an increased risk of certain autoimmune diseases [78]. In humans, alleles such as HLA (human leukocyte antigen)-DR4, HLA-DR12, and HLA-DR15 have been associated not only with the development of myocarditis but also with a higher risk of progression to DCM [79,80]. Furthermore, recent transcriptomic studies have revealed a higher prevalence of HLA-DQ1 expression in patients with myocarditis compared to those to those without. In fact, transgenic mice expressing human HLA-DQ8 or HLA-DR4 have been shown to spontaneously develop fatal autoimmune myocarditis [5,81]. Besides HLA polymorphism, recent data suggest an overlap between certain genetic cardiomyopathies and myocarditis. A population-based cohort study that included 336 consecutive myocarditis patients, mainly with a clinically suspect diagnosis, revealed that pathogenic mutations in cardiomyopathy-related genes (i.e., pathogenic or likely pathogenic variants in genes related to specific cardiomyopathies) were present in 8% of the myocarditis cases and in less than 1% of healthy controls [74]. These genetic variants were detected in both genes associated with DCM, such as TTN, and those linked to ARVC, like DSP. Importantly, patients carrying these genotype-positive mutations showed a poorer prognosis and increased 5-year mortality rates [74].
7. Immunosuppressive Regimens for Myocarditis: Current Evidence and Future Challenges
Effective management of myocarditis requires a comprehensive therapeutic approach designed to address both cardiovascular complications and the specific underlying etiology, whether viral or autoimmune.
Immunosuppressive therapy (IS) is a cornerstone in the management of biopsy-proven (BP) autoimmune myocarditis, aiming to attenuate inflammation and prevent myocardial injury [2,82]. Typical immunosuppressive regimens involve corticosteroids combined with agents like azathioprine or cyclosporine over a six-month period. Alternatively, other immunosuppressive drugs such as mycophenolate mofetil or methotrexate may be used alongside steroids [82,83,84].
Evidence supporting the efficacy of IS for treating heart failure in lymphocytic virus-negative myocarditis mainly derives retrospective studies and meta-analyses [82,83,85,86]. A recent propensity-score-based study assessed the long-term safety and effectiveness of personalized IS therapy in 91 patients with BP immune-mediated myocarditis. The study found comparable survival rates and heart function at the 5-year follow-up in IS-treated patients with BP immune-mediated myocarditis, compared to 267 controls receiving only optimal medical therapy (OMT). Minor manageable adverse reactions occurred in just 13% of IS patients [35].
For other less common histological forms of myocarditis such as GCM [44], eosinophilic myocarditis [87], and cardiac sarcoidosis [88], data on the efficacy of IS come from retrospective observational registries, and further studies are needed for a complete characterization of the optimal types and duration of a tailored IS in these settings.
Despite advances in diagnostic techniques, a standardized treatment approach for myocarditis remains elusive, and individual responses to IS treatment vary, with some patients showing significant improvement, while others remain refractory to therapy [36]. This is primarily due to the unknown mechanisms governing host immune responses, which can either eliminate the virus and resolve inflammation or lead to persistent immune-mediated damage with or without viral clearance. Therefore, predictors of a favorable response to IS in myocarditis, including peripheral non-invasive biomarkers, are still under investigation.
8. Role of Different Immune Cell Populations and Cytokines in Myocarditis
Cardiomyocytes are the major cellular component in the heart, but many other cell types are present to allow proper cardiac functionality (Figure 2, Table 1). Among these are resident immune cells, such as macrophages. Resident monocytes, mainly CX3CR1+ and of embryonic origin, establish physical contact with neighboring cardiomyocytes. At basal conditions, these cells exert a tissue remodeling role. On the other hand, cardiac dysfunction induces cardiomyocytes to secrete monocytes recruiting chemokines. These recruited monocytes differentiate into CCR2+ macrophages, which are pro-inflammatory [89] and have been identified in EMB of myocarditis patients [90]. Indeed, in experimental autoimmune myocarditis (EAM) models, as well as in human EMB, macrophages are the predominant infiltrating cells, and their pro-inflammatory M1 polarization can be driven by mitochondrial fission [91]. In EAM models, the infiltration kinetics of classical monocytes, contributing to CCR2+MHCII+ macrophage compartment, peaked at 14 days of immunization, while the non-classical monocytes peaked at 21 days [92,93]. This double recruitment of CD11b+ monocytes resulted both in a strong pro-inflammatory signal in the first peak as well as a suppression of myosin heavy chain (MyHC)-specific Th17 T cell responses in the second peak through a disease-induced limiting IFN-γ-triggered negative feedback loop [93]. Autoreactive T cells recruited monocytes either directly, as in the case of Th17 cells, or by IL-3 secretion, which incites tissue macrophages to produce monocyte-attracting chemokines [92,94]. Recently, it has been demonstrated that monocyte recruitment can be blocked by siRNA silencing of CCR2 in EAM, leading to reduced myocardial inflammation [90].
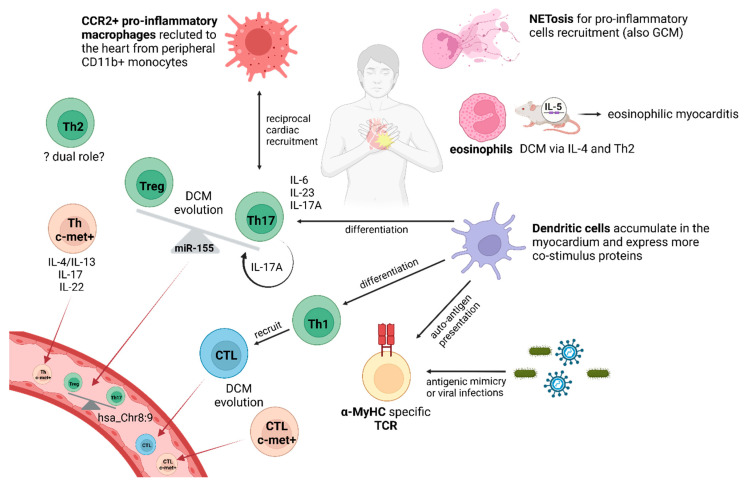
Figure 2.
Summary of the role of cellular immunity in myocarditis within the inflamed heart and peripheral blood (bottom left). Myocarditis is the result of a fine interplay of different pro-inflammatory cells. α-MyHC: myosin heavy chain isoform α; CTL: cytotoxic T cell; DCM: dilated cardiomyopathy; GCM: giant cell myocarditis; IL: interleukin; TCR: T cell receptor; Th: T helper cell; Treg: T regulatory cell. Created with Biorender.
Table 1.
Cytokines and chemokines, listed in alphabetical order, that are relevant in myocarditis pathogenesis.
| Cytokine | Role in Myocarditis |
|---|---|
| CCL5 | Pro-inflammatory: CTL chemoattracting agent |
| IFN-γ | Pro-inflammatory: secreted by infiltrating T lymphocytes and increases cardiac tissue inflammation |
| IL-1β | Pro-inflammatory: important for innate immunity |
| IL-4 | Pro-inflammatory: linked to Th2 cell differentiation |
| IL-5 | Pro-inflammatory in eosinophilic myocarditis |
| IL-6 | Pro-inflammatory: fundamental for myocarditis development and Th17 cell differentiation |
| IL-10 | Anti-inflammatory: reduced in myocarditis |
| IL-17 | Pro-inflammatory: secreted by Th17 cells and mediates myeloid cells recruitment, fibrosis, and favors DCM evolution |
| IL-22 | Pro-inflammatory: secreted by Th22 cells |
| IL-23 | Pro-inflammatory: fundamental for myocarditis development and Th17 cell differentiation |
| MIP-1α | Pro-inflammatory: macrophages and CTL chemoattracting agent |
| TGF-β1 | Pro-inflammatory: favors Th17 cell differentiation |
| TNF-α | Pro-inflammatory |
Many other myeloid cells contribute to myocarditis onset/progression, including granulocytes and dendritic cells. Regarding granulocytes, neutrophil extracellular traps (NETs) have been identified in EMB samples from patients and EAM mice, and their inhibition can reduce inflammation, including in giant cell myocarditis, the most fatal form [95,96]. Moreover, eosinophils can strongly infiltrate the myocardium, as observed in transgenic mice overexpressing IL-5, leading to fatal eosinophilic myocarditis, which is one of the most aggressive forms of myocarditis in humans [97]. Eosinophils are not essential for myocarditis initiation, but they are fundamental in mediating DCM evolution through IL-4 secretion and a Th2 deviation [97,98].
Historically, myocarditis was defined as a T-cell-mediated diseases, but given the large amount of studies proving the role of nearly all inflammatory cell types in myocarditis development, nowadays myocarditis pathogenesis should be described as a state of general immune dysregulation, including both adaptive and innate immunity, with the latter playing a fundamental role in antigen presentation to T cells [9,99,100,101]. Among the key players, dendritic cells (DCs) are a heterogeneous type of professional antigen presenting cells that might derive from myeloid precursors, as well as from monocytes. Alterations in various DC subsets have been observed in the peripheral blood of myocarditis patients. In acute myocarditis, higher levels of DCs with a stronger expression of co-stimulatory proteins have been reported compared with healthy controls, suggesting a higher immunogenic state that might prime better T cells [7]. Conversely, in a mixed cohort of patients with suspected and biopsy-proven myocarditis, with DCM evolution, a strong reduction in plasmacytoid and myeloid dendritic cells has been described in peripheral blood, with a concomitant accumulation in patients’ myocardium [8]. These opposite results could be due to different patients’ enrollment criteria, as well as different panels for DC characterization, but they are indicative of a potential pathogenetic role of DCs in myocarditis, a hypothesis supported by several myocarditis mouse models. In fact, DCs can induce T lymphocytes and exacerbate the mouse myocardial inflammation through the glycoprotein Tenascin-C, which induces inflammatory cytokine expression and activation of Th17 cells via Toll-like receptor 4 [102]. Moreover, after EAM induction, type 2 conventional DCs (cDC2) have been reported to specifically present α-myosin and induce Th1 and Th17 cell differentiation [101]. The accumulation of cDC2 and plasmacytoid DCs in inflamed myocardial tissue and their immune-related pathway activation have been recently described by an integrated single-cell RNA sequencing analysis of two different EAM model gene expression data sets [103]. The generation of tolerogenic (t)DCs demonstrated the pathogenicity of DCs and opened new potential therapeutic options. Specifically, myosin-pulsed tDCs can ameliorate EAM by antigen-specific Treg cell stimulation, as they overexpress the long noncoding RNA MALAT1 [104,105]. Treg cell stimulation is fundamental to restore normal cardiac immunity, as these cells have been demonstrated to be reduced and less functional in DCM and acute myocarditis [9,106,107,108]. Moreover, human extracellular vesicles, isolated from media conditioned with human-heart-derived stromal/progenitor cells, can induce Treg cell differentiation and promote the secretion of anti-inflammatory cytokines, as IL-10, both in vitro and in EAM models [109].
Dysfunctional Treg cells are strictly related to the strong activation and increase in Th17 cells, which so far have been the most extensively studied cells and proved to play a pathogenic role in myocarditis/DCM; thus, the Th17/Treg ratio is in favor of Th17 cells in myocarditis [9]. This imbalance might be linked to increased miR-155 levels in inflamed hearts, since in EAM models, it could facilitate Th17 cell differentiation as well as Treg suppression, and its inhibition rebalances the Th17/Treg ratio [110]. This imbalance has also been targeted with small molecules, such as fenofibrate, and with antibodies against the Th17 cell cytokines, such as IL-17 and IL-23, leading to an improvement of cardiac inflammation in models [111,112,113]. The most relevant role played by Th17 cells is the contribution to DCM evolution; in particular, IL-23 and IL-6 signaling induce Th17 cells to differentiate and infiltrate the heart, and, in fact, their silencing blocks EAM onset/evolution [114,115,116]. IL-6 is a fundamental cytokine for myocarditis development, since EAM models IL-6−/− are resistant to myocarditis development [117]. Recently, a temporal characterization of heart-infiltrating CD45+ cells in EAM mice showed that Th17 cells, overexpressing Hypoxia Inducible Factor (Hif)1α was the predominant T-cell population during the acute inflammatory phase, whereas Treg cells are detected at the subacute inflammatory phase, and γδ T cells releasing Il-17 are the main T-cell population observed at the myopathy phase [118]. IL-17A, produced by infiltrating Th17 cells, induces the production of monocyte-chemoattracting chemokines by cardiac fibroblasts to recruit inflammatory monocytes, underlining the fine immune cell cross-talk taking place in myocarditis evolution [119]. Moreover, Th17 cells further enhance their own differentiation through a positive feedback loop, since IL-17-A induces a heart-specific upregulation of IL-6, TNF-α, and IL-1 and promotes the recruitment of CD11b+ monocyte and Gr1+ granulocyte populations to the heart. Furthermore, IL-17A-deficient mice had reduced interstitial myocardial fibrosis [111]. The pathogenicity of Th17 cells has also been demonstrated in human myocarditis. A higher presence of a small noncoding RNA, i.e., hsa-Chr8:96, a homolog of the murine mmu-miR-721 produced by Th17 cells in EAM models and not in acute myocardial infarction, has been found in patients with acute myocarditis and biopsy-proven myocarditis [120]. Moreover, Th17 cells are found to be increased in the peripheral blood of suspected myocarditis/DCM patients with persistent heart failure and are detected also in EMB, correlating with higher levels of cardiac fibrosis. A proof of concept for the role of Th17 cells in disease progression can be obtained by evaluating Th17-associated cytokines in patients’ plasma: IL17-A is increased only after 6 months of disease onset, while IL-6 and TGF-β1 (cytokines relevant for Th17 cell differentiation) are increased at diagnosis. Th17 cell differentiation might be induced by cardiac myosin in human myocarditis, because human cardiac myosin S2 hinge region (S2-16 and S2-28) peptides act as endogenous ligands for Toll-like receptor 2, leading to an exaggerated response from CD14+ monocytes to secrete Th17-differentiating cytokines [9].
The relevance of other T helper subtypes has been more debated than Th17 cells, despite CD4+ cells being known to be fundamental to myocarditis pathogenesis, as the treatment of EAM rats with anti-CD4 antibodies blocks the development of the disease [100]. In a T cell receptor (TCR) transgenic mouse model specific for myosin heavy chain α (residue 614–629) spontaneously developing myocarditis, heart-infiltrating CD4+ T cells secrete IFN-γ and IL-17, indicating their Th1/Th17 phenotype. In particular, IFN-γ signaling is needed for spontaneous myocarditis development, while IL-17A, also in this model, has been linked to disease severity and DCM development [121]. The same findings have been described in in vitro stimulation of EAM mouse splenocytes with a recombinant fragment of cardiac myosin (1074–1646), obtaining lymphocytes secreting more IFN-γ, IL-6, and IL-17 than IL-4 [122]. This evidence underlines the importance of Th1 cells in myocarditis, even if their precise role is still debated. In classical EAM models, MyHC-α-specific Th cells more frequently differentiate towards IFN-γ-secreting cells [123]. Moreover, the transfer of CD4+ T cells specific to influenza hemagglutinin (HA) into transgenic mice expressing HA under the MyHC-α promoter gives rise to a cytotoxic Th1 phenotype, given the increased secretion of chemokines, i.e., Macrophage Inflammatory Protein (MIP)-1α and CCL5, which stimulates CD8+ cell migration [124]. On the other hand, Th2 lymphocytes, for the first time, have been implicated in the pathogenesis of EAM models developing eosinophilic or giant cell myocarditis, and the treatment with anti-IL-4 reduced the disease severity [125]. Conversely, in keeping with most of the studies on Th1 cells, another study with EAM rats showed that Th1 cytokines were increased in the acute phase, while they decreased during the recovery phase, when Th2 cytokines and IL-10 levels increased [126]. The clear role of Th2 cells is still to be clearly determined, but a recent study describe that, in advanced stages of EAM myocarditis, Bcl2-like protein (Bcl2L)12, by complexing the master regulator of Th2 differentiation GATA3, favors Th2 cells differentiation by IL-4 expression and blocks Th2 apoptosis inhibiting the expression of p53, leading to a Th2-mediate inflammation in the heart [127].
Other types of CD4+ lymphocytes, such as Th22 cells, have been reported to be increased in peripheral blood of DCM patients, indicating that a broader range of T helper cells might be involved in myocarditis/DCM pathogenesis [128]. Even if the clear pathogenetic mechanisms are not fully understood, this review has clearly described the double-edged sword role of CD4+ cells in both initiating and mediating recovery in myocarditis; this dual role could be due to TNF-α signaling, which promotes myocarditis development by activating cardiac endothelial cells to recruit T cells, but it can also trigger the activation-induced cell death pathway in cardiac-reactive T cells [129]. Moreover, a type of CD4+ cell that preferentially migrates to the heart has been described. These cells express hepatocyte growth factor receptor c-mesenchymal epithelial transition factor (c-Met) and chemokine receptors CXCR3 and CCR4 and present unique features, as they are able to secrete a mixture of cytokines from the different T helper cells described so far, such as IL-4/IL-13, IL-17, and IL-22, both in EAM models and acute myocarditis cases. c-Met+/CD4+ memory T cells have been identified in both inflammatory DCM and hereditary forms of other cardiomyopathies, suggesting that the immune system’s involvement should be considered even in cases where it is not the primary pathogenetic mechanism. On the other hand, c-Met+/CD8+ memory T cells are more specifically present in DCM. This could be due to the crucial role of CD8+ T cell responses in viral containment during viral infections [106]. Recently, an increased presence of exhausted CD8+ lymphocytes has been identified in both EMB and peripheral blood of patients with inflammatory DCM, with higher levels correlating with a worse prognosis in a combined cohort of inflammatory and non-inflammatory DCM cases [130]. Nonetheless, in models of T cell receptor (TCR) transgenic (Tg) mice specific to cardiac myosin heavy chain (MyHC)-α 334–352, CD4+ T cells have been shown to harbor a cytotoxic phenotype, since they express CD107a, IFN-γ, granzyme B, and natural killer cell receptors (NKG)2A and NKG2D [131].
The T cell compartment in myocarditis may develop distinct features due to expansion of cells equipped with a TCR specifically targeting myocardium. In various models, including in vitro studies, it has been shown that T cells often target the α isoform of MyHC, which is the predominant antigen detected in this context [121,123,124,131,132]. Interestingly, although α-MyHC constitutes only a small fraction of MyHC in the human heart, it has been evidenced that in EAM and in humans, medullary thymic epithelial cells, which are critical for central T cell tolerance, lack the expression of α-MyHC, leading to a defective control over α-MyHC autoantigens, allowing the increased presence of α-MyHC-T cells specifically in peripheral blood [99]. In addition to reduced immune tolerance, structural and immunological mimicry with viral and bacterial infections can contribute to the development of T cells targeting the myocardium, as streptococcal M protein-reactive T cells can target cardiac myosin [133]. Moreover, the evaluation of EMB from DCM patients reveals a preferential use of TCRVβ in infiltrating T cells, particularly in case of DCM with a viral etiology [134].
9. Future Perspectives and Clinical Applications: Targeted Immunosuppression
Among novel approaches to immune-mediated myocarditis, monoclonal antibodies represent a promising option, including rituximab, which acts against CD20+ B cells [135,136], and mepolizumab, which inhibits the binding of interleukin-5 (IL-5) to its receptors expressed on eosinophils, improving cardiac function [137,138,139].
IL-1β, a proinflammatory cytokine in the innate immune pathway that is crucial for host defense, represents another possible target for pharmacological intervention in myocardial inflammation. While neutralizing IL-1β has shown promise in reducing inflammation, interstitial fibrosis, and adverse cardiac remodeling in experimental animal studies [140,141,142], the ARAMIS (Anakinra versus placebo double-blind Randomized controlled trial for the treatment of Acute Myocarditis) phase 3 placebo-controlled trial did not show a net benefit in terms of in terms of heart failure episodes, chest pain, left ventricular ejection fraction < 50%, and ventricular arrythmias in patients with clinically suspected acute myocarditis of unspecified etiology presenting with chest pain and normal left ventricular (LV) function [143]. This is likely due to the low adverse event rate in the trial, and to the prevalent involvement of autoimmune, rather than autoinflammatory mechanisms in a sizable proportion of myocarditis cases. Further studies are needed to clarify the role of IL-1 receptor antagonists in patients with biopsy-proven immune-mediated acute myocarditis, considering the evidence supporting the role of innate immunity in myocarditis [144].
10. Conclusions
Myocarditis has been increasingly recognized as common cause of sudden cardiac death in young adults and heart failure overall.
Despite advancements in both experimental and clinical research, the immunological background of myocarditis remains only partially understood. Exploration of the specific cytokines and molecular pathways, both within myocardium and at the peripheral level, as well as the assessment of genetic predisposition, warrants further studies. Furthermore, the predictors of IS response in myocarditis are still under investigation.
The success of future trials for immunosuppressive treatments in myocarditis will depend also on immunophenotyping characterization of patients with myocarditis. This will help identify individuals with ongoing inflammation or abnormal immune responses, who are the most likely candidates to benefit from IS therapy.
Author Contributions
Conceptualization, C.V., A.S.G. and C.M.; methodology, A.B.; software, F.S.; validation, E.P. and R.M.; investigation, E.B. and M.G.P.-C.; resources, F.S.; writing—original draft preparation, C.V., A.S.G. and C.M.; writing—review and editing, A.L.P.C. and R.M.; visualization, A.S.G. and C.V.; supervision, A.L.P.C.; project administration, A.L.P.C. and R.M.; funding acquisition, A.L.P.C. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interests.
Funding Statement
A.L.P.C. acknowledges the support of Budget Integrato per la Ricerca dei Dipartimenti (BIRD, year 2019), Padua University, Padua, Italy (project title: Myocarditis: genetic background, predictors of dismal prognosis and of response to immunosuppressive therapy); of the Italian Ministry of Health, Target Research, Rome, Italy, year 2019, RF-2019-12370183 (project title: Biopsy-proven myocarditis: genetic background, predictors of dismal prognosis and of response to immunosuppressive therapy and preclinical evaluation of innovative immunomodulatory therapies); and by the European Union—Next Generation EU—NRRP M6C2—Investment: 2.1 “Enhancement and strengthening of biomedical research in the NHS” (project title: Biopsy-proven pediatric and adult giant cell and other rare immune-mediated forms of myocarditis: creation of a prospective multicenter Italian registry and a biobank network to identify clinical, immune, and genetic predictors of dismal prognosis, relapse, and response to immunosuppressive therapy; code PNRR-MR1-2022-12375693, Cup: I93C22000560006). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Richardson P., McKenna W., Bristow M., Maisch B., Mautner B., O’Connell J., Olsen E., Thiene G., Goodwin J., Gyarfas I., et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 2.Caforio A.L.P., Pankuweit S., Arbustini E., Basso C., Gimeno-Blanes J., Felix S.B., Fu M., Heliö T., Heymans S., Jahns R., et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013;34 doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 3.Caforio A.L.P., Calabrese F., Angelini A., Tona F., Vinci A., Bottaro S., Ramondo A., Carturan E., Iliceto S., Thiene G., et al. A Prospective Study of Biopsy-Proven Myocarditis: Prognostic Relevance of Clinical and Aetiopathogenetic Features at Diagnosis. Eur. Heart J. 2007;28:1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 4.Tschöpe C., Ammirati E., Bozkurt B., Caforio A.L.P., Cooper L.T., Felix S.B., Hare J.M., Heidecker B., Heymans S., Hübner N., et al. Myocarditis and Inflammatory Cardiomyopathy: Current Evidence and Future Directions. Nat. Rev. Cardiol. 2021;18:169–193. doi: 10.1038/s41569-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Şelli M.E., Thomas A.C., Wraith D.C., Newby A.C. A Humanized HLA-DR4 Mouse Model for Autoimmune Myocarditis. J. Mol. Cell. Cardiol. 2017;107:22–26. doi: 10.1016/j.yjmcc.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller A.-M., Fischer A., Katus H.A., Kaya Z. Mouse Models of Autoimmune Diseases—Autoimmune Myocarditis. Curr. Pharm. Des. 2015;21:2498–2512. doi: 10.2174/1381612821666150316123711. [DOI] [PubMed] [Google Scholar]
- 7.Guerra-de-Blas P.D.C., Cruz-González D., Martínez-Shio E.B., González-Amaro R., González-Pacheco H., Layseca-Espinosa E., Escobedo-Uribe C.D., Monsiváis-Urenda A.E. Altered Phenotype of Circulating Dendritic Cells and Regulatory T Cells from Patients with Acute Myocarditis. J. Immunol. Res. 2022;2022:8873146. doi: 10.1155/2022/8873146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pistulli R., Andreas E., König S., Drobnik S., Kretzschmar D., Rohm I., Lichtenauer M., Heidecker B., Franz M., Mall G., et al. Characterization of Dendritic Cells in Human and Experimental Myocarditis. ESC Heart Fail. 2020;7:2305–2317. doi: 10.1002/ehf2.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers J.M., Cooper L.T., Kem D.C., Stavrakis S., Kosanke S.D., Shevach E.M., Fairweather D., Stoner J.A., Cox C.J., Cunningham M.W. Cardiac Myosin-Th17 Responses Promote Heart Failure in Human Myocarditis. JCI Insight. 2016;1:e85851. doi: 10.1172/jci.insight.85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith S.C., Allen P.M. Myosin-Induced Acute Myocarditis Is a T Cell-Mediated Disease. J. Immunol. 1991;147:2141–2147. doi: 10.4049/jimmunol.147.7.2141. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P., Cox C.J., Alvarez K.M., Cunningham M.W. Cutting Edge: Cardiac Myosin Activates Innate Immune Responses through TLRs. J. Immunol. 2009;183:27–31. doi: 10.4049/jimmunol.0800861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson U., Ricci R., Hunziker L., Kurrer M.O., Oudit G.Y., Watts T.H., Sonderegger I., Bachmaier K., Kopf M., Penninger J.M. Dendritic Cell-Induced Autoimmune Heart Failure Requires Cooperation between Adaptive and Innate Immunity. Nat. Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 13.Younis A., Matetzky S., Mulla W., Masalha E., Afel Y., Chernomordik F., Fardman A., Goitein O., Ben-Zekry S., Peled Y., et al. Epidemiology Characteristics and Outcome of Patients with Clinically Diagnosed Acute Myocarditis. Am. J. Med. 2020;133:492–499. doi: 10.1016/j.amjmed.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Castrichini M., Porcari A., Baggio C., Gagno G., Maione D., Barbati G., Medo K., Mestroni L., Merlo M., Sinagra G. Sex Differences in Natural History of Cardiovascular Magnetic Resonance- and Biopsy-Proven Lymphocytic Myocarditis. ESC Heart Fail. 2022;9:4010–4019. doi: 10.1002/ehf2.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brociek E., Tymińska A., Giordani A.S., Caforio A.L.P., Wojnicz R., Grabowski M., Ozierański K. Myocarditis: Etiology, Pathogenesis, and Their Implications in Clinical Practice. Biology. 2023;12:874. doi: 10.3390/biology12060874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won T., Song E.J., Kalinoski H.M., Moslehi J.J., Čiháková D. Autoimmune Myocarditis, Old Dogs and New Tricks. Circ. Res. 2024;134:1767–1790. doi: 10.1161/CIRCRESAHA.124.323816. [DOI] [PubMed] [Google Scholar]
- 17.Ammirati E., Cipriani M., Moro C., Raineri C., Pini D., Sormani P., Mantovani R., Varrenti M., Pedrotti P., Conca C., et al. Clinical Presentation and Outcome in a Contemporary Cohort of Patients with Acute Myocarditis: Multicenter Lombardy Registry. Circulation. 2018;138:1088–1099. doi: 10.1161/CIRCULATIONAHA.118.035319. [DOI] [PubMed] [Google Scholar]
- 18.Pahuja M., Adegbala O., Mishra T., Akintoye E., Chehab O., Mony S., Singh M., Ando T., Abubaker H., Yassin A., et al. Trends in the Incidence of In-Hospital Mortality, Cardiogenic Shock, and Utilization of Mechanical Circulatory Support Devices in Myocarditis (Analysis of National Inpatient Sample Data, 2005–2014) J. Card. Fail. 2019;25:457–467. doi: 10.1016/j.cardfail.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Seferović P.M., Tsutsui H., McNamara D.M., Ristić A.D., Basso C., Bozkurt B., Cooper L.T., Jr., Filippatos G., Ide T., Inomata T., et al. Heart Failure Association of the ESC, Heart Failure Society of America and Japanese Heart Failure Society Position Statement on Endomyocardial Biopsy. Eur. J. Heart Fail. 2021;23:854–871. doi: 10.1002/ejhf.2190. [DOI] [PubMed] [Google Scholar]
- 20.Y-Hassan S. Myocarditis and Takotsubo Syndrome: Are They Mutually Exclusive? Int. J. Cardiol. 2014;177:149–151. doi: 10.1016/j.ijcard.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 21.Redfors B., Shao Y., Lyon A.R., Omerovic E. Diagnostic Criteria for Takotsubo Syndrome: A Call for Consensus. Int. J. Cardiol. 2014;176:274–276. doi: 10.1016/j.ijcard.2014.06.094. [DOI] [PubMed] [Google Scholar]
- 22.Bariani R., Cipriani A., Rizzo S., Celeghin R., Bueno Marinas M., Giorgi B., De Gaspari M., Rigato I., Leoni L., Zorzi A., et al. “Hot Phase” Clinical Presentation in Arrhythmogenic Cardiomyopathy. Europace. 2021;23:907–917. doi: 10.1093/europace/euaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bariani R., Rigato I., Cipriani A., Bueno Marinas M., Celeghin R., Basso C., Corrado D., Pilichou K., Bauce B. Myocarditis-like Episodes in Patients with Arrhythmogenic Cardiomyopathy: A Systematic Review on the so-Called Hot-Phase of the Disease. Biomolecules. 2022;12:1324. doi: 10.3390/biom12091324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corrado D., Basso C. Arrhythmogenic Left Ventricular Cardiomyopathy. Heart. 2022;108:733–743. doi: 10.1136/heartjnl-2020-316944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basso C., Thiene G., Corrado D., Angelini A., Nava A., Valente M. Arrhythmogenic Right Ventricular Cardiomyopathy. Dysplasia, Dystrophy, or Myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.CIR.94.5.983. [DOI] [PubMed] [Google Scholar]
- 26.Leone O., Veinot J.P., Angelini A., Baandrup U.T., Basso C., Berry G., Bruneval P., Burke M., Butany J., Calabrese F., et al. 2011 Consensus Statement on Endomyocardial Biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U., Levine G.N., Narula J., Starling R.C., Towbin J., et al. The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur. Heart J. 2007;28:3076–3093. doi: 10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 28.Aretz H.T. Myocarditis: The Dallas Criteria. Hum Pathol. 1987;18:619–624. doi: 10.1016/S0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 29.Aretz H.T., Billingham M.E., Edwards W.D., Factor S.M., Fallon J.T., Fenoglio J.J., Jr., Olsen E.G., Schoen F.J. Myocarditis. A Histopathologic Definition and Classification. Am. J. Cardiovasc. Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 30.Caforio A.L.P. Myocarditis: Pathogenesis, Diagnosis and Treatment. Springer Nature; Cham, Switzerland: 2020. [Google Scholar]
- 31.Mahrholdt H., Goedecke C., Wagner A., Meinhardt G., Athanasiadis A., Vogelsberg H., Fritz P., Klingel K., Kandolf R., Sechtem U. Cardiovascular Magnetic Resonance Assessment of Human Myocarditis: A Comparison to Histology and Molecular Pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich M.G., Sechtem U., Schulz-Menger J., Holmvang G., Alakija P., Cooper L.T., White J.A., Abdel-Aty H., Gutberlet M., Prasad S., et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira V.M., Schulz-Menger J., Holmvang G., Kramer C.M., Carbone I., Sechtem U., Kindermann I., Gutberlet M., Cooper L.T., Liu P., et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018;72:3158–3176. doi: 10.1016/j.jacc.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 34.Vicenzetto C., Giordani A.S., Menghi C., Baritussio A., Peloso Cattini M.G., Pontara E., Bison E., Rizzo S., De Gaspari M., Basso C., et al. The Role of the Immune System in Pathobiology and Therapy of Myocarditis: A Review. Biomedicines. 2024;12:1156. doi: 10.3390/biomedicines12061156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caforio A.L.P., Giordani A.S., Baritussio A., Marcolongo D., Vicenzetto C., Tarantini G., Napodano M., Toscano G., Gregori D., Brigiari G., et al. Long-term Efficacy and Safety of Tailored Immunosuppressive Therapy in Immune-mediated Biopsy-proven Myocarditis: A Propensity-weighted Study. Eur. J. Heart Fail. 2024;26:1175–1185. doi: 10.1002/ejhf.3220. [DOI] [PubMed] [Google Scholar]
- 36.Baritussio A., Schiavo A., Basso C., Giordani A.S., Cheng C.-Y., Pontara E., Cattini M.G., Bison E., Gallo N., De Gaspari M., et al. Predictors of Relapse, Death or Heart Transplantation in Myocarditis before the Introduction of Immunosuppression: Negative Prognostic Impact of Female Gender, Fulminant Onset, Lower Ejection Fraction and Serum Autoantibodies. Eur. J. Heart Fail. 2022;24:1033–1044. doi: 10.1002/ejhf.2496. [DOI] [PubMed] [Google Scholar]
- 37.Eichhorn C., Koeckerling D., Reddy R.K., Ardissino M., Rogowski M., Coles B., Hunziker L., Greulich S., Shiri I., Frey N., et al. Risk Stratification in Nonischemic Dilated Cardiomyopathy Using CMR Imaging: A Systematic Review and Meta-Analysis. JAMA. 2024;332:1535–1550. doi: 10.1001/jama.2024.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baritussio A., Cheng C.-Y., Simeti G., Ocagli H., Lorenzoni G., Giordani A.S., Basso C., Rizzo S., De Gaspari M., Motta R., et al. CMR Predictors of Favorable Outcome in Myocarditis: A Single-Center Experience. J. Clin. Med. 2024;13:1229. doi: 10.3390/jcm13051229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kusano K.F., Satomi K. Diagnosis and Treatment of Cardiac Sarcoidosis. Heart. 2016;102:184–190. doi: 10.1136/heartjnl-2015-307877. [DOI] [PubMed] [Google Scholar]
- 40.Hamzeh N., Steckman D.A., Sauer W.H., Judson M.A. Pathophysiology and Clinical Management of Cardiac Sarcoidosis. Nat. Rev. Cardiol. 2015;12:278–288. doi: 10.1038/nrcardio.2015.22. [DOI] [PubMed] [Google Scholar]
- 41.Sharma R., Kouranos V., Cooper L.T., Metra M., Ristic A., Heidecker B., Baksi J., Wicks E., Merino J.L., Klingel K., et al. Management of Cardiac Sarcoidosis. Eur. Heart J. 2024;45:2697–2726. doi: 10.1093/eurheartj/ehae356. [DOI] [PubMed] [Google Scholar]
- 42.Ammirati E., Buono A., Moroni F., Gigli L., Power J.R., Ciabatti M., Garascia A., Adler E.D., Pieroni M. State-of-the-Art of Endomyocardial Biopsy on Acute Myocarditis and Chronic Inflammatory Cardiomyopathy. Curr. Cardiol. Rep. 2022;24:597–609. doi: 10.1007/s11886-022-01680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gracia E., Monach P., Shih J. Monitoring of Disease Activity in Eosinophilic Myocarditis via Pet Scans. J. Am. Coll. Cardiol. 2020;75:2559. doi: 10.1016/S0735-1097(20)33186-7. [DOI] [Google Scholar]
- 44.Kandolin R., Lehtonen J., Salmenkivi K., Räisänen-Sokolowski A., Lommi J., Kupari M. Diagnosis, Treatment, and Outcome of Giant-Cell Myocarditis in the Era of Combined Immunosuppression. Circ. Heart Fail. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 45.Lamacie M.M., Almufleh A., Nair V., Stadnick E., Birnie D., Beanlands R.S.B., Chih S. Serial 18F-Fluorodeoxyglucose Positron Emission Tomography Imaging in a Patient with Giant Cell Myocarditis. Circ. Cardiovasc. Imaging. 2020;13:e009940. doi: 10.1161/CIRCIMAGING.119.009940. [DOI] [PubMed] [Google Scholar]
- 46.Ederhy S., Devos P., Pinna B., Funck-Brentano E., Abbar B., Fenioux C., Cohen A.A., Moslehi J., Bretagne M., Allenbach Y., et al. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Imaging for the Diagnosis of Immune Checkpoint Inhibitor-Associated Myocarditis. Arch. Cardiovasc. Dis. 2022;115:114–116. doi: 10.1016/j.acvd.2021.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Mahfoud F., Gärtner B., Kindermann M., Ukena C., Gadomski K., Klingel K., Kandolf R., Böhm M., Kindermann I. Virus Serology in Patients with Suspected Myocarditis: Utility or Futility? Eur. Heart J. 2011;32:897–903. doi: 10.1093/eurheartj/ehq493. [DOI] [PubMed] [Google Scholar]
- 48.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., Burri H., Butler J., Čelutkienė J., Chioncel O., et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 49.Iacucci M., de Silva S., Ghosh S. Mesalazine in Inflammatory Bowel Disease: A Trendy Topic Once Again? Can. J. Gastroenterol. 2010;24:127–133. doi: 10.1155/2010/586092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inamul Haq M., Ahmed S., Pasha W., Anwer Ali Zaidi S. Mesalazine-Induced Cardiotoxicity. J. Rare Disord. Diagn. Ther. 2018;4:24. doi: 10.21767/2380-7245.100192. [DOI] [Google Scholar]
- 51.Giordani A.S., Candelora A., Fiacca M., Cheng C., Barberio B., Baritussio A., Marcolongo R., Iliceto S., Carturan E., De Gaspari M., et al. Myocarditis and Inflammatory Bowel Diseases: A Single-Center Experience and a Systematic Literature Review. Int. J. Cardiol. 2023;376:165–171. doi: 10.1016/j.ijcard.2023.01.071. [DOI] [PubMed] [Google Scholar]
- 52.Naqash A.R., Moey M.Y.Y., Cherie Tan X.-W., Laharwal M., Hill V., Moka N., Finnigan S., Murray J., Johnson D.B., Moslehi J.J., et al. Major Adverse Cardiac Events with Immune Checkpoint Inhibitors: A Pooled Analysis of Trials Sponsored by the National Cancer Institute-Cancer Therapy Evaluation Program. J. Clin. Oncol. 2022;40:3439–3452. doi: 10.1200/JCO.22.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson D.B., Balko J.M., Compton M.L., Chalkias S., Gorham J., Xu Y., Hicks M., Puzanov I., Alexander M.R., Bloomer T.L., et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng C.-Y., Baritussio A., Giordani A.S., Iliceto S., Marcolongo R., Caforio A.L.P. Myocarditis in Systemic Immune-Mediated Diseases: Prevalence, Characteristics and Prognosis. A Systematic Review. Autoimmun. Rev. 2022;21:103037. doi: 10.1016/j.autrev.2022.103037. [DOI] [PubMed] [Google Scholar]
- 55.Caforio A.L.P., Adler Y., Agostini C., Allanore Y., Anastasakis A., Arad M., Böhm M., Charron P., Elliott P.M., Eriksson U., et al. Diagnosis and Management of Myocardial Involvement in Systemic Immune-Mediated Diseases: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. Eur. Heart J. 2017;38:2649–2662. doi: 10.1093/eurheartj/ehx321. [DOI] [PubMed] [Google Scholar]
- 56.Mahr A., Moosig F., Neumann T., Szczeklik W., Taillé C., Vaglio A., Zwerina J. Eosinophilic Granulomatosis with Polyangiitis (Churg–Strauss): Evolutions in Classification, Etiopathogenesis, Assessment and Management. Curr. Opin. Rheumatol. 2014;26:16. doi: 10.1097/BOR.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 57.Comarmond C., Pagnoux C., Khellaf M., Cordier J.-F., Hamidou M., Viallard J.-F., Maurier F., Jouneau S., Bienvenu B., Puéchal X., et al. Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss): Clinical Characteristics and Long-Term Followup of the 383 Patients Enrolled in the French Vasculitis Study Group Cohort. Arthritis Rheum. 2013;65:270–281. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 58.Caforio A.L.P., Goldman J.H., Haven A.J., Baig K.M., Dalla L., McKenna W.J. Circulating Cardiac-Specific Autoantibodies as Markers of Autoimmunity in Clinical and Biopsy-Proven Myocarditis. Eur. Heart J. 1997;18:270–275. doi: 10.1093/oxfordjournals.eurheartj.a015230. [DOI] [PubMed] [Google Scholar]
- 59.Warraich R.S., Dunn M.J., Yacoub M.H. Subclass Specificity of Autoantibodies against Myosin in Patients with Idiopathic Dilated Cardiomyopathy: Pro-Inflammatory Antibodies in DCM Patients. Biochem. Biophys. Res. Commun. 1999;259:255–261. doi: 10.1006/bbrc.1999.0761. [DOI] [PubMed] [Google Scholar]
- 60.Caforio A.L., Grazzini M., Mann J.M., Keeling P.J., Bottazzo G.F., McKenna W.J., Schiaffino S. Identification of Alpha- and Beta-Cardiac Myosin Heavy Chain Isoforms as Major Autoantigens in Dilated Cardiomyopathy. Circulation. 1992;85:1734–1742. doi: 10.1161/01.CIR.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 61.Lauer B., Schannwell M., Kühl U., Strauer B.E., Schultheiss H.P. Antimyosin Autoantibodies Are Associated with Deterioration of Systolic and Diastolic Left Ventricular Function in Patients with Chronic Myocarditis. J. Am. Coll. Cardiol. 2000;35:11–18. doi: 10.1016/S0735-1097(99)00485-4. [DOI] [PubMed] [Google Scholar]
- 62.Caforio A.L., Bonifacio E., Stewart J.T., Neglia D., Parodi O., Bottazzo G.F., McKenna W.J. Novel Organ-Specific Circulating Cardiac Autoantibodies in Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 1990;15:1527–1534. doi: 10.1016/0735-1097(90)92821-I. [DOI] [PubMed] [Google Scholar]
- 63.Schulze K., Becker B.F., Schauer R., Schultheiss H.P. Antibodies to ADP-ATP Carrier--an Autoantigen in Myocarditis and Dilated Cardiomyopathy--Impair Cardiac Function. Circulation. 1990;81:959–969. doi: 10.1161/01.CIR.81.3.959. [DOI] [PubMed] [Google Scholar]
- 64.Wallukat G., Morwinski M., Kowal K., Forster A., Boewer V., Wollenberger A. Autoantibodies against the -Adrenergic Receptor in Human Myocarditis and Dilated Cardiomyopathy: -Adrenergic Agonism without Desensitization. Eur. Heart J. 1991;12:178–181. doi: 10.1093/eurheartj/12.suppl_D.178. [DOI] [PubMed] [Google Scholar]
- 65.Caforio A.L.P., Mahon N.G., Baig M.K., Tona F., Murphy R.T., Elliott P.M., McKenna W.J. Prospective Familial Assessment in Dilated Cardiomyopathy: Cardiac Autoantibodies Predict Disease Development in Asymptomatic Relatives. Circulation. 2007;115:76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]
- 66.Warraich R.S., Griffiths E., Falconar A., Pabbathi V., Bell C., Angelini G., Suleiman M.-S., Yacoub M.H. Human Cardiac Myosin Autoantibodies Impair Myocyte Contractility: A Cause-and-Effect Relationship. FASEB J. 2006;20:651–660. doi: 10.1096/fj.04-3001com. [DOI] [PubMed] [Google Scholar]
- 67.Mahon N.G., Murphy R.T., MacRae C.A., Caforio A.L.P., Elliott P.M., McKenna W.J. Echocardiographic Evaluation in Asymptomatic Relatives of Patients with Dilated Cardiomyopathy Reveals Preclinical Disease. Ann. Intern. Med. 2005;143:108–115. doi: 10.7326/0003-4819-143-2-200507190-00009. [DOI] [PubMed] [Google Scholar]
- 68.Caforio A.L.P., De Luca G., Baritussio A., Seguso M., Gallo N., Bison E., Cattini M.G., Pontara E., Gargani L., Pepe A., et al. Serum Organ-Specific Anti-Heart and Anti-Intercalated Disk Autoantibodies as New Autoimmune Markers of Cardiac Involvement in Systemic Sclerosis: Frequency, Clinical and Prognostic Correlates. Diagnostics. 2021;11:2165. doi: 10.3390/diagnostics11112165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caforio A.L.P., Baritussio A., Marcolongo R., Cheng C.-Y., Pontara E., Bison E., Cattini M.G., Gallo N., Plebani M., Iliceto S., et al. Serum Anti-Heart and Anti-Intercalated Disk Autoantibodies: Novel Autoimmune Markers in Cardiac Sarcoidosis. J. Clin. Med. 2021;10:2476. doi: 10.3390/jcm10112476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caforio A.L.P., Angelini A., Blank M., Shani A., Kivity S., Goddard G., Doria A., Schiavo A., Testolina M., Bottaro S., et al. Passive Transfer of Affinity-Purified Anti-Heart Autoantibodies (AHA) from Sera of Patients with Myocarditis Induces Experimental Myocarditis in Mice. Int. J. Cardiol. 2015;179:166–177. doi: 10.1016/j.ijcard.2014.10.165. [DOI] [PubMed] [Google Scholar]
- 71.Giordani A.S., Pontara E., Vicenzetto C., Baritussio A., Peloso Cattini M.G., Bison E., Re F., Marcolongo R., Joseph S., Chatterjee D., et al. Prevalence and Correlates of Anti-DSG2 Antibodies in Arrhythmogenic Right Ventricular Cardiomyopathy and Myocarditis: Immunological Insights from a Multicenter Study. J. Clin. Med. 2024;13:6736. doi: 10.3390/jcm13226736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suna G., Kolios A., Chatterjee D., Fatah M., Gasperetti A., Casella M., Sommariva E., Franzen D., Manka R., Pazhenkottil A., et al. Anti-Desmoglein2 Autoantibodies Are Present in Patients with Cardiac Sarcoidosis and Correlate with Cardiac Inflammation. Europace. 2021;23:euab116.539. doi: 10.1093/europace/euab116.539. [DOI] [Google Scholar]
- 73.Chatterjee D., Fatah M., Akdis D., Spears D.A., Koopmann T.T., Mittal K., Rafiq M.A., Cattanach B.M., Zhao Q., Healey J.S., et al. An Autoantibody Identifies Arrhythmogenic Right Ventricular Cardiomyopathy and Participates in Its Pathogenesis. Eur. Heart J. 2018;39:3932–3944. doi: 10.1093/eurheartj/ehy567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lota A.S., Hazebroek M.R., Theotokis P., Wassall R., Salmi S., Halliday B.P., Tayal U., Verdonschot J., Meena D., Owen R., et al. Genetic Architecture of Acute Myocarditis and the Overlap with Inherited Cardiomyopathy. Circulation. 2022;146:1123–1134. doi: 10.1161/CIRCULATIONAHA.121.058457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marinas M.B., Baritussio A., Cason M., Celeghin R., Giordani A., Carturan E., De Gaspari M., Rizzo S., Tarantini G., Marcolongo R., et al. 510 Genetic Characterization of Biopsy-Proven Myocarditis: A Pilot Study. Eur. Heart J. Suppl. 2022;24:suac121.416. doi: 10.1093/eurheartjsupp/suac121.416. [DOI] [Google Scholar]
- 76.Monda E., Bakalakos A., Cannie D., O’Mahony C., Syrris P., Kaski J.P., Limongelli G., Elliott P.M. Prevalence of Pathogenic Variants in Cardiomyopathy-Associated Genes in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2024;12:1101–1111. doi: 10.1016/j.jchf.2024.02.012. [DOI] [PubMed] [Google Scholar]
- 77.Baggio C., Gagno G., Porcari A., Paldino A., Artico J., Castrichini M., Dal Ferro M., Bussani R., Merlo M. Myocarditis: Which Role for Genetics? Curr. Cardiol. Rep. 2021;23:58. doi: 10.1007/s11886-021-01492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruestle K., Hackner K., Kreye G., Heidecker B. Autoimmunity in Acute Myocarditis: How Immunopathogenesis Steers New Directions for Diagnosis and Treatment. Curr. Cardiol. Rep. 2020;22:28. doi: 10.1007/s11886-020-01278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martinetti M., Dugoujon J.M., Caforio A.L., Schwarz G., Gavazzi A., Graziano G., Arbustini E., Lorini R., McKenna W.J., Bottazzo G.F. HLA and Immunoglobulin Polymorphisms in Idiopathic Dilated Cardiomyopathy. Hum. Immunol. 1992;35:193–199. doi: 10.1016/0198-8859(92)90105-V. [DOI] [PubMed] [Google Scholar]
- 80.Carlquist J.F., Menlove R.L., Murray M.B., O’Connell J.B., Anderson J.L. HLA Class II (DR and DQ) Antigen Associations in Idiopathic Dilated Cardiomyopathy. Validation Study and Meta-Analysis of Published HLA Association Studies. Circulation. 1991;83:515–522. doi: 10.1161/01.CIR.83.2.515. [DOI] [PubMed] [Google Scholar]
- 81.Elliott J.F., Liu J., Yuan Z.-N., Bautista-Lopez N., Wallbank S.L., Suzuki K., Rayner D., Nation P., Robertson M.A., Liu G., et al. Autoimmune Cardiomyopathy and Heart Block Develop Spontaneously in HLA-DQ8 Transgenic IAbeta Knockout NOD Mice. Proc. Natl. Acad. Sci. USA. 2003;100:13447–13452. doi: 10.1073/pnas.2235552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frustaci A., Russo M.A., Chimenti C. Randomized Study on the Efficacy of Immunosuppressive Therapy in Patients with Virus-Negative Inflammatory Cardiomyopathy: The TIMIC Study. Eur. Heart J. 2009;30:1995–2002. doi: 10.1093/eurheartj/ehp249. [DOI] [PubMed] [Google Scholar]
- 83.Escher F., Kühl U., Lassner D., Poller W., Westermann D., Pieske B., Tschöpe C., Schultheiss H.-P. Long-Term Outcome of Patients with Virus-Negative Chronic Myocarditis or Inflammatory Cardiomyopathy after Immunosuppressive Therapy. Clin. Res. Cardiol. 2016;105:1011–1020. doi: 10.1007/s00392-016-1011-z. [DOI] [PubMed] [Google Scholar]
- 84.Cheng C.-Y., Cheng G.-Y., Shan Z.-G., Baritussio A., Lorenzoni G., Tyminska A., Ozieranski K., Iliceto S., Marcolongo R., Gregori D., et al. Efficacy of Immunosuppressive Therapy in Myocarditis: A 30-Year Systematic Review and Meta Analysis. Autoimmun. Rev. 2021;20:102710. doi: 10.1016/j.autrev.2020.102710. [DOI] [PubMed] [Google Scholar]
- 85.Chimenti C., Russo M.A., Frustaci A. Immunosuppressive Therapy in Virus-Negative Inflammatory Cardiomyopathy: 20-Year Follow-up of the TIMIC Trial. Eur. Heart J. 2022;43:3463–3473. doi: 10.1093/eurheartj/ehac348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Merken J., Hazebroek M., Van Paassen P., Verdonschot J., Van Empel V., Knackstedt C., Abdul Hamid M., Seiler M., Kolb J., Hoermann P., et al. Immunosuppressive Therapy Improves Both Short- and Long-Term Prognosis in Patients with Virus-Negative Nonfulminant Inflammatory Cardiomyopathy. Circ. Heart Fail. 2018;11:e004228. doi: 10.1161/CIRCHEARTFAILURE.117.004228. [DOI] [PubMed] [Google Scholar]
- 87.Zhong Z., Yang Z., Peng Y., Wang L., Yuan X. Diagnosis and Treatment of Eosinophilic Myocarditis. J Transl Autoimmun. 2021;4:100118. doi: 10.1016/j.jtauto.2021.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fazelpour S., Sadek M.M., Nery P.B., Beanlands R.S., Tzemos N., Toma M., Birnie D.H. Corticosteroid and Immunosuppressant Therapy for Cardiac Sarcoidosis: A Systematic Review. J. Am. Heart Assoc. 2021;10:e021183. doi: 10.1161/JAHA.121.021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang P., Chen Z., Huang W., Zhang J., Zou L., Wang H. Communications between Macrophages and Cardiomyocytes. Cell Commun. Signal. 2023;21:206. doi: 10.1186/s12964-023-01202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leuschner F., Courties G., Dutta P., Mortensen L.J., Gorbatov R., Sena B., Novobrantseva T.I., Borodovsky A., Fitzgerald K., Koteliansky V., et al. Silencing of CCR2 in Myocarditis. Eur. Heart J. 2015;36:1478–1488. doi: 10.1093/eurheartj/ehu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin L., Wei J., Xue J., Fan G., Zhu W., Zhu Y., Wu R. Drp1 Promotes Macrophage M1 Polarization and Inflammatory Response in Autoimmune Myocarditis by Driving Mitochondrial Fission. J. Cardiovasc. Transl. Res. 2024 doi: 10.1007/s12265-024-10570-2. [DOI] [PubMed] [Google Scholar]
- 92.Hou X., Chen G., Bracamonte-Baran W., Choi H.S., Diny N.L., Sung J., Hughes D., Won T., Wood M.K., Talor M.V., et al. The Cardiac Microenvironment Instructs Divergent Monocyte Fates and Functions in Myocarditis. Cell Rep. 2019;28:172–189.e7. doi: 10.1016/j.celrep.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valaperti A., Marty R.R., Kania G., Germano D., Mauermann N., Dirnhofer S., Leimenstoll B., Blyszczuk P., Dong C., Mueller C., et al. CD11b+ Monocytes Abrogate Th17 CD4+ T Cell-Mediated Experimental Autoimmune Myocarditis. J. Immunol. 2008;180:2686–2695. doi: 10.4049/jimmunol.180.4.2686. [DOI] [PubMed] [Google Scholar]
- 94.Anzai A., Mindur J.E., Halle L., Sano S., Choi J.L., He S., McAlpine C.S., Chan C.T., Kahles F., Valet C., et al. Self-Reactive CD4+ IL-3+ T Cells Amplify Autoimmune Inflammation in Myocarditis by Inciting Monocyte Chemotaxis. J. Exp. Med. 2019;216:369–383. doi: 10.1084/jem.20180722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weckbach L.T., Grabmaier U., Uhl A., Gess S., Boehm F., Zehrer A., Pick R., Salvermoser M., Czermak T., Pircher J., et al. Midkine Drives Cardiac Inflammation by Promoting Neutrophil Trafficking and NETosis in Myocarditis. J. Exp. Med. 2019;216:350–368. doi: 10.1084/jem.20181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu Z., Hua X., Mo X., Chang Y., Chen X., Xu Z., Tao M., Hu G., Song J. Inhibition of NETosis via PAD4 Alleviated Inflammation in Giant Cell Myocarditis. iScience. 2023;26:107162. doi: 10.1016/j.isci.2023.107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diny N.L., Baldeviano G.C., Talor M.V., Barin J.G., Ong S., Bedja D., Hays A.G., Gilotra N.A., Coppens I., Rose N.R., et al. Eosinophil-Derived IL-4 Drives Progression of Myocarditis to Inflammatory Dilated Cardiomyopathy. J. Exp. Med. 2017;214:943–957. doi: 10.1084/jem.20161702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barin J.G., Baldeviano G.C., Talor M.V., Wu L., Ong S., Fairweather D., Bedja D., Stickel N.R., Fontes J.A., Cardamone A.B., et al. Fatal Eosinophilic Myocarditis Develops in the Absence of IFN-γ and IL-17A. J. Immunol. 2013;191:4038–4047. doi: 10.4049/jimmunol.1301282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lv H., Havari E., Pinto S., Gottumukkala R.V.S.R.K., Cornivelli L., Raddassi K., Matsui T., Rosenzweig A., Bronson R.T., Smith R., et al. Impaired Thymic Tolerance to α-Myosin Directs Autoimmunity to the Heart in Mice and Humans. J. Clin. Investig. 2011;121:1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Q.-Q., Wang Y.-L., Yuan H.-T., Liu F.-Q., Jin Y.-P., Han B. Immune Tolerance to Cardiac Myosin Induced by Anti-CD4 Monoclonal Antibody in Autoimmune Myocarditis Rats. J. Clin. Immunol. 2006;26:213–221. doi: 10.1007/s10875-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 101.Van der Borght K., Scott C.L., Martens L., Sichien D., Van Isterdael G., Nindl V., Saeys Y., Boon L., Ludewig B., Gillebert T.C., et al. Myocarditis Elicits Dendritic Cell and Monocyte Infiltration in the Heart and Self-Antigen Presentation by Conventional Type 2 Dendritic Cells. Front. Immunol. 2018;9:2714. doi: 10.3389/fimmu.2018.02714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Machino-Ohtsuka T., Tajiri K., Kimura T., Sakai S., Sato A., Yoshida T., Hiroe M., Yasutomi Y., Aonuma K., Imanaka-Yoshida K. Tenascin-C Aggravates Autoimmune Myocarditis via Dendritic Cell Activation and Th17 Cell Differentiation. J. Am. Heart Assoc. 2014;3:e001052. doi: 10.1161/JAHA.114.001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gong Q., Huang J., Wu Q. Integrated Single-Cell and RNA Sequencing Analysis Identifies Key Immune Cell and Dendritic Cells Associated Genes Participated in Myocarditis. J. Immunol. Res. 2022;2022:8655343. doi: 10.1155/2022/8655343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee E.Y., Lee H.L., Kim H.T., Lee H.D., Park J.A. Clinical Features and Short-Term Outcomes of Pediatric Acute Fulminant Myocarditis in a Single Center. Korean J. Pediatr. 2014;57:489–495. doi: 10.3345/kjp.2014.57.11.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu B., Li J., Ni H., Zhuang X., Qi Z., Chen Q., Wen Z., Shi H., Luo X., Jin B. TLR4 Activation Promotes the Progression of Experimental Autoimmune Myocarditis to Dilated Cardiomyopathy by Inducing Mitochondrial Dynamic Imbalance. Oxid. Med. Cell. Longev. 2018;2018:3181278. doi: 10.1155/2018/3181278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fanti S., Stephenson E., Rocha-Vieira E., Protonotarios A., Kanoni S., Shahaj E., Longhi M.P., Vyas V.S., Dyer C., Pontarini E., et al. Circulating C-Met-Expressing Memory T Cells Define Cardiac Autoimmunity. Circulation. 2022;146:1930–1945. doi: 10.1161/CIRCULATIONAHA.121.055610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu X., Zhang W., Han Z. Decreased Circulating Follicular Regulatory T Cells in Patients with Dilated Cardiomyopathy. Braz. J. Med. Biol. Res. 2021;54:e11232. doi: 10.1590/1414-431x2021e11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei Y., Yu K., Wei H., Su X., Zhu R., Shi H., Sun H., Luo Q., Xu W., Xiao J., et al. CD4+ CD25+ GARP+ Regulatory T Cells Display a Compromised Suppressive Function in Patients with Dilated Cardiomyopathy. Immunology. 2017;151:291–303. doi: 10.1111/imm.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Akhmerov A., Rogers R., de Couto G., Valle J., Li L., Ibrahim A., Sanchez L., Zhang R., Lin Y.-N., Liu W., et al. Regulatory T Cell Activation, Proliferation, and Reprogramming Induced by Extracellular Vesicles. J. Heart Lung Transplant. 2021;40:1387–1395. doi: 10.1016/j.healun.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yan L., Hu F., Yan X., Wei Y., Ma W., Wang Y., Lu S., Wang Z. Inhibition of MicroRNA-155 Ameliorates Experimental Autoimmune Myocarditis by Modulating Th17/Treg Immune Response. J. Mol. Med. 2016;94:1063–1079. doi: 10.1007/s00109-016-1414-3. [DOI] [PubMed] [Google Scholar]
- 111.Baldeviano G.C., Barin J.G., Talor M.V., Srinivasan S., Bedja D., Zheng D., Gabrielson K., Iwakura Y., Rose N.R., Cihakova D. Interleukin-17A Is Dispensable for Myocarditis but Essential for the Progression to Dilated Cardiomyopathy. Circ. Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 112.Cheng C., Baritussio A., Giordani A.S., Marcolongo R., Caforio A.L.P., Iliceto S. Role of T Cells in Viral and Immune-Mediated Myocarditis. Cardiol. Discov. 2024;4:43–54. doi: 10.1097/CD9.0000000000000116. [DOI] [Google Scholar]
- 113.Sonderegger I., Röhn T.A., Kurrer M.O., Iezzi G., Zou Y., Kastelein R.A., Bachmann M.F., Kopf M. Neutralization of IL-17 by Active Vaccination Inhibits IL-23-Dependent Autoimmune Myocarditis. Eur. J. Immunol. 2006;36:2849–2856. doi: 10.1002/eji.200636484. [DOI] [PubMed] [Google Scholar]
- 114.Chen P., Baldeviano G.C., Ligons D.L., Talor M.V., Barin J.G., Rose N.R., Cihakova D. Susceptibility to Autoimmune Myocarditis Is Associated with Intrinsic Differences in CD4(+) T Cells. Clin. Exp. Immunol. 2012;169:79–88. doi: 10.1111/j.1365-2249.2012.04598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rangachari M., Mauermann N., Marty R.R., Dirnhofer S., Kurrer M.O., Komnenovic V., Penninger J.M., Eriksson U. T-Bet Negatively Regulates Autoimmune Myocarditis by Suppressing Local Production of Interleukin 17. J. Exp. Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamashita T., Iwakura T., Matsui K., Kawaguchi H., Obana M., Hayama A., Maeda M., Izumi Y., Komuro I., Ohsugi Y., et al. IL-6-Mediated Th17 Differentiation through RORγt Is Essential for the Initiation of Experimental Autoimmune Myocarditis. Cardiovasc. Res. 2011;91:640–648. doi: 10.1093/cvr/cvr148. [DOI] [PubMed] [Google Scholar]
- 117.Eriksson U., Kurrer M.O., Schmitz N., Marsch S.C., Fontana A., Eugster H.-P., Kopf M. Interleukin-6-Deficient Mice Resist Development of Autoimmune Myocarditis Associated with Impaired Upregulation of Complement C3. Circulation. 2003;107:320–325. doi: 10.1161/01.CIR.0000043802.38699.66. [DOI] [PubMed] [Google Scholar]
- 118.Hua X., Hu G., Hu Q., Chang Y., Hu Y., Gao L., Chen X., Yang P.-C., Zhang Y., Li M., et al. Single-Cell RNA Sequencing to Dissect the Immunological Network of Autoimmune Myocarditis. Circulation. 2020;142:384–400. doi: 10.1161/CIRCULATIONAHA.119.043545. [DOI] [PubMed] [Google Scholar]
- 119.Wu L., Ong S., Talor M.V., Barin J.G., Baldeviano G.C., Kass D.A., Bedja D., Zhang H., Sheikh A., Margolick J.B., et al. Cardiac Fibroblasts Mediate IL-17A-Driven Inflammatory Dilated Cardiomyopathy. J. Exp. Med. 2014;211:1449–1464. doi: 10.1084/jem.20132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blanco-Domínguez R., Sánchez-Díaz R., de la Fuente H., Jiménez-Borreguero L.J., Matesanz-Marín A., Relaño M., Jiménez-Alejandre R., Linillos-Pradillo B., Tsilingiri K., Martín-Mariscal M.L., et al. A Novel Circulating MicroRNA for the Detection of Acute Myocarditis. N. Engl. J. Med. 2021;384:2014–2027. doi: 10.1056/NEJMoa2003608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nindl V., Maier R., Ratering D., De Giuli R., Züst R., Thiel V., Scandella E., Di Padova F., Kopf M., Rudin M., et al. Cooperation of Th1 and Th17 Cells Determines Transition from Autoimmune Myocarditis to Dilated Cardiomyopathy. Eur. J. Immunol. 2012;42:2311–2321. doi: 10.1002/eji.201142209. [DOI] [PubMed] [Google Scholar]
- 122.Daniels M.D., Hyland K.V., Wang K., Engman D.M. Recombinant Cardiac Myosin Fragment Induces Experimental Autoimmune Myocarditis via Activation of Th1 and Th17 Immunity. Autoimmunity. 2008;41:490–499. doi: 10.1080/08916930802167902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maier R., Miller S., Kurrer M., Krebs P., de Giuli R., Kremer M., Scandella E., Ludewig B. Quantification and Characterization of Myosin Peptide-Specific CD4+ T Cells in Autoimmune Myocarditis. J. Immunol. Methods. 2005;304:117–125. doi: 10.1016/j.jim.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 124.Song H.K., Noorchashm H., Lin T.H., Moore D.J., Greeley S.A., Caton A.J., Naji A. Specialized CC-Chemokine Secretion by Th1 Cells in Destructive Autoimmune Myocarditis. J. Autoimmun. 2003;21:295–303. doi: 10.1016/S0896-8411(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 125.Afanasyeva M., Wang Y., Kaya Z., Park S., Zilliox M.J., Schofield B.H., Hill S.L., Rose N.R. Experimental Autoimmune Myocarditis in A/J Mice Is an Interleukin-4-Dependent Disease with a Th2 Phenotype. Am. J. Pathol. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fuse K., Kodama M., Ito M., Okura Y., Kato K., Hanawa H., Aoki S., Aizawa Y. Polarity of Helper T Cell Subsets Represents Disease Nature and Clinical Course of Experimental Autoimmune Myocarditis in Rats. Clin. Exp. Immunol. 2003;134:403–408. doi: 10.1111/j.1365-2249.2003.02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chen X., Zeng X.-H., Wang M., Chen L., Zhang N., Rao M., Yang P.-C., Song J. Bcl2-Like Protein 12 Is Required for the Aberrant T Helper-2 Polarization in the Heart by Enhancing Interleukin-4 Expression and Compromising Apoptotic Machinery in CD4+ T Cells. Circulation. 2018;138:22. doi: 10.1161/CIRCULATIONAHA.118.033890. [DOI] [PubMed] [Google Scholar]
- 128.Kong Q., Li X., Wu W., Yang F., Liu Y., Lai W., Pan X., Gao M., Xue Y. Increased Circulating T-helper 22 Cells in Patients with Dilated Cardiomyopathy. Mol. Med. Rep. 2014;10:359–364. doi: 10.3892/mmr.2014.2146. [DOI] [PubMed] [Google Scholar]
- 129.Rolski F., Tkacz K., Węglarczyk K., Kwiatkowski G., Pelczar P., Jaźwa-Kusior A., Bar A., Kuster G.M., Chłopicki S., Siedlar M., et al. TNF-α Protects from Exacerbated Myocarditis and Cardiac Death by Suppressing Expansion of Activated Heart-Reactive CD4+ T Cells. Cardiovasc. Res. 2024;120:82–94. doi: 10.1093/cvr/cvad158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sikking M.A., Harding D., Henkens M.T.H.M., Stroeks S.L.V.M., Venner M.F.G.H.M., Nihant B., van Leeuwen R.E.W., Fanti S., Li X., van Paassen P., et al. Cytotoxic T-Cells Drive Outcome in Inflammatory Dilated Cardiomyopathy. Circ. Res. 2024;135:12. doi: 10.1161/CIRCRESAHA.124.325183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sur M., Rasquinha M.T., Mone K., Massilamany C., Lasrado N., Gurumurthy C., Sobel R.A., Reddy J. Investigation into Cardiac MyHC-α 334-352-Specific TCR Transgenic Mice Reveals a Role for Cytotoxic CD4 T Cells in the Development of Cardiac Autoimmunity. Cells. 2024;13:234. doi: 10.3390/cells13030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kohlgruber A.C., Dezfulian M.H., Sie B.M., Wang C.I., Kula T., Laserson U., Larman H.B., Elledge S.J. High-Throughput Discovery of MHC Class I- and II-Restricted T Cell Epitopes Using Synthetic Cellular Circuits. Nat. Biotechnol. 2024 doi: 10.1038/s41587-024-02248-6. [DOI] [PubMed] [Google Scholar]
- 133.Cunningham M.W. T Cell Mimicry in Inflammatory Heart Disease. Mol. Immunol. 2004;40:1121–1127. doi: 10.1016/j.molimm.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 134.Noutsias M., Rohde M., Göldner K., Block A., Blunert K., Hemaidan L., Hummel M., Blohm J.-H., Lassner D., Kühl U., et al. Expression of Functional T-Cell Markers and T-Cell Receptor Vbeta Repertoire in Endomyocardial Biopsies from Patients Presenting with Acute Myocarditis and Dilated Cardiomyopathy. Eur. J. Heart Fail. 2011;13:611–618. doi: 10.1093/eurjhf/hfr014. [DOI] [PubMed] [Google Scholar]
- 135.Hot A., Guerry M.J., Smith R., Sivasothy P., Guillevin L., Merkel P., Jayne D. A Multicenter Survey of Rituximab for Eosinophilic Granulomatosis with Polyangiitis (Churg-Strauss) Presse Med. 2013;42:698. doi: 10.1016/j.lpm.2013.02.109. [DOI] [Google Scholar]
- 136.Toscano G., Tartaro P., Fedrigo M., Angelini A., Marcolongo R. Rituximab in Recurrent Idiopathic Giant Cell Myocarditis after Heart Transplantation: A Potential Therapeutic Approach. Transpl. Int. 2014;27:e38–e42. doi: 10.1111/tri.12270. [DOI] [PubMed] [Google Scholar]
- 137.Kim S., Marigowda G., Oren E., Israel E., Wechsler M.E. Mepolizumab as a Steroid-Sparing Treatment Option in Patients with Churg-Strauss Syndrome. J. Allergy Clin. Immunol. 2010;125:1336–1343. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 138.Moosig F., Gross W.L., Herrmann K., Bremer J.P., Hellmich B. Targeting Interleukin-5 in Refractory and Relapsing Churg-Strauss Syndrome. Ann. Intern. Med. 2011;155:341–343. doi: 10.7326/0003-4819-155-5-201109060-00026. [DOI] [PubMed] [Google Scholar]
- 139.Song T., Jones D.M., Homsi Y. Therapeutic Effect of Anti-IL-5 on Eosinophilic Myocarditis with Large Pericardial Effusion. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2016-218992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kraft L., Erdenesukh T., Sauter M., Tschöpe C., Klingel K. Blocking the IL-1β Signalling Pathway Prevents Chronic Viral Myocarditis and Cardiac Remodeling. Basic Res. Cardiol. 2019;114:11. doi: 10.1007/s00395-019-0719-0. [DOI] [PubMed] [Google Scholar]
- 141.Bonaventura A., Moroni F., Golino M., Del Buono M.G., Vecchié A., Potere N., Abbate A. IL-1 Blockade in Cardiovascular Disease: An Appraisal of the Evidence across Different Inflammatory Paradigms. Minerva Cardiol. Angiol. 2024;72:477–488. doi: 10.23736/S2724-5683.23.06390-1. [DOI] [PubMed] [Google Scholar]
- 142.Heron C., Lemarcis T., Laguerre O., Valet M., Michel J.-B., Mulder P., Tardif San Martin V., Brakenhielm E. Blocking Interleukin-1β Transiently Limits Left Ventricular Dilation and Modulates Cardiac Lymphangiogenesis in a Mouse Pressure-Overload Model. bioRxiv. 2023 doi: 10.1101/2023.04.01.535056. [DOI] [Google Scholar]
- 143.Kerneis M., Cohen F., Combes A., Amoura Z., Pare C., Brugier D., Puymirat E., Abtan J., Lattuca B., Dillinger J.-G., et al. Rationale and Design of the ARAMIS Trial: Anakinra versus Placebo, a Double Blind Randomized Controlled Trial for the Treatment of Acute Myocarditis. Arch. Cardiovasc. Dis. 2023;116:460–466. doi: 10.1016/j.acvd.2023.07.004. [DOI] [PubMed] [Google Scholar]
- 144.Golino M., Harding D., Del Buono M.G., Fanti S., Mohiddin S., Toldo S., Smyth J., Sanna T., Marelli-Berg F., Abbate A. Innate and Adaptive Immunity in Acute Myocarditis. Int. J. Cardiol. 2024;404:131901. doi: 10.1016/j.ijcard.2024.131901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.