Abstract
Abstract
Importance
Immunocompromised status is a risk factor for severe SARS-CoV-2 infection. Little is known about how systemic corticosteroid dose and concurrent use of immunosuppressants are associated with COVID-19 outcomes.
Objective
To assess the association between corticosteroid dose/duration and concurrent immunosuppressant use on COVID-19 hospitalisation and death in the era of COVID-19 vaccinations.
Design
This is a retrospective cohort study using a deidentified insurance claims database from 1 July 2020 to 30 June 30, 2022, with the risk period starting on 1 July 2021. Impact of corticosteroid exposures and concurrent use of other immunosuppressants was assessed with attributable risk analysis and Cox regression that included COVID-19 vaccination status and time-updated dichotomous immunosuppressive medication exposures.
Participants
There were 10 109 596 eligible patients enrolled during the risk period, each with at least 365 days of continuous enrolment prior to 1 July 2021.
Exposures
Systemic corticosteroids, disease-modifying antirheumatic drugs (DMARDs), tumour necrosis factor-alpha inhibitors (TNFis) and other immunosuppressive drug categories.
Main outcomes
Incidence rate ratios and hazard ratios for COVID-19 hospitalisation and death.
Results
Corticosteroids were prescribed to 1 379 049 (13.6%) of 10 109 596 individuals. After adjustment, corticosteroids were associated with an increased risk of COVID-19 hospitalisation (HR: 5.40; 95% CI 5.27 to 5.53; p<0.0001) and death (HR: 5.90; 95% CI 5.59 to 6.22; p<0.0001). Among individuals exposed to corticosteroids without a record of COVID-19 vaccination, risks for COVID-19 hospitalisation and death were increased by 3- and 14.5-fold. The population attributable risk of corticosteroid use for COVID-19 hospitalisations was 13.9% (95% CI 13.5 to 14.3%). There was a significantly increased risk of COVID-19 hospitalisation associated with the use of corticosteroids plus DMARDs (HR: 1.55; 95% CI 1.42 to 1.70; p<0.0001) or plus TNFis (HR: 1.60; 95% CI 1.15 to 2.22; p=0.005).
Conclusions
Corticosteroids are associated with greater risk of COVID-19 hospitalisation and death, especially among unvaccinated individuals. Concurrent use of DMARDs and TNFis with corticosteroids confers greater risk.
Keywords: COVID-19, EPIDEMIOLOGIC STUDIES, Epidemiology, Public health, Rheumatology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Large sample size.
Diverse population from the USA.
Linked medical and pharmacy claims data.
May not be generalisable to people without health insurance.
COVID-19 vaccinations may be undercaptured.
Introduction
The Centres for Disease Control (CDC) cites immunocompromised status as a risk factor for severe SARS-CoV-2 infection. Due to impaired immune defences from both underlying disease and immunosuppressive treatments, these patients are at higher risk of infection and severe COVID-19 outcomes.1 Although immunosuppressive medications reduce inflammation from underlying disease, they also increase the risk of infection.2,4
Previous studies investigating associations between immunosuppressive therapies and COVID-19 outcomes mostly focused on immunocompromised patients.5,16 Furthermore, many of these studies were conducted prior to COVID-19 vaccination availability and Omicron and Delta variant predominance. Patients who are taking corticosteroids and other immunosuppressive therapies for chronic immune-mediated conditions are immunocompromised because of their treatments and may face higher risks of infections and potentially worse COVID-19 outcomes. Rituximab, an anti-CD20 monoclonal antibody, has been associated with increased odds of COVID-19-related death compared with methotrexate monotherapy in patients with rheumatic diseases likely due to B-cell depletion and compromised immunity against viruses, including less development of SARS-CoV-2 antibodies. As COVID-19 variants evolve, understanding the role of immunosuppressants on outcomes remains critical, yet there is limited information about their effect on the general population. Although studies have shown an association between corticosteroids and worse COVID-19 outcomes, information on specific doses and durations of systemic corticosteroid (SC) use and concurrent use of multiple immunosuppressants is scarce. To address these gaps, the aims of this study were to assess the association between SC exposure, including dose and duration, non-corticosteroid immunosuppressants and concurrent immunosuppressant use on COVID-19 hospitalisation and death in the era of widespread COVID-19 vaccination and Delta and Omicron predominance.
Methods
Study design and setting
This is a retrospective cohort study that used Optum Labs Data Warehouse (OLDW; Optum Labs, Eden Prairie, Minnesota), a deidentified administrative claims and electronic health record database consisting of demographic information, medical claims and outpatient pharmacy claims of commercial and Medicare Advantage enrollees of all ages since 1994 in the USA.17 Medical claims comprised of diagnosis codes, that is, International Classification of Disease 10th revision (ICD-10), Current Procedural Terminology codes, dates of service and provider specialty codes. Pharmacy information included the National Drug Code, brand name, generic name, quantity, days’ supply, drug strength, drug administration route and the prescription fill date.
Patient and public involvement
None.
Study population
Patients enrolled in OLDW between 1 July 2021 and 30 June 2022 were included. Each patient had at least 365 days of continuous enrolment with medical and pharmacy coverage before 1 July 2021, to capture baseline comorbidities. Medical and pharmacy coverage refers to health insurance benefits that provide access to healthcare services such as physician visits, hospital care, medical treatments (medical coverage), as well as prescription medications or vaccinations (pharmacy coverage). The risk period started on 1 July 2021, which is the date designated by the US CDC as the start of the Delta variant dominant period. Cohort selection details are shown in online supplemental eAppendix 1.
Covariates
Baseline covariates included age in 2021, sex, race/ethnicity, geographic region and comorbidities. Comorbidities were captured based on ICD-10 codes (online supplemental eAppendix 2). Outpatient immunosuppressant use during the risk period was categorised into the following groups: SCs, disease-modifying antirheumatic drugs (DMARDs), tumour necrosis factor-alpha inhibitors (TNFis), interleukin-6 (IL-6) inhibitors, anti-CD20 monoclonal antibodies, other biologic immunosuppressive therapies and other non-biologic immunosuppressive drugs that did not fit into the previous categories. Prescription fills for immunosuppressants and COVID-19 treatments were identified by text search of drug names in pharmacy claims (onlinesupplemental eAppendices 3 4). Records of previous COVID-19 infection and vaccination were also included (onlinesupplemental eAppendices 5 6).
Assessment of immunosuppressant exposure episodes
Time-varying dichotomous exposure was calculated for each immunosuppressant category based on outpatient pharmacy claims using methods previously described by our group (onlinesupplemental methods 1, eAppendices 7 8undefined).13 18
Outcomes
Outcomes of interest included COVID-19 hospitalisation and COVID-19 in-hospital death, which were identified during the risk period (1 July 2021–30 June 2022) as described previously (online supplemental eAppendix 5).19 The first COVID-19 hospitalisation date was taken if there were multiple hospitalisations. Patients were censored if disenrolled from the medical plan, experienced death unrelated to COVID-19 or reached the end of the risk period.
Statistical analysis
Baseline covariates, clinical characteristics and immunosuppressant exposure during the risk period up to COVID-19 hospitalisation or censoring were summarised using descriptive statistics. Incidence rates (IRs) of COVID-19 outcomes (hospitalisation, in-hospital death) were calculated based on immunosuppressant categories (exposed vs non-exposed to a medication in that category) and COVID-19 vaccination status. Time-varying Cox regression using robust standard errors was applied to estimate associations of COVID-19 outcomes with time-updated covariates for each immunosuppressant exposure, baseline demographics (age, sex, region, race/ethnicity) and comorbidities, which were included as binary variables. Exposure to immunosuppressants dispensed was considered ‘ever exposed’ to that medication and included as a dichotomous variable in the models. The average daily prednisone dose (mg) during periods of SC exposure was included as a continuous covariate and reported per 10 mg unit change, in addition to the dichotomous SC exposure variable.
A risk-stratification-based method was performed as a secondary analysis to better understand the association between SC dose and duration and risk of COVID-19 outcomes (online supplemental methods 2).
We also determined the attributable risk proportion (ARP) and the population ARP (PARP) of corticosteroid exposure on COVID-19 hospitalisations.
To examine the effect of concurrent usage of immunosuppressants on COVID-19 hospitalisation, we evaluated the following combinations of drug exposures compared with DMARDs only: (1) DMARDs+SCs, (2) DMARDs+TNF-alpha inhibitors (TNFis) and (3) DMARDs+TNFis+SCs. We also assessed combinations compared with TNFis only: (1) TNFis+SCs, (2) TNFis+DMARDs and (3) TNFis+DMARDs+SCs. Categories were defined as ≥1 day of exposure; individuals who had no exposures or different exposure categories were excluded. Cox models were run separately, adjusting for baseline demographics and comorbidities.
Statistical analyses were performed in R (V.4.2.1, R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/). P values <0.05 were considered statistically significant. This study was approved by the Institutional Review Board of the University of California, San Francisco and was conducted in adherence with the tenets of the Declaration of Helsinki.
Results
Characteristics of the study population
There were 10 109 596 patients included in the cohort. More female patients were exposed to SCs than male patients (57.9% vs 42.1%). Patients who were exposed to SCs were older (mean: 54.3 years, SD: 20.7 years) and generally had a higher prevalence of comorbidities compared with those who were not exposed (mean: 45.9 years, SD: 23.5 years) (table 1, online supplemental table S1).
Table 1. Baseline characteristics of general population cohort by systemic corticosteroid exposure status* (n=10 109 596).
| Never exposed† (n=8 730 547) | Ever exposed† (n=1 379 049) | All (n=10 109 596) | |
| Age | |||
| Mean (SD) | 45.9 (23.5) | 54.3 (20.7) | 47.1 (23.3) |
| Median (Q1, Q3) | 47.0(27.0, 67.0) | 58.0(41.0, 70.0) | 49.0(29.0, 67.0) |
| Sex | |||
| Female | 4 383 754 (50.2) | 798 134 (57.9) | 5 181 888 (51.3) |
| Male | 4 345 127 (49.8) | 580 774 (42.1) | 4 925 901 (48.7) |
| Unknown | 1666 (0.0) | 141 (0.0) | 1807 (0.0) |
| Race/ethnicity | |||
| White | 5 341 439 (61.2) | 955 571 (69.3) | 6 297 010 (62.3) |
| Asian | 420 151 (4.8) | 33 571 (2.4) | 453 722 (4.5) |
| Black | 814 700 (9.3) | 145 078 (10.5) | 959 778 (9.5) |
| Hispanic | 974 510 (11.2) | 130 337 (9.5) | 1 104 847 (10.9) |
| Unknown/missing | 1 179 747 (13.5) | 114 492 (8.3) | 1 294 239 (12.8) |
| Region | |||
| South | 3 538 880 (40.5) | 723 435 (52.5) | 4 262 215 (42.2) |
| Midwest | 2 353 195 (27.0) | 358 920 (26.0) | 2 712 115 (26.8) |
| Northeast | 1 029 139 (11.8) | 143 594 (10.4) | 1 172 733 (11.6) |
| West | 1 559 402 (17.9) | 152 264 (11.0) | 1 711 666 (16.9) |
| Other/unknown | 249 931 (2.9) | 936 (0.1) | 250 867 (2.5) |
| Charlson Comorbidity Index | |||
| Mean (SD) | 1.07 (1.33) | 1.46 (1.36) | 1.12 (1.34) |
| Median (Q1, Q3) | 0(0, 2.00) | 1.00(0, 3.00) | 0(0, 2.00) |
| Asthma | 351 975 (4.0) | 157 189 (11.4) | 509 164 (5.0) |
| Another autoimmune disease | 422 443 (4.8) | 174 541 (12.7) | 596 984 (5.9) |
| Cancer | 364 298 (4.2) | 108 703 (7.9) | 473 001 (4.7) |
| Cerebrovascular disease | 316 892 (3.6) | 87 661 (6.4) | 404 553 (4.0) |
| Chronic kidney disease | 537 689 (6.2) | 144 283 (10.5) | 681 972 (6.7) |
| Chronic liver disease | 158 334 (1.8) | 49 959 (3.6) | 208 293 (2.1) |
| Chronic lung disease | 388 453 (4.4) | 186 581 (13.5) | 575 034 (5.7) |
| Diabetes (any type) | 1 107 062 (12.7) | 247 442 (17.9) | 1 354 504 (13.4) |
| Disabilities | 271 983 (3.1) | 47 453 (3.4) | 319 436 (3.2) |
| Heart disease | 707 919 (8.1) | 209 509 (15.2) | 917 428 (9.1) |
| HIV/AIDS | 19 895 (0.2) | 3563 (0.3) | 23 458 (0.2) |
| Primary immunodeficiency | 11 894 (0.1) | 6778 (0.5) | 18 672 (0.2) |
| Mental health disorder | 429 149 (4.9) | 112 825 (8.2) | 541 974 (5.4) |
| Neurologic disease | 143 514 (1.6) | 21 512 (1.6) | 165 026 (1.6) |
| Obesity | 1 173 666 (13.4) | 313 131 (22.7) | 1 486 797 (14.7) |
| Pregnancy (risk period) | 129 298 (1.5) | 15 251 (1.1) | 144 549 (1.4) |
| Solid organ transplantation | 16 927 (0.2) | 11 887 (0.9) | 28 814 (0.3) |
| Number of comorbidities category | |||
| 0 | 5 221 105 (59.8) | 518 624 (37.6) | 5 739 729 (56.8) |
| 1 | 1 847 801 (21.2) | 357 485 (25.9) | 2 205 286 (21.8) |
| 2–3 | 1 302 572 (14.9) | 358 131 (26.0) | 1 660 703 (16.4) |
| 4+ | 359 069 (4.1) | 144 809 (10.5) | 503 878 (5.0) |
| Smoking status (baseline or risk period) | |||
| Never | 1 280 469 (14.7) | 272 796 (19.8) | 1 553 265 (15.4) |
| Current/former | 1 217 425 (13.9) | 375 686 (27.2) | 1 593 111 (15.8) |
| Unknown | 6 232 653 (71.4) | 730 567 (53.0) | 6 963 220 (68.9) |
| COVID-19 infection (baseline or risk period) | 1 158 862 (13.3) | 350 187 (25.4) | 1 509 049 (14.9) |
| Had ≥1 COVID-19 vaccination records (24 December 2020 up to end date) | 3 896 643 (44.6) | 619 133 (44.9) | 4 515 776 (44.7) |
| Received any outpatient COVID-19 treatments (risk period) | 89 856 (1.0) | 45 315 (3.3) | 135 171 (1.3) |
| Received tixagevimab/cilgavimab, preventative COVID-19 treatment (risk period) | 1975 (0.0) | 2282 (0.2) | 4257 (0.0) |
| Received any inpatient COVID-19 treatments (risk period) | 118 (0.0) | 20 230 (1.4) | 20 348 (0.2) |
Systemic corticosteroid (SC) exposure during the risk period up to COVID-19 hospitalizationhospitalisation or censoring is reported in this table. Patients with at least one prescription dispensing during risk period were considered ‘ever exposed’.
Numbers are reported as No. (%) unless otherwise specified.
Immunosuppressant use
There were 1 474 862 (14.6%) patients who had at least one immunosuppressant exposure during the risk period. There were 1 379 049 (13.6%) individuals prescribed SCs and 96 201 (0.95%) received DMARDs. <0.5% of the cohort received a medication from each of the other immunosuppressive categories (table 2).
Table 2. Immunosuppressive drug prescriptions during the risk period* (n=10 109 596).
| Drug class/generic name | Number (%) of cohort with ≥1 prescription fill† |
| Overall | 1 474 862 (14.6) |
| Systemic corticosteroids | 1 379 049 (13.6) |
| Prednisone | 814 085 (8.1) |
| Methylprednisolone | 539 234 (5.3) |
| Dexamethasone | 120 773 (1.2) |
| Budesonide | 11 604 (0.1) |
| Hydrocortisone | 8037 (0.08) |
| Prednisolone | 49 410 (0.5) |
| Disease-modifying antirheumatic drugs | 96 201 (0.95) |
| Methotrexate | 53 658 (0.5) |
| Mycophenolic acid (mycophenolate mofetil, enteric-coated mycophenolate sodium) | 17 129 (0.2) |
| Tacrolimus | 12 566 (0.1) |
| Leflunomide | 12 381 (0.1) |
| Azathioprine | 11 631 (0.1) |
| Cyclosporine | 2150 (0.02) |
| Tumour necrosis factor-alpha inhibitors | 31 147 (0.3) |
| Interleukin-6 inhibitors | 1611 (0.02) |
| Anti-CD20 monoclonal antibodies | 843 (0.008) |
| Ofatumumab | 556 (0.005) |
| Rituximab | 150 (0.001) |
| Ocrelizumab | >128 (0.001) |
| Obinutuzumab | <11 (0.0) |
| Other biologics | 34 553 (0.3) |
| Dupilumab | 10 482 (0.1) |
| Ustekinumab | 5806 (0.06) |
| Secukinumab | 4467 (0.04) |
| Risankizumab | 3533 (0.04) |
| Guselkumab | 3211 (0.03) |
| Abatacept | 2161 (0.02) |
| Omalizumab | 1993 (0.02) |
| Interferon beta-1a | 1462 (0.01) |
| Ixekizumab | 1140 (0.01) |
| Belimumab | 751 (0.007) |
| Interferon beta-1b | 214 (0.002) |
| Vedolizumab | 161 (0.002) |
| Anakinra | 159 (0.002) |
| Tildrakizumab | 65 (0.001) |
| Natalizumab | 41 (0.0) |
| Brodalumab | 40 (0.0) |
| Eculizumab | 31 (0.0) |
| Rilonacept | 28 (0.0) |
| Canakinumab‡ | >11 (0.0) |
| Alemtuzumab‡ | <11 (0.0) |
| Other immunosuppressive drugs | 35 727 (0.4) |
| Sulfasalazine | 13 214 (0.1) |
| Apremilast | 6258 (0.06) |
| Tofacitinib citrate | 4003 (0.04) |
| Upadacitinib | 2334 (0.02) |
| Mercaptopurine | 2060 (0.02) |
| Glatiramer acetate | 1981 (0.02) |
| Dimethyl fumarate | 1893 (0.02) |
| Teriflunomide | 1518 (0.02) |
| Ruxolitinib phosphate | 1068 (0.01) |
| Fingolimod hydrochloride | 979 (0.01) |
| Cyclophosphamide | 479 (0.005) |
| Diroximel farate | 257 (0.003) |
| Baricitinib | 218 (0.002) |
| Cladribine | 210 (0.002) |
| Ozanimod hydrochloride | 177 (0.002) |
| Siponimod | 166 (0.002) |
| Monomethyl fumarate | 65 (0.001) |
Frequencies and percentages reflect the proportion of patients who filled one or more prescriptions during risk period up to COVID-19 hospitalizationhospitalisation outcome date (results differ slightly for death outcome).
Numbers are reported as No. (%) unless otherwise specified.
In order to protect patient privacy, medications with small cell numbers (<11 patients) were masked.
The average SC prescription duration was 15 days, and the median was 6 days (IQR: 5–16 days). The median prednisone equivalent average daily dose per prescription was 253.4 mg/day (IQR: 44.6–1250 mg/day) for intravenous steroids and 17.95 mg/day (IQR: 15–40 mg/day) for oral steroids. SCs were further classified by dosage and length of exposure (online supplemental table S2).
Exposures associated with COVID-19 hospitalisation and death
There were 77 647 COVID-19 hospitalisations within 8 786 685 person-years (PY) in the study cohort (overall IR of 8.8 per 1000 PY). The IR ratio (IRR) of hospitalizstions among those exposed versus unexposed to SCs was 12.29. For all other immunosuppressant categories, IRs remained higher in the exposed person-time (table 3).
Table 3. Incidence of COVID-19 outcomes (hospitalisation and death) per 1000 person-years by immunosuppressive medication exposure.
| Exposed | Unexposed | Incidence rate ratio | |||||
| Number of cases | Number of person-Years | Incidence rate | Number of cases | Number of person-years | Incidence rate | ||
| COVID-19 hospitalisationOverall: 77 647 cases within 8786685.0 person-years, corresponding to an incidence rate of 8.8 per 1000 person-years | |||||||
| Systemic corticosteroids | 8986 | 92 549.6 | 97.1 | 68 661 | 8694135.4 | 7.9 | 12.29 |
| DMARDs | 2127 | 61 621.2 | 34.5 | 75 520 | 8725063.8 | 8.7 | 3.97 |
| TNF-α inhibitors* | 202 | 19 714.4 | 10.2 | 77 445 | 8766970.5 | 8.8 | 1.16 |
| IL-6 inhibitors | 13 | 866.6 | 15.0 | 77 634 | 8785818.4 | 8.8 | 1.70 |
| Anti-CD20 monoclonal antibodies | 12 | 357.4 | 33.6 | 77 635 | 8786327.6 | 8.8 | 3.82 |
| Other biologic therapies | 194 | 19 095.8 | 10.2 | 77 453 | 8767589.2 | 8.8 | 1.16 |
| Other immunosuppressive drugs | 408 | 20 922.0 | 19.5 | 77 239 | 8765763.0 | 8.8 | 2.22 |
| COVID-19 deathOverall: 13 783 cases within 8817480.0 person-years, corresponding to an incidence rate of 1.6 per 1000 person-years | |||||||
| Systemic corticosteroids | 2027 | 94 361.6 | 21.5 | 11 756 | 8723118.3 | 1.3 | 16.54 |
| DMARDs | 430 | 62 362.3 | 6.9 | 13 353 | 8755117.7 | 1.5 | 4.60 |
| TNF-α inhibitors | 31 | 19 787.2 | 1.567 | 13 752 | 8797692.8 | 1.563 | 1.003 |
| IL-6 inhibitors | 0 | 872.9 | 0.0 | 13 783 | 8816607.1 | 1.6 | 0.00 |
| Anti-CD20 monoclonal antibodies* | / | / | 2.8 | / | / | 1.6 | 1.75 |
| Other biologic therapies | 31 | 19 174.2 | 1.62 | 13 752 | 8798305.8 | 1.56 | 1.04 |
| Other immunosuppressive drugs | 79 | 21 076.3 | 3.7 | 13 704 | 8796403.7 | 1.6 | 2.31 |
To protect patient privacy, Optum Labs does not allow reporting of the true value of a count if it is less than <11. Only incidence rate was reported because the number of cases in the category is less than <11. The corresponding person-years are also not reported to prevent back calculation.
Anti-CD20anti-CD20 monoclonal antibodiesDMARDdisease-modifying antirheumatic drugsIL-6interleukin 6TNF-αtumour necrosis factor alpha
There were 13 783 COVID-19 deaths within 8 817 480 PY (IR: 1.6 per 1000 PY). The IRR of COVID-19 death among those exposed versus unexposed to SCs was 16.54. IRs remained higher in the exposed person-time for DMARDs and anti-CD20 monoclonal antibodies. There were zero deaths in those exposed to IL-6 inhibitors (table 3).
The HR compares the likelihood of an event (eg, COVID-19 death) occurring at any given time in the treatment group versus the control group (eg, SC exposed vs SC unexposed). The unadjusted HR for COVID-19 hospitalisation and death comparing SC exposed and unexposed person-time was 12.11 (95% CI 11.85–12.38; p<0.0001) and 15.77 (95% CI 15.04 to 16.53; p<0.0001), respectively, and remained significant in the adjusted models (table 4). In the adjusted analyses, there was a 3.7% increased risk of COVID-19 hospitalisation (HR: 1.037; 95% CI 1.035 to 1.038; p<0.0001) and a 2.4% increased risk of death (HR: 1.024; 95% CI 1.022 to 1.026; p<0.0001) with every 10 mg average daily dose increase in prednisone equivalent SC use (table 4). Anti-CD20 monoclonal antibodies (HR: 3.47; 95% CI 1.97 to 6.11; p<0.0001) and other non-biologic immunosuppressive medications (HR: 1.21; 95% CI.10 to 1.34; p=0.0001) were significantly associated with an increased risk for hospitalisation, and DMARDs (HR: 0.91; 95% CI 0.86 to 0.96; p=0.0003) and TNF-alpha inhibitors (HR: 0.86; 95% CI 0.75 to 0.99; p=0.04) were significantly associated with decreased risk for hospitalisation. IL-6 inhibitors and other biologic therapies were not significantly associated with hospitalisation. Other non-biologic immunosuppressive medications (HR: 1.33; 95% CI 1.06 to 1.66; p=0.01) were significantly associated with an increased risk for death (table 4).
Table 4. Unadjusted and adjusted hazard ratios showing associations between immunosuppressive medication exposure and COVID-19 outcomes (hospitalisation and death) in the cohort.
| Immunosuppressive medication | Unadjusted HR (95% CI) | P value* | Adjusted HR (95% CI) | P value* |
| COVID-19 hospitalisation | ||||
| Systemic corticosteroids (SC) (any exposure) | 12.11 (11.85, 12.38) | <0.0001 | 5.40 (5.27, 5.53) | <0.0001 |
| SC average daily dose (per 10 mg) | 2.52 (2.50, 2.53) | <0.0001 | 1.037 (1.035, 1.038) | <0.0001 |
| DMARDs | 4.006 (3.84, 4.18) | <0.0001 | 0.91 (0.86, 0.96) | 0.0003 |
| TNF-α inhibitors | 1.17 (1.02, 1.34) | 0.029 | 0.86 (0.75, 0.99) | 0.038 |
| IL-6 inhibitors | 1.72 (0.998, 2.96) | 0.051 | 0.62 (0.36, 1.07) | 0.088 |
| Anti-CD20 monoclonal antibodies | 3.99 (2.27, 7.01) | <0.0001 | 3.47 (1.97, 6.11) | <0.0001 |
| Other biologic therapies | 1.18 (1.025, 1.36) | 0.021 | 0.94 (0.82, 1.09) | 0.43 |
| Other immunosuppressive drugs | 2.23 (2.02, 2.45) | <0.0001 | 1.21 (1.10, 1.34) | 0.0001 |
| COVID-19 death | ||||
| SC (any exposure) | 15.77 (15.04, 16.53) | <0.0001 | 5.90 (5.59, 6.22) | <0.0001 |
| SC average daily dose (per 10 mg) | 2.71 (2.67, 2.76) | <0.0001 | 1.024 (1.022, 1.026) | <0.0001 |
| DMARDs | 4.56 (4.14, 5.02) | <0.0001 | 0.90 (0.80, 1.01) | 0.075 |
| TNF-α inhibitors | 1.01 (0.71, 1.44) | 0.96 | 0.84 (0.59, 1.20) | 0.33 |
| IL-6 inhibitors† | / | / | / | / |
| Anti-CD20 monoclonal antibodies | 1.90 (0.27, 13.49) | 0.52 | 2.08 (0.29, 14.77) | 0.46 |
| Other biologic therapies | 1.07 (0.75, 1.52) | 0.71 | 1.04 (0.73, 1.48) | 0.83 |
| Other immunosuppressive drugs | 2.43 (1.95, 3.03) | <0.0001 | 1.33 (1.06, 1.66) | 0.013 |
P -values calculated from Cox proportional hazards models.
We were not able to estimate hazard ratioHR and corresponding p- value due to zero outcome event in the exposed group.
Anti-CD20, anti-CD20 monoclonal antibodiesDMARD, disease-modifying antirheumatic drug; IL-6, interleukin 6; SC, systemic corticosteroid; TNF-α, tumour necrosis factor-α
COVID-19 outpatient treatment was associated with a higher risk of COVID-19 hospitalisation (HR: 3.29; 95% CI 3.16 to 3.43; p<0.0001). Tixagevimab/cilgavimab (Evusheld), a preventative COVID-19 treatment, was associated with a 98% reduced risk of hospitalisation (HR: 0.02; 95% CI 0.01 to 0.04; p<0.0001). There was a 72.4% and 64.6% reduced risk with previous COVID-19 infection (HR: 0.276; 95% CI 0.268 to 0.284; p<0.0001) and with at least one COVID-19 vaccination (HR: 0.354; 95% CI 0.347 to 0.360; p<0.0001), respectively (online supplemental table S3). There were zero deaths in those who had exposure to tixagevimab/cilgavimab. COVID-19 inpatient treatment was associated with a higher risk of COVID-19 death (HR: 19.46; 95% CI 17.83 to 21.23; p<0.0001). There was a 72% and 92% reduced risk of death with previous COVID-19 infection (HR: 0.28; 95% CI 0.27 to 0.30; p<0.0001) and with at least one COVID-19 vaccination (HR: 0.08; 95% CI 0.07 to 0.09; p<0.0001) (online supplemental table S4). Among those exposed to SCs, the IRR of those without a record of COVID-19 vaccination was 3.1- and 14.5-fold higher for hospitalisation and death, respectively.
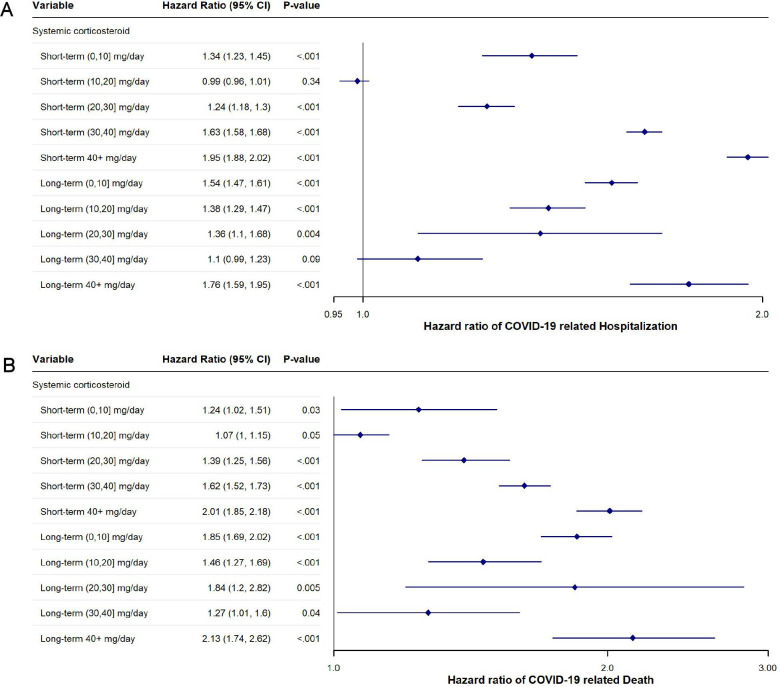
In the risk-stratification secondary adjusted analysis, the estimates suggested an increased risk of hospitalisation and death for most exposure levels of corticosteroids (figure 1).
Figure 1. (A) Adjusted hazard ratios of coronavirus disease 2019 hospitalisation associated with systemic corticosteroid dose and duration. (B) Adjusted hazard ratios of coronavirus disease 2019 death associated with systemic corticosteroid dose and duration. A duration of <30 days was considered short term and ≥30 days was considered long term. All systemic corticosteroid (SC) doses were converted into prednisone equivalents and broken down by 10 mg increments.
Attributable risk proportion of corticosteroid exposure on COVID-19 hospitalisations
In the ARP analysis, 54.2% (95% CI 53.5 to 54.9%) of the COVID-19 hospitalisations could be directly attributed to corticosteroid use in those who took SCs. The PARP was 13.9% (95% CI 13.5 to 14.3%).
Subgroup analyses of concurrent immunosuppressant exposures and COVID-19 hospitalisation risk
Compared with those who were only exposed to DMARDs, those who had overlapping exposures with SCs had a 55% greater hazard of COVID-19 hospitalisation (HR: 1.55; 95% CI 1.42 to 1.70; p<0.0001). There was a reduced hazard in those exposed to TNFis and DMARDs (HR: 0.88; 95% CI 0.64 to 1.21; p=0.43) and an increased hazard in those exposed to TNFis, DMARDs and SCs (HR: 1.19; 95% CI 0.89 to 1.59; p=0.24).
Compared with those who were only exposed to TNFis, those who were exposed to SCs and TNFis had a 60% greater hazard of COVID-19 hospitalisation (HR: 1.60; 95% CI 1.15 to 2.22; p=0.005). Those who were exposed to TNFis, DMARDs and SCs had a 58% greater hazard of COVID-19 hospitalisation (HR: 1.58; 95% CI 1.09 to 2.29; p=0.02). Exposure to DMARDs and TNFis was not significantly associated with higher risk (online supplemental table S5).
Discussion
In this large retrospective cohort study of the general population using a US claims database, exposure to SCs was associated with an increased risk of COVID-19 hospitalisation and death in an era of COVID-19 vaccination. Among those exposed to SCs, there was a 5.4- and 5.9-fold increase in the risk of hospitalisation and death, respectively.
Studies addressing the associations between SCs and COVID-19 outcomes have yielded mixed results, and many of these studies were conducted prior to the availability of COVID-19 vaccinations and during the time period of earlier COVID variants.5,12 In the current era of COVID-19 vaccination, a study by Risk et al found an increased risk of COVID-19 hospitalisation among individuals taking DMARDs and glucocorticoids.20 Our finding that over 50% of COVID-19 hospitalisations in those exposed to SCs were attributable to that exposure highlights the clinical significance of this risk factor.
For hospitalisation, both DMARDs and TNF-alpha inhibitors were significantly associated with a decreased risk in the adjusted model. The HRs were also in the protective direction for death but were not significant. It is unclear how conventional DMARDs may be protective against COVID-19 hospitalisation and death, though it has been postulated that DMARDs may be protective against COVID-19 infection via downregulation of angiotensin-converting enzyme 2,21 which the virus uses for entry into epithelial cells.22 Other studies have found that TNF-alpha inhibitors may be associated with a decreased risk of COVID-19 hospitalisation, intensive care unit (ICU) admission and death.6 14 16 23 Mechanistically, TNF-alpha is increased in early and late stages of COVID-19 infection, so inhibiting this cytokine may help decrease the effects of a cytokine storm.24 We found that anti-CD20 monoclonal antibodies, including rituximab, obinutuzumab and ofatumumab, were associated with increased risk, in line with prior studies.512 16 25,27 B- and T-cells protect against SARS-CoV-2 by playing a key role in viral clearance as well as protection against reinfection by eliciting a memory response.28 Anti-CD20 therapies result in selective depletion of B-cells and to some degree T-cells,29 so it is biologically plausible that taking these therapies increases risk. Furthermore, it may take 6–12 months after treatment to recover from this depletion.30
This study also provides insight into the risks of concurrent immunosuppressant use and COVID-19 hospitalisation, which has been lacking in previous studies. Our results point towards an increased risk, specifically when SCs are added to DMARDS and TNF-alpha inhibitors. The harmful effect of outpatient SC use on COVID-19 outcomes is a consistent finding in all our analyses. Combination therapy with DMARDs and TNFis was in the protective direction for COVID-19 hospitalisation. This suggests that outpatient use of TNFis may have a protective effect, whether used alone or in combination with DMARDs. A single-centre study by Veenstra et al, including 213 individuals on immunosuppressants, found that those taking multiple immunosuppressive medications (DMARD plus a biologic or multiple DMARDs) were associated with increased odds of COVID-19 positivity and hospitalisation.14 However, that study was not able to attribute this increased association to any particular medication or medication category; therefore, it is unclear why this association was observed. In contrast, our study included SCs in a multidrug analysis and found that the addition of SCs accounted for the increased association with COVID-19 hospitalisation in all combinations, indicating the safety of DMARDs and TNFis when used together or alone.
Previous studies on the effect of dose and duration of SCs on COVID-19 outcomes have been scarce and have yielded mixed results. Some studies have found that prednisone doses ≥7.5 and ≥10 mg/day were associated with an increased risk of hospitalisation, while other studies found that risk was only increased when doses were ≥20 mg/day.5 6 15 31 32 Our results point towards a dose-dependent increased risk of SCs and worse COVID-19 outcomes. In our primary analyses, there was a 3.7% increased risk of COVID-19 hospitalisation and a 2.4% increased risk of COVID-19 death for every 10 mg prednisone equivalent increase in the average daily dose of SCs. In the risk-stratification analysis on SC dose and duration, we found a non-linear increased risk of both hospitalisation and death regardless of dose and duration. This non-linearity may be attributed to tapering protocols, which are common in SC management, complicating the dose-response relationship. Furthermore, the relationship between corticosteroid doses and outcomes may not be directly proportional. These complex dynamics may explain why intermediate doses show higher HRs than expected and support the need for caution with any dose of SC.
Although overall coverage of COVID-19 vaccination was 45%, it was still associated with a reduced risk of hospitalisation and death. The lower-than-expected coverage can be partly attributed to the initial mass vaccination campaigns in the USA in 2021, which provided vaccinations that were covered by the US government. These vaccinations were not always linked to an insurance claim, and thus would not be captured in this database. Additionally, among individuals exposed to SCs who did not have a record of COVID-19 vaccination, the risk for COVID-19 hospitalisation and death was increased by 3-fold and nearly 15-fold, respectively, suggesting that COVID-19 vaccination was protective among those who were exposed to SCs. COVID-19 vaccination helps prevent severe disease among those who are immunosuppressed, even though the antibody response to vaccination in these individuals is lower than in those who are immunocompetent.33 34 It is crucial for physicians to continue advising immunosuppressed patients to be up-to-date with their COVID-19 vaccinations.
Our study also adjusted for receipt of pre-exposure prophylaxis with tixagevimab/cilgavimab as well as outpatient and inpatient COVID-19 treatments as they may affect hospitalisation and death, although it is no longer authorised by the US Food and Drug Administration as of 26 January 2023. Receipt of tixagevimab/cilgavimab was significantly associated with a decreased risk of COVID-19 hospitalisation. Notably, none of the individuals who had a COVID-19-related death had taken tixagevimab/cilgavimab. Receipt of outpatient and inpatient COVID-19 treatments was associated with an increased risk of both outcomes, likely indicating that these treatments were given to individuals with more severe infection, who were already at greater risk for worse outcomes. Furthermore, delays in initiating therapy or reduced effectiveness of conventional treatments such as monoclonal antibodies or antiviral medications in immunocompromised patients may have contributed to these results.
Strengths and limitations
This study includes a large, diverse population in the USA with over 10 million individuals. This sample size allowed for adjustment for many potential confounders, to assess many different immunosuppressive therapies, and to assess the risk of concurrent therapy exposure. Additionally, classifying exposure to the immunosuppressant categories as time-updated variables avoids immortal-time bias and provides more accurate effect size estimates.13 35 36
There are several limitations to this study. This study only includes data from individuals who have commercial insurance or Medicare Advantage; therefore, those with basic Medicare, Medicaid, or who are uninsured could not be assessed. As the study includes those with Medicare Advantage, our study population may skew towards an older population. Identification of COVID-19 outcomes relied on the use of ICD-10 codes; therefore, outcomes could be potentially misclassified. Additionally, it is not possible to assess patients’ medication adherence since pharmacy claims only reflect prescription fills. Some immunosuppressive therapies have longer-lasting effects after stopping, but it was not feasible to account for this in our study. Finally, COVID-19 vaccination records are incompletely captured in claims databases,37 so the protective effects of vaccination may be underestimated.
Conclusions
This study demonstrates that outpatient SC exposure is associated with greater risk of COVID-19 hospitalisation and death in an era of widespread COVID-19 vaccination. Among those taking combinations of DMARDs and TNFis, risk increased when SCs were added. Thus, it may be important for physicians to minimise the use of SCs when possible. Our results also indicate that unvaccinated individuals exposed to immunosuppressants have a greater risk of severe outcomes. COVID-19 vaccination should be strongly encouraged in patients taking immunosuppressive therapies.
supplementary material
Footnotes
Funding: This study was funded by grant R01 EY028739 from the National Eye Institute and the Office of Research on Women’s Health at the National Institutes of Health (Dr Acharya). The Department of Ophthalmology at the University of California, San Francisco, is supported by core grant EY06190 from the National Eye Institute and an unrestricted grant from the Research to Prevent Blindness Foundation.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-087467).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Data availability free text: The data supporting these findings are not available. The Optum Labs data is proprietary and is available through a partnership between the University of California and Optum Labs.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Ethics approval: This study was approved by the Institutional Review Board (IRB) of the University of California, San Francisco (IRB no.: 17-22323) and was conducted in adherence with the tenets of the Declaration of Helsinki. Since this study was done using deidentified data, no additional ethics committee approval was required.
Contributor Information
Samantha J Sechrist, Email: sam.sechrist@gmail.com.
Emily Tang, Email: emily.tang2@ucsf.edu.
Benjamin F Arnold, Email: Ben.Arnold@ucsf.edu.
Nisha R Acharya, Email: nisha.acharya@ucsf.edu.
Data availability statement
No data are available.
References
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature New Biol. 2020;584:430–6. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Keeley A, Mallen C, et al. Incidence of infections associated with oral glucocorticoid dose in people diagnosed with polymyalgia rheumatica or giant cell arteritis: a cohort study in England. CMAJ. 2019;191:E680–8. doi: 10.1503/cmaj.190178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irving PM, de Lusignan S, Tang D, et al. Risk of common infections in people with inflammatory bowel disease in primary care: a population-based cohort study. BMJ Open Gastroenterol. 2021;8:e000573. doi: 10.1136/bmjgast-2020-000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fardet L, Petersen I, Nazareth I. Common Infections in Patients Prescribed Systemic Glucocorticoids in Primary Care: A Population-Based Cohort Study. PLoS Med. 2016;13:e1002024. doi: 10.1371/journal.pmed.1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–42. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–66. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481–91. doi: 10.1053/j.gastro.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adir Y, Humbert M, Saliba W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: Nationwide real-world evidence. J Allergy Clin Immunol. 2021;148:361–7. doi: 10.1016/j.jaci.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nørgård BM, Nielsen J, Knudsen T, et al. Hospitalization for COVID-19 in patients treated with selected immunosuppressant and immunomodulating agents, compared to the general population: A Danish cohort study. Br J Clin Pharmacol. 2021;87:2111–20. doi: 10.1111/bcp.14622. [DOI] [PubMed] [Google Scholar]
- 10.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann Neurol. 2021;89:780–9. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen KM, Mehta HB, Palamuttam N, et al. Association Between Chronic Use of Immunosuppresive Drugs and Clinical Outcomes From Coronavirus Disease 2019 (COVID-19) Hospitalization: A Retrospective Cohort Study in a Large US Health System. Clin Infect Dis. 2021;73:e4124–30. doi: 10.1093/cid/ciaa1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen KM, Bates BA, Rashidi ES, et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol . 2022;4:e33–41. doi: 10.1016/S2665-9913(21)00325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y, Miller DC, Akpandak I, et al. Association between Immunosuppressive Drugs and Coronavirus Disease 2019 Outcomes in Patients with Noninfectious Uveitis in a Large US Claims Database. Ophthalmology . 2022;129:1096–106. doi: 10.1016/j.ophtha.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veenstra J, Buechler CR, Robinson G, et al. Antecedent immunosuppressive therapy for immune-mediated inflammatory diseases in the setting of a COVID-19 outbreak. J Am Acad Dermatol. 2020;83:1696–703. doi: 10.1016/j.jaad.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodin R, van der Werff SD, Hedberg P, et al. The association between pre-exposure to glucocorticoids and other immunosuppressant drugs with severe COVID-19 outcomes. Clin Microbiol Infect. 2022;28:1477–85. doi: 10.1016/j.cmi.2022.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Y, Yue X, Krueger WS, et al. Factors Associated with Severe COVID-19 Among Patients with Rheumatoid Arthritis: A Large, Nationwide Electronic Health Record Cohort Study in the United States. Adv Ther. 2023;40:3723–38. doi: 10.1007/s12325-023-02533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OptumLabs OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. OptumLabs OptumLabs Data Wareh OLDW Descr Cit n.d. [Google Scholar]
- 18.Sechrist SJ, Tang E, Sun Y, et al. Immunosuppressive Medications and COVID-19 Outcomes in Patients with Noninfectious Uveitis in the Era of COVID-19 Vaccinations. Ophthalmol Sci . 2024;4:100411. doi: 10.1016/j.xops.2023.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller DC, Sun Y, Chen EM, et al. The Association between Noninfectious Uveitis and Coronavirus Disease 2019 Outcomes: An Analysis of United States Claims-Based Data. Ophthalmology. 2022;129:334–43. doi: 10.1016/j.ophtha.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risk M, Hayek SS, Schiopu E, et al. COVID-19 vaccine effectiveness against omicron (B.1.1.529) variant infection and hospitalisation in patients taking immunosuppressive medications: a retrospective cohort study. Lancet Rheumatol . 2022;4:e775–84. doi: 10.1016/S2665-9913(22)00216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schälter F, Dürholz K, Bucci L, et al. Does methotrexate influence COVID-19 infection? Case series and mechanistic data. Arthritis Res Ther. 2021;23:166. doi: 10.1186/s13075-021-02464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–80. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokkotis G, Kitsou K, Xynogalas I, et al. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment Pharmacol Ther. 2022;55:154–67. doi: 10.1111/apt.16717. [DOI] [PubMed] [Google Scholar]
- 24.Jamilloux Y, Henry T, Belot A, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19:S1568-9972(20)30129-4. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh N, Madhira V, Hu C, et al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: A retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C) Semin Arthritis Rheum. 2023;58:S0049-0172(22)00200-1. doi: 10.1016/j.semarthrit.2022.152149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liew DFL, Robinson PC. What does endemic COVID-19 mean for the future of rituximab? Lancet Rheumatol . 2022;4:e3–5. doi: 10.1016/S2665-9913(21)00362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol . 2021;3:e419–26. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52:910–41. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mélet J, Mulleman D, Goupille P, et al. Rituximab-Induced T Cell Depletion in Patients With Rheumatoid Arthritis: Association With Clinical Response: Rituximab-Induced T Cell Depletion and Clinical Response in RA. Arthritis Rheum. 2013;65:2783–90. doi: 10.1002/art.38107. [DOI] [PubMed] [Google Scholar]
- 30.Worch J, Makarova O, Burkhardt B. Immunreconstitution and infectious complications after rituximab treatment in children and adolescents: what do we know and what can we learn from adults? Cancers (Basel) 2015;7:305–28. doi: 10.3390/cancers7010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Álvarez-Troncoso J, López-Caballero L, Robles-Marhuend Á, et al. Influence of vaccination and immunosuppressive treatments on the coronavirus disease 2019 outcomes in patients with systemic autoimmune diseases. Eur J Intern Med. 2023;108:114–6. doi: 10.1016/j.ejim.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hyrich KL, Machado PM. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17:71–2. doi: 10.1038/s41584-020-00562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mason A, Anver H, Lwin M, et al. Lupus, vaccinations and COVID-19: What we know now. Lupus (Los Angel) 2021;30:1541–52. doi: 10.1177/09612033211024355. [DOI] [PubMed] [Google Scholar]
- 34.Embi PJ, Levy ME, Naleway AL, et al. Effectiveness of 2-Dose Vaccination with mRNA COVID-19 Vaccines Against COVID-19-Associated Hospitalizations Among Immunocompromised Adults - Nine States, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1553–9. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolkewitz M, Lambert J, von Cube M, et al. Statistical Analysis of Clinical COVID-19 Data: A Concise Overview of Lessons Learned, Common Errors and How to Avoid Them. Clin Epidemiol. 2020;12:925–8. doi: 10.2147/CLEP.S256735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lévesque LE, Hanley JA, Kezouh A, et al. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:bmj.b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Medicare & Medicaid Services (CMS) Assessing the completeness of medicare claims data for measuring covid-19 vaccine administration. https://www.cms.gov/files/document/assessing-completeness-medicare-claims-data-measuring-covid-19-vaccine-administration.pdf n.d. Available.