Abstract
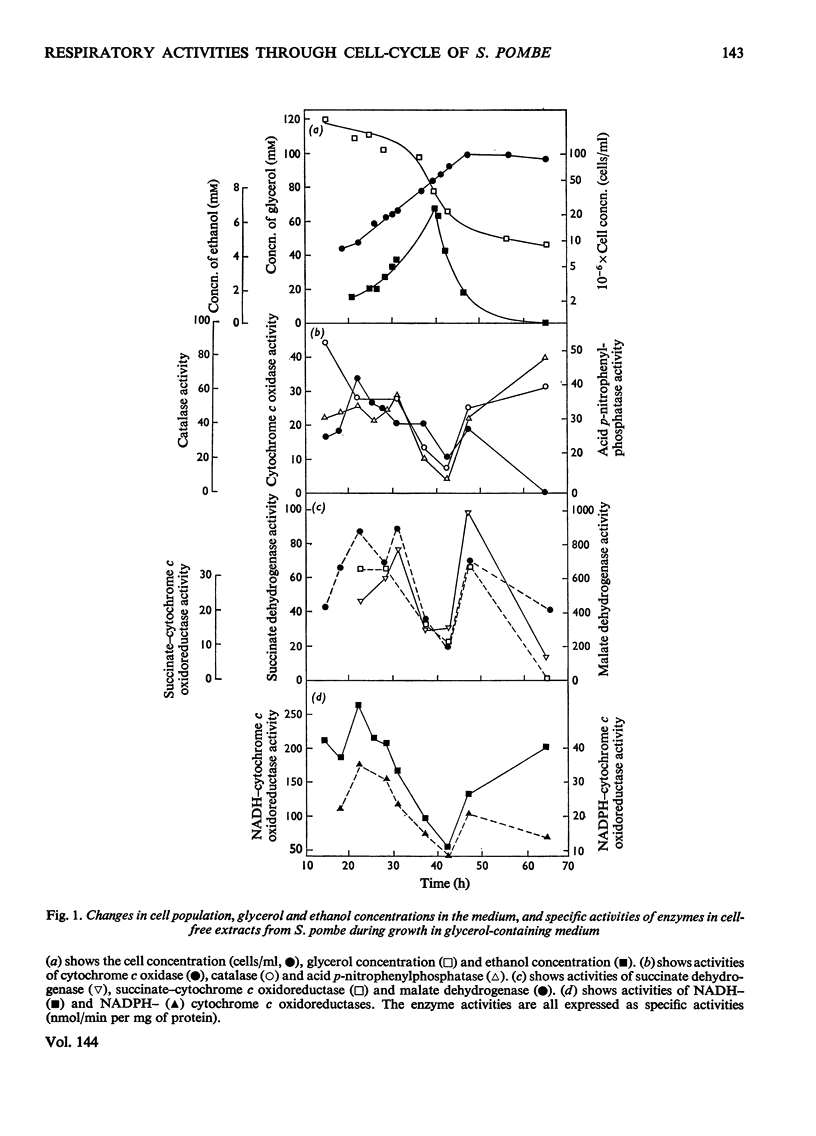
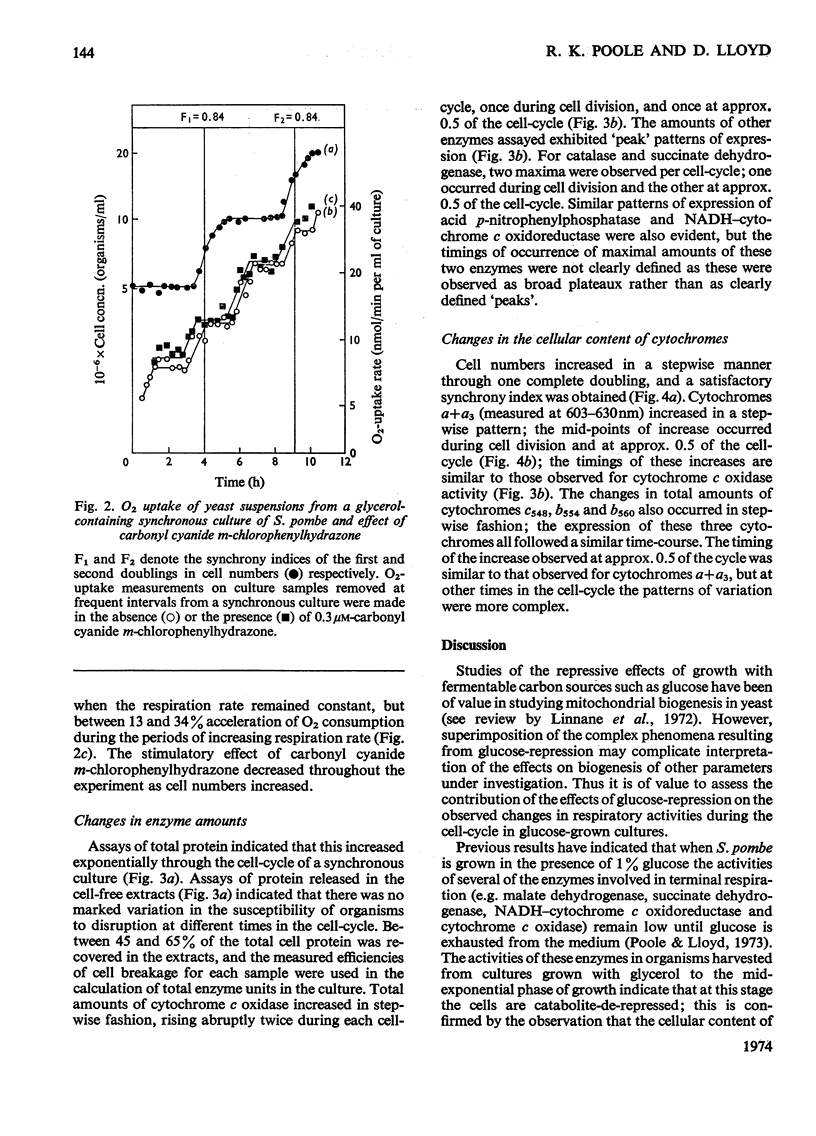
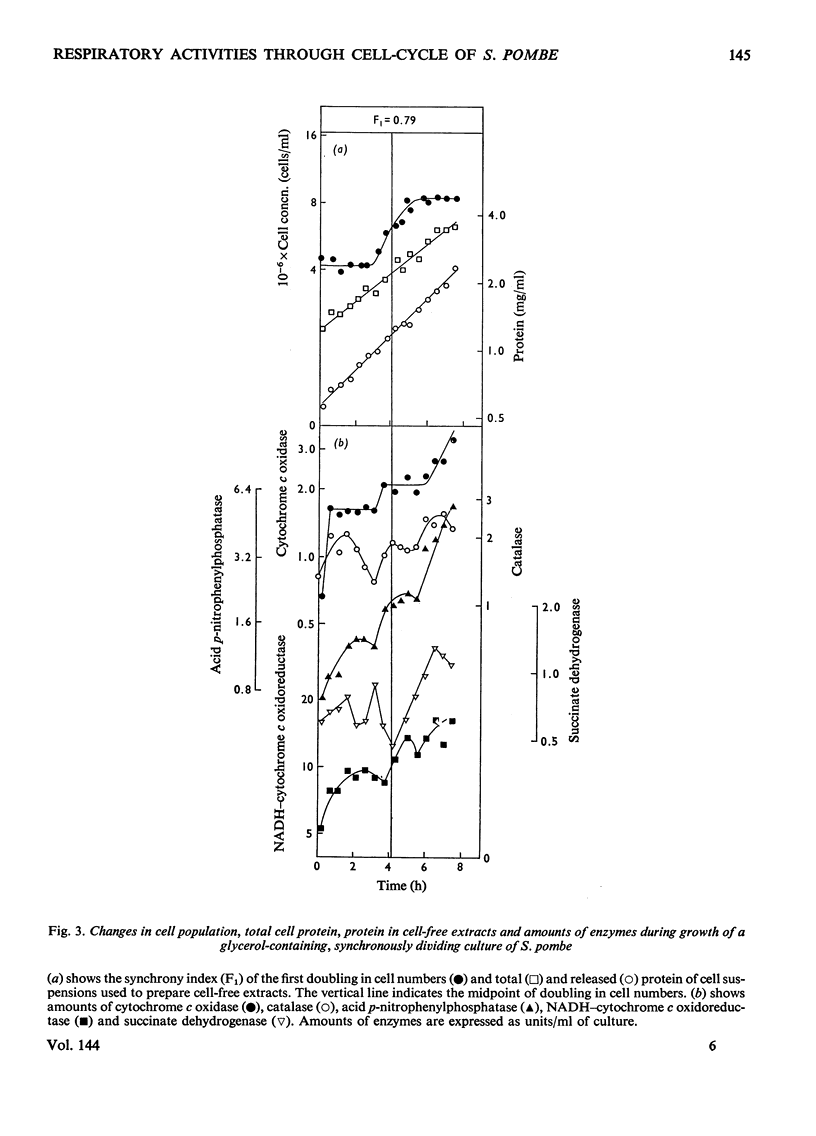
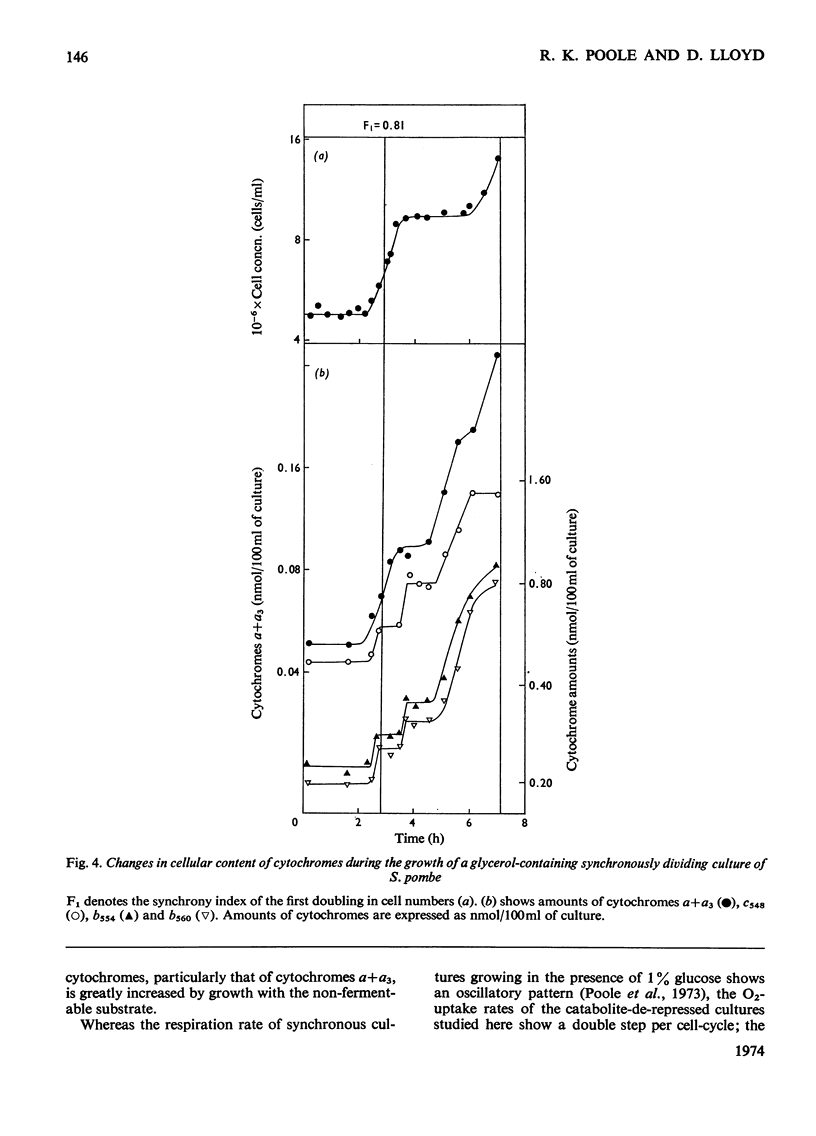
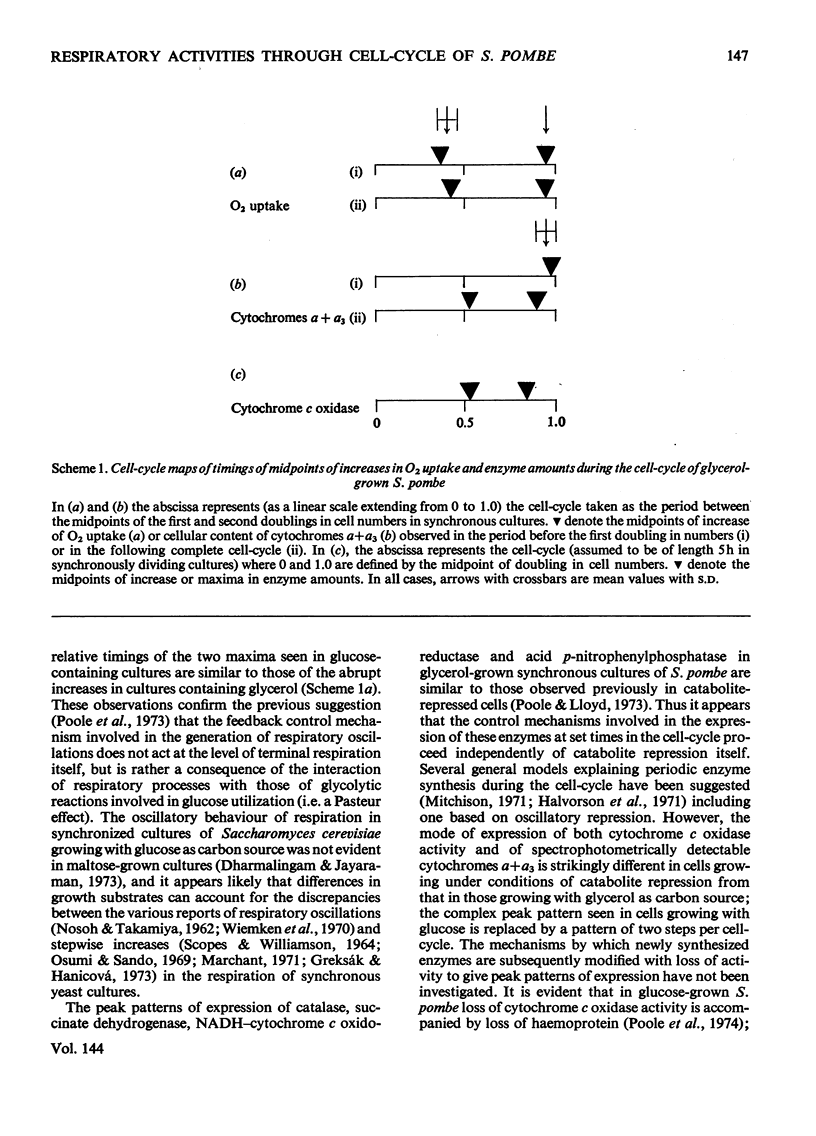
1. The specific activities of cytochrome c oxidase, catalase, succinate dehydrogenase, succinate–cytochrome c oxidoreductase, NADH–cytochrome c oxidoreductase, and NADPH–cytochrome c oxidoreductase in mid-exponential-phase batch cultures of glycerol-grown Schizosaccharomyces pombe indicated that the organisms were catabolite-de-repressed. 2. In cultures growing synchronously in the presence of glycerol as sole carbon source, the respiration rate showed two abrupt increases at about 0.45 and 0.95 of the cell-cycle and remained constant in the periods between successive rises. 3. Catalase, succinate dehydrogenase, NADH–cytochrome c oxidoreductase and acid p-nitrophenyl-phosphatase all showed peak patterns of expression in synchronous cultures. 4. Cytochrome c oxidase and cytochromes a+a3 both showed step patterns of expression with two rises per cell-cycle. 5. Cytochromes c548, b554 and b560 all followed similar time-courses in step patterns of expression, but these were distinct from, and more complex than, that of cytochromes a+a3. 6. These results are compared with those previously obtained with glucose-grown cultures, and the part played by catabolite repression in the expression of respiratory activities in the cell-cycle is assessed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLUMENTHAL L. K., ZAHLER S. A. Index for measurement of synchronization of cell populations. Science. 1962 Mar 2;135(3505):724–724. doi: 10.1126/science.135.3505.724. [DOI] [PubMed] [Google Scholar]
- Dharmalingam K., Jayaraman J. Mitochondriogenesis in synchronous cultures of yeast. I. Oscillatory pattern of respiration. Arch Biochem Biophys. 1973 Jul;157(1):197–202. doi: 10.1016/0003-9861(73)90405-0. [DOI] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Carter B. L., Tauro P. Synthesis of enzymes during the cell cycle. Adv Microb Physiol. 1971;6(0):47–106. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linnane A. W., Haslam J. M., Lukins H. B., Nagley P. The biogenesis of mitochondria in microorganisms. Annu Rev Microbiol. 1972;26:163–198. doi: 10.1146/annurev.mi.26.100172.001115. [DOI] [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- Marchant R. The initiation of cell wall synthesis in parasynchronous cultures of Schizosaccharomyces pombe. Arch Mikrobiol. 1971;78(3):205–213. doi: 10.1007/BF00424894. [DOI] [PubMed] [Google Scholar]
- Mitchison J. M., Creanor J. Linear synthesis of sucrase and phosphatases during the cell cycle of Schizosaccharomyces pombe. J Cell Sci. 1969 Sep;5(2):373–391. doi: 10.1242/jcs.5.2.373. [DOI] [PubMed] [Google Scholar]
- Osumi M., Sando N. Division of yeast mitochondria in synchronous culture. J Electron Microsc (Tokyo) 1969;18(1):47–56. [PubMed] [Google Scholar]
- Poole R. K., Lloyd D., Chance B. The development of cytochromes during the cell cycle of a glucose-repressed fission yeast, Schizosaccharomyces pombe 972h-. Biochem J. 1974 Feb;138(2):201–210. doi: 10.1042/bj1380201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Lloyd D. Oscillations of enzyme activities during the cell-cycle of a glucose-repressed fission-yeast Schizosaccharomyces pombe 972h-. Biochem J. 1973 Sep;136(1):195–207. doi: 10.1042/bj1360195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOPES A. W., WILLIAMSON D. H. THE GROWTH AND OXYGEN UPTAKE OF SYNCHRONOUSLY DIVIDING CULTURES OF SACCHAROMYCES CEREVISIAE. Exp Cell Res. 1964 Jul;35:361–371. doi: 10.1016/0014-4827(64)90102-8. [DOI] [PubMed] [Google Scholar]


