Abstract
Background
To investigate the joint associations between various body fat distribution parameters and high blood pressure (HBP) using the Bayesian Kernel Machine Regression (BKMR) model in school-aged children.
Methods
A diverse sample of 7 ∼ 17 years old (N = 1423; 50.25% boys) was recruited for this study. Fat distribution parameters for multiple body parts, including trunk, legs, android region, and gynoid region fat percentage were measured. HBP was defined as either systolic or diastolic blood pressure exceeded age-, sex- and height-specific 95th percentiles. The chi-square test was utilized to compare differences between groups. The BKMR model was employed to analyze the joint effects of body fat indicators on HBP while accounting for potential confounders. Weighted Quantile Sum (WQS) model was used to characterize the relative weights of each body fat distribution parameter for HBP. Additionally, stratified analyses were performed by sexes and overweight/non overweight groups.
Results
HBP prevalence was 46.86% and 35.10% for overweight and obese (OB) boys and girls, and was 17.96% and 17.28% for non-overweight and obese (non-OB) boys and girls, respectively. Increased fat percentages of trunk, android, and gynoid parts are associated with a higher risk of HBP, while increased fat percentage of the leg was associated with lower HBP risk. Android fat percentage contributed the most HBP risk in OB boys (weight = 0.34), OB girls (weight = 0.39), and non-OB girls (weight = 0.56). Leg fat percentage had significant protective effect on HBP for non-OB boys (weight=-0.22) and OB boys (weight=-0.44), while gynoid fat percentage had significant protective effect for OB girls (weight=-0.27).
Conclusions
Fat distribution of various body parts have inconsistent roles and directions in their association with HBP risk in children of different sex and weight status. We recommend that children of different sexes and weight statuses be provided with body-part-specific exercise recommendations for optimal chronic disease prevention and control benefits.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20702-7.
Keywords: Body fat distribution, High blood pressure, Bayesian Kernel Machine Regression, Weighted quantile Sum, Children and adolescents
Introduction
Body fat distribution, such as the hips, trunks, and legs, plays a crucial and fundamental role in influencing the overall metabolic and cardiovascular health of individuals [1, 2]. By recognizing the importance of body fat distribution and its impact on cardiovascular health, individuals can make informed decisions about their lifestyle choices, to optimize their overall well-being and reduce the risk of associated health complications [3]. While body mass index (BMI) has conventionally served as a surrogate measure of overall adiposity [4], depending solely on it to establish weight deviation limits can be restrictive, as it does not differentiate between excess body weight and fat deposits [5]. However, recent research has emphasized the importance of considering specific measures of different parts of body fat percentage, as they provide insights into the regional deposition of fat and its associated health implications [6, 7]. Among the various techniques employed for analyzing body composition, dual-energy X-ray absorptiometry (DXA) stands out as an accurate tool with excellent applicability, particularly in pediatric patients [8].
In recent years, there has been growing concern regarding the impact of body fat distribution on the development of high blood pressure (HBP) in children and adolescents [9, 10]. HBP is a prevalent cardiovascular risk factor among the younger population, with significant implications for long-term health outcomes. Particularly in Asian countries, the findings indicate that the prevalence of HBP in children and adolescents has escalated from 4.3% at the age of 6 to 7.9% at the age of 14 [11]. Based on previous national survey data in China [12], it was found that the prevalence of overweight students showed a significant rise from 4.3% in 1995 to 18.4% in 2014. On the other hand, the prevalence of HBP exhibited fluctuations within the range of 4.4–6.4% during the same period. These findings highlight a concerning trend of increasing overweight cases over time, while the prevalence of HBP remained relatively stable albeit with slight variations.
Existing studies examining the connection between body fat distribution and HBP have predominantly focused on the impact of individual variables on outcomes [10, 13, 14]. The absence of evidence concerning the joint associations and relative contributions of multiple body fat distribution parameters to HBP would limit further insights into the underlying mechanisms and aid in the development of targeted interventions. To investigate the complex interplay between body fat distribution parameters in multiple body parts and HBP in children and adolescents, advanced statistical modeling approaches are required. Bayesian Kernel Machine Regression (BKMR) has emerged as a powerful and flexible modeling technique for assessing the joint associations of multiple predictors with an outcome of interest [15]. The BKMR model allows for the incorporation of non-linear and interactive effects [16], which would be beneficial for examining both the individual and joint associations between body fat distribution parameters and HBP. The weighted quantile sum (WQS) model, a statistical approach commonly used in epidemiological research, offers a novel and comprehensive way to evaluate the combined effects of multiple exposures on a particular outcome [17]. By assigning weights to each body fat distribution parameter, determined by their respective associations with blood pressure levels, the WQS model facilitates the quantification of their relative contributions. This approach provides a more comprehensive understanding of how body fat distribution affects blood pressure under real physiological conditions.
In this cross-sectional survey, by utilizing BKMR and WQS models, we aim to fill a critical knowledge gap in the field by providing valuable insights into the joint associations between multiple body fat distribution parameters and HBP over 7–17 years in Beijing, China. Our primary hypotheses centered around the predominant body fat distribution parameters with greater contributions to HBP among children and adolescents, with considerations on overweight or obesity and biological sex differences.
Methods
Study design and population
The data utilized in this study were obtained from a cross-sectional survey conducted in 2021 in Beijing among children and adolescents. A comprehensive description of the sampling procedure has been previously published [18]. In summary, we conducted a comprehensive pre-survey of all participants from four elementary and junior high schools, which was meticulously designed to guarantee a representative sample that encompassed a diverse range of age groups, both sexes, and different nutritional statuses, including normal weight, overweight, and obesity. This approach enabled a more robust analysis of the potential influences of age, sex, and body composition on the risk of HBP across a broad pediatric population.
All eligible participants in our study underwent a comprehensive medical examination prior to data collection. Those who met any of the following criteria were excluded from the study: (1) a history of severe vital organ diseases such as liver and kidney failure, or the presence of tumors; (2) recent surgical procedures, physical developmental defects, or the presence of medical devices containing metal in their bodies; (3) disagreement to participate in the study from either the participant or their guardians. In our study, we initially recruited a total of 1597 participants. However, we excluded 174 participants (including 58 without basic information, 45 without body fat distribution data, 15 outliers of body fat and blood pressure levels (within the range of Mean ± 3SD), and 56 not meeting the inclusion and exclusion criteria), which were essential for our analyses. This represents approximately 10.9% of the overall sample. Eventually, a total of 1423 children and adolescents with complete individual information met the inclusion criteria and were included in the final analysis.
Ethical approval for this study was obtained from the Medical Research Ethics Committee of Peking University Health Science Center (IRB00001052-20024), and written informed consent was obtained from both the participating students and their parents or guardians.
Measurements and classifications
Body fat distribution
Measurements of the whole body and regional fat distribution were performed by skilled medical personnel using the GE Healthcare Lunar iDXA dual-energy X-ray bone densitometer. The device we employed is calibrated regularly according to the manufacturer’s specifications to maintain its accuracy. The precision error for the DEXA scanner is typically less than 1% for body fat measurements, which is considered excellent in the field of body composition analysis [19] The measurements were carried out in accordance with the instruction manual and program guidelines, with each participant’s assessment taking approximately 5 to 7 min. Participants were asked to wear lightweight, comfortable clothing and to remove all metal objects or accessories, such as jewelry, zippers, and clasps, prior to the assessment. Body fat distribution data were analyzed using enCORE software (version 16).
Avoiding overeating or engaging in strenuous exercise before the examination. Not having any medical devices, such as pacemakers or cochlear implants. Ensuring that they had not undergone gastrointestinal or angiographic procedures within the previous week. Participants were then positioned according to the requirements outlined in the manual. Subsequently, whole-body and regional fat distribution indicators, including the fat mass and fat percentage of the trunk, legs, android region, and gynoid region, were analyzed for children and adolescents. Additionally, the formula of fat percentage as: Fat percentage = Total fat mass/ Total body mass*100%.
BMI
All participants underwent a comprehensive physical examination conducted by trained medical staff following a standardized protocol. Participants’ height was measured barefoot using a uniform and calibrated mechanical stadiometer (model TZG, Jiangyin No. 2 Medical Equipment Factory, Jiangsu, China) with an accuracy of 0.1 centimeters. Weight was measured while wearing light clothes using a uniform and calibrated electronic scale (model RGT-140, Shanghai Dachuan Electronic Weighing Apparatus Co. Ltd., Shanghai, China) with an accuracy of 0.1 kg. Each of the aforementioned measurements was taken twice, and the average values were recorded for further analysis. The instruments used for measurements were calibrated and designed to ensure accuracy and consistency in data collection.
BMI was determined by dividing weight (in kilograms) by the square of height (in meters). Subsequently, the BMI-z-score, a standardized score based on age and sex according to the WHO standard reference population (http://www.who.int/childgrowth/standards/en/) [20], was calculated for each person. Overweight or obesity was assessed using the BMI-z-score, with a value of ≤ 1 indicating non-overweight and obesity (non-OB), and a value of ≥ 1 indicating overweight and obesity (OB) [20]. These calculations were performed using the “zanthro” module in STATA 14.0.
High blood pressure
BP was measured in the right arm using the guidelines of the National High Blood Pressure Education Program (NHBPEP) Working Group in Children and Adolescents [21]. The measurements were conducted using an electronic blood pressure monitor with an appropriately sized cuff, mercury sphygmomanometers (model XJ11D, Shanghai Medical Instruments Co. Ltd., Shanghai, China), and stethoscopes (model TZ-1, Shanghai Medical Instruments Co. Ltd., Shanghai, China). Systolic blood pressure (SBP) was determined by the onset of the first Korotkoff sound, while diastolic blood pressure (DBP) was determined by the fifth Korotkoff sound. BP was measured twice with a 5-minute interval between measurements. The average values of SBP and DBP were calculated, respectively.
BP levels were evaluated based on the 2017 Guideline from the American Academy of Pediatrics (AAP) titled “Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents” [22]. Normotensive individuals were identified as those with SBP and DBP values below the 95th percentile for their sex, age, and height. High systolic blood pressure (HSBP) and high diastolic blood pressure (HDBP) were defined as SBP or DBP values equal to or exceeding the 95th percentile for age, sex, and height among children younger than 13 years, and as equal to or exceeding 130/80 mmHg for those aged 13 years or older. Participants meeting either the HSBP or HDBP criteria were categorized as having HBP.
Questionnaires data collection
The project team distributed questionnaires to the students and parents through school doctors prior to the physical examination. To ensure accuracy, thorough preparations were made, including pre-investigation of all questionnaire questions. For participants in grades above third, the questionnaires were filled out in the classroom, with professional investigators available for any necessary explanations. For participants in third grade and below, the questionnaires were completed at home under the supervision of their primary guardian.
The children’s questionnaire encompassed sociodemographic variables, basic information, and lifestyle factors. This included gathering data such as date of birth, age, sex, monthly family income, as well as lifestyle behaviors such as sleeping time, smoking, and drinking. Dietary behaviors, including the consumption of fruits, vegetables, sugar-sweetened beverages, and physical activity time (Table 1). Detailly, our study employed a structured self-reported questionnaire to collect data on participants’ drinking and smoking behaviors. Sample questions included: (1) Do you smoke/drink now? (2) How much you smoked/drank alcohol per day in the past seven days?
Table 1.
Definition of questionnaire information
| Questionnaire information | Variables | Classification criteria |
|---|---|---|
| Basic Information | Date of birth | ID number |
| Sex | Boys or Girls | |
| Age | (Examine date -Birth date)/365.25 | |
| Sociodemographic | Monthly family income(yuan) | < 8,000, 8,000–18,000, and ≥ 18,000 yuan |
| Lifestyle factors | Sleeping time (PSQI) [24] (hour) | Continuous variable |
| Smoking | Yes or no | |
| Drinking | Yes or no | |
| Fruits(days) | (Days× (consumption in each of those days))/7) | |
| Vegetables(days) | (Days× (consumption in each of those days))/7) | |
| Sugar-sweetened beverages(days) | (Days× (consumption in each of those days))/7) | |
| Physical activity time (IPAQ) [25] (days) | Days× (time in each of those days)/7) |
The dietary intake was assessed using a structured self-reported questionnaire to collect data on their consumption of a comprehensive list of food and beverage items in the past week. The question pertaining to meat, vegetables, and fruits intake was phrased as follows: “In the past 7 days, how many days have you eaten them?” Participants were provided with options ranging from “none” to “seven days” to capture a detailed profile of their diet consumption patterns. The questionnaire employed in this study has demonstrated strong reliability and validity, and it has been previously utilized in several published studies [23].
Statistical analysis
Continuous variables were presented as means and standard deviations, while categorical variables were reported as numbers and percentages. The T-test was used for continuous variables, and the Chi-square test was used for categorical variables. The BKMR model was used to assess the joint associations of the four body fat distribution parameters (i.e., trunk, legs, android region, and gynoid region fat percentage) with BP levels. The BKMR model could address the deal with the complexity of multiple variables of interest with potentially correlated structures and interacting effects [15, 26]. The model could simulate the combined effect estimates of multiple variables of interests using kernel functions through Markov Chain Monte Carlo (MCMC) methods [15, 26]. The formula used in this study could be expressed in supplementary materials. MCMC methods with 5000 iterations were adopted to generate stable parameters [15]. The results of the BKMR models in this study included the following aspects:
Linear and non-linear exposure-response associations between one fat distribution parameter (e.g. android region percentage) of interest and the outcome (e.g. HBP) when the other fat distribution parameters (e.g. trunk, legs, and gynoid region fat percentage) were fixed at a particular quantile (e.g. median). In this study, the results were presented for one body fat percentage indicator when the other body fat percentage indicators were fixed at their 50th percentiles [26, 27].
The posterior inclusion probability (PIP) value of each body fat distribution parameter for each BP level. The PIP values of the BKMR model could demonstrate the relative importance of each fat distribution parameter, and a PIP value larger than 0.5 is generally acknowledged as an important variable associated with BP levels [28].
WQS model was further used to characterize the relative contributions (weights) of each body fat distribution parameter as for each BP levels [29], and the formula used in this study could be expressed in supplementary materials. In brief, as the WQS model requires that all exposure variables exhibit effects in the same direction [29], we adjusted for body fat indicators demonstrating inverse associations with BP (e.g., leg fat percentage showing a negative association with HBP) by utilizing their inverse values [30, 31]. This adjustment ensured compliance with the assumption of uniform effect direction.
The results of the WQS model in this study included the weights and directions of each body fat distribution parameter as for each BP level The weights derived from the WQS model were initially positive for all parameters. For indicators where inverse values were applied, a negative sign was assigned to indicate their opposite direction of effect. Accordingly, the absolute values of the weights indicated the relative importance of each body fat distribution indicator, while the direction is reflected by the positive/negative signs.
All the statistical analyses were performed using Stata 14.0 (College Station, TX, USA) and R software (version 3.6.3, Comprehensive R Archive Network) incorporated with the packages “bkmr”, “gWQS”, and “wqspt” [15, 32]. A two-sided P < 0.05 was considered as statistical significance.
Sensitivity analysis
Furthermore, to ensure the generalizability of our findings, we conducted sensitivity analyses on the entire population as well as subgroups stratified by nutrition status and sex in the BKMR models. First, we analyze the correlations between whole-body fat percentage, trunk fat percentage, leg fat percentage, android fat percentage, and gynoid fat percentage among boys and girls in Figure S1. Second, we plotted the univariate exposure effect curves of the associations between four body fat percentage distribution parameters and BP levels stratified by nutrition status and sex, to further characterize their potential non-linear associations in Figure S2-S5. Third, we plotted the bivariate cross-sections by investigating the exposure effect curves of one body fat percentage parameter where the other parameters were fixed at 5th, 25th, 50th, 75th, and 95th [32]in Figure S6-S8.
Results
Participant characteristics
Demographic characteristics of 1423 (715 boys versus 708 girls) participants among overweight or obesity are presented in Table 2. The age of the participants ranged between 12.24 (SD = 2.90) and 12.68 (SD = 3.24) years. There were statistically significant differences in weight and BMI based on overweight or obesity observed in both boys and girls (P < 0.05). In boys, the prevalence of HBP was found to be 17.96% among non-OB individuals and 46.86% among those who were OB. However, the prevalence of HBP among girls was found to be 17.28% among non-OB participants and 35.10% among those classified as OB. Significant differences in different parts of body fat percentage were also identified between non-OB and OB participants within the different sex groups (P < 0.05).
Table 2.
Characteristics of participants (N = 1423), stratified by sex among overweight or obesity
| Variables | Boys (N = 715) | Girls (N = 708) | ||||
|---|---|---|---|---|---|---|
| Non-OB | OB | P value | Non-OB | OB | P value | |
| N (%) | 412(57.62) | 303(42.38) | 463(65.40) | 245(34.60) | ||
| Age (years) | 12.68 ± 3.24 | 12.24 ± 2.90 | 0.063 | 12.53 ± 3.19 | 12.54 ± 2.92 | 0.956 |
| Height (cm) | 156.95 ± 17.91 | 156.24 ± 16.09 | 0.581 | 151.22 ± 13.98 | 152.29 ± 12.14 | 0.310 |
| Weight (kg) | 45.78 ± 15.27 | 61.57 ± 21.59 | < 0.001 | 41.85 ± 11.98 | 58.91 ± 16.96 | < 0.001 |
| BMI (kg/m2) | 17.93 ± 2.59 | 24.36 ± 4.53 | < 0.001 | 17.84 ± 2.59 | 24.8 ± 4.21 | < 0.001 |
| Body fat mass (kg) | ||||||
| Trunk fat mass | 1.15 ± 0.38 | 2.38 ± 0.94 | < 0.001 | 1.47 ± 0.56 | 2.62 ± 0.92 | < 0.001 |
| Leg fat mass | 3.78 ± 1.35 | 7.69 ± 3.16 | < 0.001 | 4.91 ± 1.77 | 8.42 ± 2.91 | < 0.001 |
| Android fat mass | 0.47 ± 0.30 | 1.61 ± 0.95 | < 0.001 | 0.63 ± 0.34 | 1.59 ± 0.71 | < 0.001 |
| Gynoid fat mass | 1.56 ± 0.64 | 3.37 ± 1.45 | < 0.001 | 2.21 ± 0.93 | 3.87 ± 1.44 | < 0.001 |
| Body fat percentage (%) | ||||||
| Trunk fat percentage | 25.53 ± 8.36 | 37.75 ± 8.67 | < 0.001 | 35.16 ± 5.87 | 43.90 ± 6.03 | < 0.001 |
| Leg fat percentage | 24.03 ± 7.20 | 34.36 ± 7.29 | < 0.001 | 32.04 ± 4.90 | 38.82 ± 4.92 | < 0.001 |
| Android fat percentage | 17.18 ± 7.08 | 37.39 ± 8.85 | < 0.001 | 24.21 ± 7.33 | 40.60 ± 6.91 | < 0.001 |
| Gynoid fat percentage | 23.47 ± 7.24 | 35.77 ± 6.85 | < 0.001 | 32.82 ± 5.43 | 41.03 ± 4.39 | < 0.001 |
| Blood pressure level (mmHg) | ||||||
| Systolic blood pressure | 114.48 ± 12.48 | 122.33 ± 14.12 | < 0.001 | 111.1 ± 10.82 | 118.79 ± 12.67 | < 0.001 |
| Diastolic blood pressure | 66.96 ± 7.35 | 69.14 ± 7.70 | < 0.001 | 67.85 ± 7.49 | 70.63 ± 8.57 | < 0.001 |
| Blood pressure status (%) | ||||||
| Normal blood pressure | 338(82.04) | 161(53.14) | < 0.001 | 383(82.72) | 159(64.90) | < 0.001 |
| High blood pressure | 74(17.96) | 142(46.86) | 80(17.28) | 86(35.10) | ||
| Life behaviors | ||||||
| Daily sleeping time (h) | 8.00 ± 1.23 | 8.00 ± 1.17 | 0.624 | 8.00 ± 1.33 | 8.00 ± 1.22 | 0.325 |
| Daily middle and high physical activity (h) | 1.00 ± 0.89 | 0.88 ± 0.81 | 0.063 | 0.76 ± 0.67 | 0.73 ± 0.65 | 0.675 |
| Daily fruit consumption (serving) | 1.16 ± 0.90 | 1.18 ± 0.92 | 0.739 | 1.26 ± 0.99 | 1.31 ± 0.83 | 0.501 |
| Daily vegetable consumption (serving) | 1.79 ± 1.30 | 1.83 ± 1.34 | 0.733 | 1.84 ± 1.27 | 1.94 ± 1.19 | 0.332 |
| Daily meat consumption (serving) | 9.71 ± 7.58 | 10.02 ± 6.73 | 0.589 | 8.37 ± 5.65 | 8.65 ± 5.00 | 0.517 |
| Daily SSB consumption (serving) | 2.67 ± 4.55 | 2.13 ± 3.30 | 0.088 | 1.90 ± 2.63 | 2.13 ± 3.22 | 0.305 |
| Family income | ||||||
| Below 8000 yuan | 48(12.66) | 46(16.14) | 0.441 | 63(14.52) | 38(16.31) | 0.374 |
| 8000–18,000 yuan | 153(40.37) | 109(38.25) | 162(37.33) | 96(41.20) | ||
| Above 18,000 yuan | 178(46.97) | 130(45.61) | 209(48.16) | 99(42.49) | ||
| Smoking | ||||||
| Yes | 7(1.82) | 5(1.74) | 0.938 | 0(0.00) | 0(0.00) | |
| No | 377(98.18) | 282(98.26) | 441(100.00) | 239(100.00) | ||
| Drinking | ||||||
| Yes | 14(3.65) | 5(1.74) | 0.141 | 5(1.13) | 4(1.67) | 0.556 |
| No | 370(96.35) | 282(98.26) | 436(98.87) | 235(98.33) | ||
OB: overweight and obesity; BMI: body mass index
SSB: sugar-sweetened beverage; One serving of SSBs is approximately 250 mL. One serving of meat is approximately 50 g
Associations of the four body fat distribution parameters with BP levels
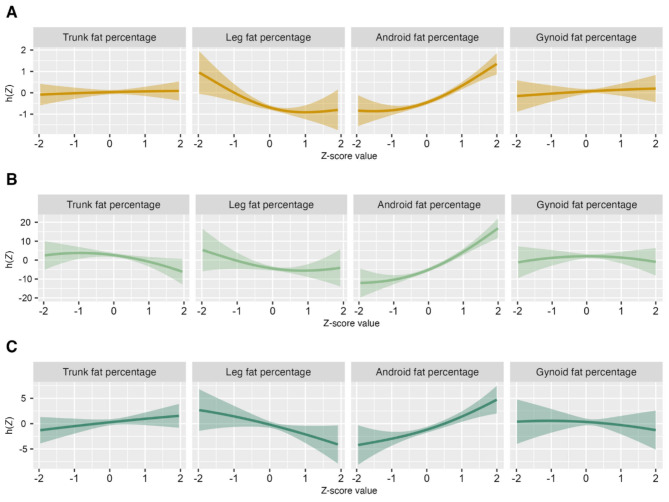
Figure 1 shows the univariate exposure effect curves of the associations of four body fat percentage distributions on BP levels (the model was adjusted for age, sex, family income, sleeping time, smoking, drinking, physical activity time, sugar, meat, vegetables, and fruits). Overall, higher percentage of android fat was associated with an increased risk of HBP, and there is a flat trend between trunk fat and gynoid fat with HBP. As the body fat percentage increases, the risk of HBP increases. However, the trend was reversed when it came to the percentage of leg fat. What’s more, the univariate exposure effect curves of the associations between four body fat percentage distribution parameters and BP levels stratified by nutrition status and sex, to further characterize their potential non-linear associations were shown in Supplementary Figs. 1–4.
Fig. 1.
Univariate exposure effect curves of the associations of four body fat percentage distributions on BP levels with all participants while all other body fat percentages were held at their median
Note: A, HBP; B, SBP; C, DBP; HBP, high blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; x-axis, four body fat percentage Z-score values; y-axis, exposure effect value estimated by the function, where a gaussian link function was used for SBP or DBP, and a binomial link function was utilized for HBP. The results were presented for one body fat percentage indicator when the other body fat percentage indicators were fixed at their 50th percentiles
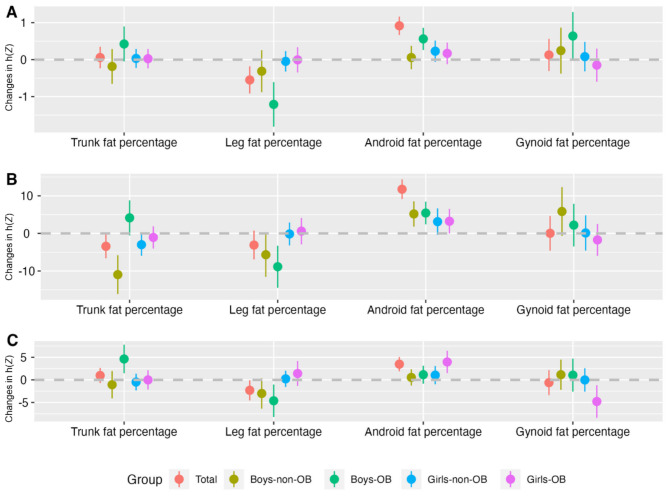
As shown in Fig. 2 and Table S1-S3, the effects of four body fat percentage distribution parameters were similar when compared to the associations between BP levels and a single body fat percentage. During this analysis, all other body fat percentages were fixed at their 50th percentile. In OB boys, HBP risk was significantly related to trunk fat percentage (estimate = 0.42, 95%CI: -0.05, 0.90), android fat percentage (estimate = 0.56, 95%CI: 0.26, 0.86), gynoid fat percentage (estimate = 0.64, 95%CI: -0.01, 1.29). Notably, the associations between leg fat percentage with HBP were significant, with an estimate value of -1.21 (95%CI: -1.81, -0.61). In non-OB boys, an inverse correlation was seen between trunk fat percentage and SBP levels. No significant relationship between HBP risk and four body fat percentage distribution parameters was found in girls while other body fat percentages were fixed at their 50th percentile.
Fig. 2.
The effect estimates and 95%CI of four body fat percentage distribution parameters on BP levels of participants
Note: A, HBP; B, SBP; C, DBP; HBP, high blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; x-axis, four body fat percentage indicators; y-axis, changes in exposure effect value estimated by the function, where a gaussian link function was used for SBP or DBP, and a binomial link function was used for HBP. The results were presented as changes in exposure effect value when one body fat percentage indicator was at the 75th vs. 25th percentile, while the other body fat percentages were fixed at their 50th percentiles
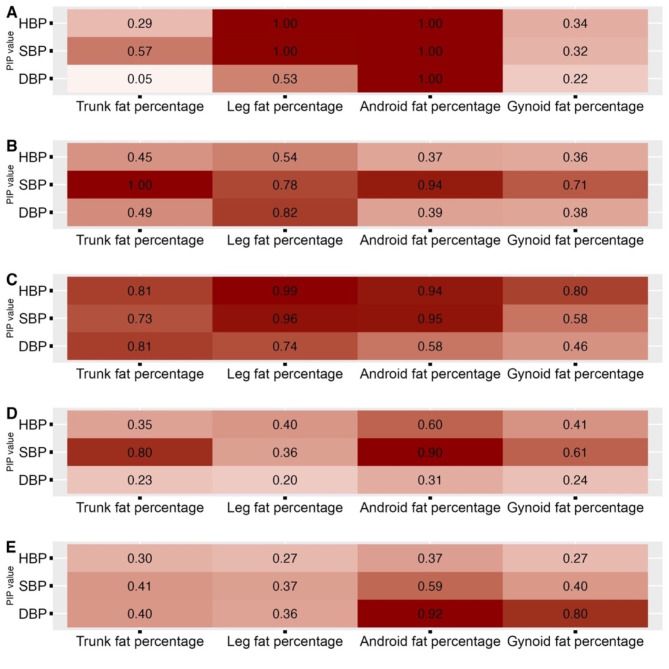
The PIP values for the BKMR model were present in Fig. 3, the larger value indicated the higher relative importance of each fat distribution parameter. For HBP, SBP, and DBP of all participants, the android fat percentage had the highest PIP (1.00) compared to the other three parts of body fat percentage. In non-OB boys, trunk (PIP = 1.00), leg (PIP = 0.78) and android (PIP = 0.94) fat percentage had high PIPs in SBP. Leg fat percentage also had a high PIP of 0.82in DBP. In OB boys, leg fat percentage had a high PIP in HBP (PIP = 0.99), SBP (PIP = 0.96), and DBP (PIP = 0.74); PIPs > 0.5 were also observed in android fat percentage with HBP (PIP = 0.94), SBP (0.95), and DBP (PIP = 0.58). Android fat percentage had a higher PIP in HBP (PIP = 0.60) in non-OB girls, but not in OB girls.
Fig. 3.
PIP value from BKMR model for four body fat distribution parameters
Note: A, Total; B, Boys-non-OB; C, Boys-OB; D, Girls-non-OB; E, Girls-OB; HBP, high blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure
Characterization of the relative weights of each body fat distribution parameter for BP levels
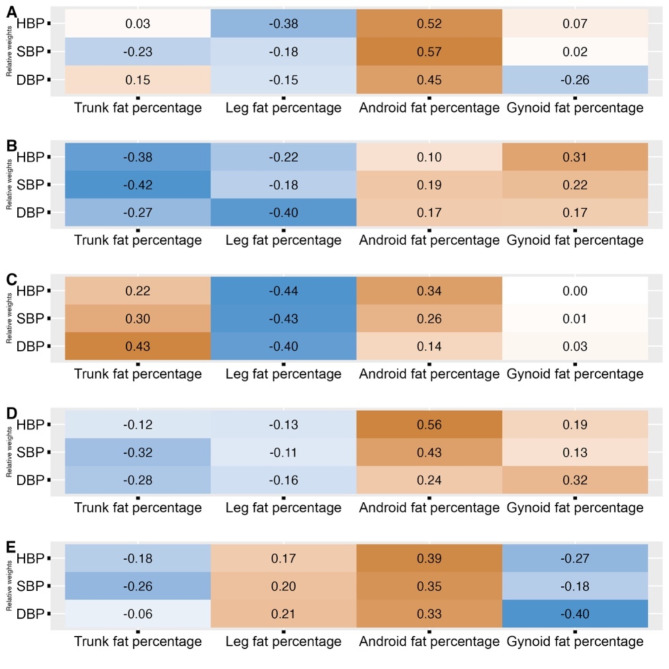
We further performed the WQS regression model to analyze the joint effects of four body fat distribution parameters on the risk of BP levels. The covariates adjusted in this model were age, family income, sleeping time, smoking, drinking, physical activity time, sugar, meat, vegetables, and fruits. Figure 4 depicts the distribution of weights assigned to different body fat parameters concerning BP levels. The absolute values of the weights indicated the relative importance of each body fat distribution indicator, while the direction is reflected by the positive/negative signs. Overall, increased android fat percentage contributed the most to HBP risk with a relative weight of 0.52, while increased leg fat percentage contributed to lowered HBP risk with the weight of -0.38. For OB boys, android (weight = 0.34), trunk (weight = 0.22) and leg (weight=-0.44) fat percentage contributed to the highest harmfulness and protectiveness on HBP risk, respectively. For non-OB boys, the right amount of trunk fat percentage (weight=-0.38), as well as leg fat percentage (weight=-0.22) could be protective from HBP instead. Android fat percentage had the highest weight on HBP risk in both non-OB (weight = 0.56) and OB (weight = 0.39) girls, while leg (weight=-0.13 in non-OB girls and weight = 0.17 in OB girls) and gynoid (weight = 0.19 in non-OB girls and weight=-0.27 in OB girls) fat percentages played opposing roles in the two groups.
Fig. 4.
Characterization of the relative weights of each body fat distribution parameter for BP levels using WQS
Note: A, Total; B, Boys-non-OB; C, Boys-OB; D, Girls-non-OB; E, Girls-OB HBP, high blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure
Sensitivity analyses
In Figure S1, there was a strong correlation whole-body fat percentage, trunk fat percentage, leg fat percentage, android fat percentage, and gynoid fat percentage among boys and girls. The univariate exposure effect curves of the associations between four body fat percentage distribution parameters and BP levels stratified by nutrition status and sex showed approximately linear trends (Figures S2-S5). Meanwhile, the bivariate cross-sections showed similar trends for exposure effect curves of one body fat percentage parameter when the other parameters were fixed at different percentiles, indicating that there might be no potential interactive effects between the multiple body fat percentage parameters (Figures S6-S8).
Discussion
The main focus of our study was to examine the joint associations between multiple body fat distribution parameters with HBP among children and adolescents in China. Our analysis indicates that there is an association between elevated trunk fat, android fat, and gynoid fat percentages and a higher prevalence of HBP. Furthermore, the android fat might play a predominant role in the joint associations between multiple body fat distribution parameters with HBP. Meanwhile, when considering the percentage of leg fat, an intriguing reversal in the trend was observed. The stratified analyses highlighted the importance of closely monitoring the android fat distribution, especially in OB boys, as it was found to have a significant influence on the development of HBP.
Previous research exploring the relationship between body fat distribution and HBP has primarily concentrated on the effects of isolated factors on the results [10, 13, 14]. Nevertheless, under actual physiological conditions, the body fat distribution in different body parts may have complex interplay and simultaneously impact on BP levels. To our knowledge, our study for the first time investigated the joint associations between multiple body fat distribution parameters with HBP as well as their relative importance by quantitatively estimating the weights. The results revealed that the android fat might play a predominant role in the joint associations between multiple body fat distribution parameters with HBP. Notably, there was an intriguing reversal in the observed trend when considering the percentage of leg fat. These findings provide valuable insights into comprehending the intricate relationship between body fat distribution and HBP and have the potential to provide a strong basis for the development of precise prevention and control strategies for HBP in children and adolescents. These results provide consistent and compelling evidence that postmenopausal women with normal BMI at 161 clinical centers in the United States revealed a significant association between leg fat and a decreased risk of cardiovascular disease (HR = 0.62) [33]. On the other hand, according to a study conducted by Taksali [34], an increase in the proportion of visceral fat and a decrease in android fat were associated with higher levels of triglyceride, BP, and insulin resistance. Fat deposits in different areas of the body can have distinct effects on vascular function. One possible explanation was that android and visceral fat deposits are known risk factors for vascular function, whereas fat deposits in the legs and buttocks may confer protective benefits for vascular function [35]. Another possible explanation was that higher abdominal fat associated positively with HBP due to its link with insulin resistance, inflammation, and dyslipidemia, while higher leg fat may provide protection by scavenging circulating lipids and glucose, thereby mitigating HBP risk factors [36, 37].
Our study emphasizes the significance of paying special attention to fat distribution patterns, particularly in OB boys. While obesity is known to be associated with increased BP, the distribution of body fat can further influence this relationship [38, 39]. These findings suggest that interventions targeting fat distribution patterns may be particularly beneficial for OB boys in reducing the risk of HBP, not non-OB boys. Among the potential mechanisms are changes in metabolic profiles, hormonal influences, genetic predispositions and lifestyle factors that contribute to differences in outcomes between the two groups [40]. Further research is warranted to explore the underlying mechanisms driving this specific association and to develop tailored strategies for managing fat distribution in this vulnerable population [41, 42]. The mechanisms by which fat distribution affects HBP can differ in children and adolescents of different sexes [13, 43]. Previous studies have provided evidence-based hormonal variations, such as sex hormones and growth factors, which play a crucial role in the regulation of fat distribution and BP regulation. Estrogen can promote adipose tissue deposition either directly or by activating its receptors in adipocytes and adipose tissue [44–46]. On the other hand, androgens can influence the number of adipocytes and the distribution of adipose tissue [47]. Additionally, differences in body composition, physical activity levels, and dietary habits between different sexes may further influence the relationship between fat distribution and HBP [48, 49]. Moreover, emerging evidence suggests that genetic and epigenetic factors may interact with fat distribution to modulate the risk of high BP differently in boys and girls. Future studies should aim to elucidate the underlying pathways involved and investigate potential sex-specific interventions targeting fat distribution to manage hypertension effectively in children and adolescents.
This study possessed several noteworthy strengths that merit acknowledgment. Firstly, the utilization of DXA to comprehensively scan the entire body of participants served as the gold standard for body composition measurement. This approach surpassed the limitations of relying solely on BMI and offered more accurate predictions of body size dissatisfaction in children and adolescents. Secondly, our study for the first time investigated the joint associations between multiple body fat distribution parameters with HBP as well as their relative importance by quantitatively estimating the weights using the BKMR and WQS models. These algorithms excel in providing detailed and interpretable results and facilitating joint effect analyses. Significantly, these strengths establish a robust foundation for the development of precise prevention and control strategies for HBP in children and adolescents, underscoring the critical importance of considering body composition measures in such endeavors. However, it is important to acknowledge several limitations in this study that should be taken into consideration. Firstly, due to the cross-sectional nature of the study, it is unable to establish a causal relationship between the variables investigated. Secondly, the measurement of BP was performed as a one-time single-point assessment, which may lead to an overestimation of HBP when making judgments. Thirdly, our study did not assess the pubertal developmental stage of participants at the time of data collection and was unable to detail sex differences in body fat distribution in children and adolescents at the pubertal stage. Consequently, the utility of body composition indicators for predictive purposes remains uncertain within the context of this study. Furthermore, the adjustment for confounding factors was constrained by the limited inclusion of lifestyle factors, and the dietary questionnaire employed was relatively simplistic, lacking more objective and accurate dietary indicators such as nutrient content.
Conclusions
In summary, our findings revealed an increased risk of HBP associated with higher percentages of trunk fat, android fat, and gynoid fat percentages, where the android fat might play a predominant role in the joint associations between multiple body fat distribution parameters with HBP. Given the fact that these fat indicators from various body parts have inconsistent roles in the association with HBP risk, we recommend that it might be beneficial to offer children body-part-specific exercise guidance to promote more effective HBP prevention strategies. These findings could contribute to evidence-based interventions and public health policies to mitigate the burden of cardiovascular diseases in later life.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our gratitude for the support received from our dedicated team members, as well as the enthusiastic participation of students, teachers, parents, and local education and health personnel involved in the programs.
Author contributions
MC originated the study, conducted initial analyses, and composed the initial manuscript. MC, along with XW, YL, DG, YM, TM, YZ, LC, and JL, collaborated on designing the study, developing data collection instruments, and critically reviewing and revising the manuscript. QM, MC, XW, TG, and WY took on the responsibility of designing data collection instruments and reviewing the manuscript. XW, JM, and YD provided invaluable feedback, making significant revisions to the manuscript in terms of intellectual content and language. All authors thoroughly examined and endorsed the final manuscript in its submitted form and have granted consent for the published version.
Funding
The present study was supported by National Key R&D Program of China (Grant number 2022YFC2705300) to Yanhui Dong,and the National Natural Science Foundation (No. 82204067 to Xijie Wang.).
Data availability
The data utilized for the analyses are presented within the paper, and upon reasonable request, the underlying data for this article will be made available by contacting the corresponding author.
Declarations
Ethics approval and consent to participate
The research involving human participants underwent scrutiny and received approval from the Ethics Committee of Peking University (Reference Number: IRB00001052 20024). Before participation, written informed consent was obtained from both students and their parents.
Consent for publication
Not applicable.
Conflict of interest
The authors declared no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xijie Wang, Email: xijie_wang@126.com.
Yanhui Dong, Email: dongyanhui@bjmu.edu.cn.
References
- 1.Lang PO, Trivalle C, Vogel T, Proust J, Papazyan JP, Dramé M. Determination of cutoff values for DEXA-Based body composition measurements for determining metabolic and Cardiovascular Health. Biores Open Access. 2015;4(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariff S, Aamir A, Young A, Sikanderali L, Rizvi A, Shaheen F, Khan GN, Soofi S, Fernandes M. Differential associations between body composition indices and neurodevelopment during early life in term-born infants: findings from the Pakistan cohort: multi-center body composition reference study. Eur J Clin Nutr 2023. [DOI] [PMC free article] [PubMed]
- 3.Holmes CJ, Racette SB. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: an overview of current methodology. Nutrients. 2021;13(8):2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bramante CT, Palzer EF, Rudser KD, Ryder JR, Fox CK, Bomberg EM, Bensignor MO, Gross AC, Sherwood NE, Kelly AS. BMI metrics and their association with adiposity, cardiometabolic risk factors, and biomarkers in children and adolescents. Int J Obes (Lond). 2022;46(2):359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frühbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, Halford JGC, Farpour-Lambert NJ, Blaak EE, Woodward E, et al. The ABCD of obesity: an EASO position Statement on a Diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hind K, Oldroyd B, Truscott JG. In vivo precision of the GE Lunar iDXA densitometer for the measurement of total body composition and fat distribution in adults. Eur J Clin Nutr. 2011;65(1):140–2. [DOI] [PubMed] [Google Scholar]
- 7.Dos Santos RRG, Forte GC, Mundstock E, Amaral MA, da Silveira CG, Amantéa FC, Variani JF, Booij L, Mattiello R. Body composition parameters can better predict body size dissatisfaction than body mass index in children and adolescents. Eat Weight Disord. 2020;25(5):1197–203. [DOI] [PubMed] [Google Scholar]
- 8.Lifshitz F, Hecht JP, Bermúdez EF, Gamba CA, Reinoso JM, Casavalle PL, Friedman SM, Rodriguez PN. Body composition analysis by dual-energy X-ray absorptiometry in young preschool children. Eur J Clin Nutr. 2016;70(10):1203–9. [DOI] [PubMed] [Google Scholar]
- 9.Pucci G, Martina MR, Bianchini E, D’Abbondanza M, Curcio R, Battista F, Anastasio F, Crapa ME, Sanesi L, Gemignani V, et al. Relationship between measures of adiposity, blood pressure and arterial stiffness in adolescents. The MACISTE study. J Hypertens. 2023;41(7):1100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales-Ghinaglia N, Larsen M, He F, Calhoun SL, Vgontzas AN, Liao J, Liao D, Bixler EO, Fernandez-Mendoza J. Circadian Misalignment Impacts the Association of Visceral adiposity with elevated blood pressure in adolescents. Hypertension. 2023;80(4):861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, Rudan I. Global prevalence of hypertension in children: a systematic review and Meta-analysis. JAMA Pediatr. 2019;173(12):1154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y, Ma J, Song Y, Ma Y, Dong B, Zou Z, Prochaska JJ. Secular trends in blood pressure and overweight and obesity in Chinese boys and girls aged 7 to 17 years from 1995 to 2014. Hypertension. 2018;72(2):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao H, Shu W, Li M, Xu L, Amaerjiang N, Zunong J, Vermund SH, Huang D, Chong M, Hu Y. Sex-specific differences in Left Ventricular Mass and volumes with body Mass Index among children aged 6 to 8: a cross-sectional study in China. Nutrients. 2023;15(13):3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao LW, Huang YW, Cheng H, Wang X, Dong HB, Xiao P, Yan YK, Shan XY, Zhao XY, Mi J. Prevalence of hypertension and its associations with body composition across Chinese and American children and adolescents. World J Pediatr 2023. [DOI] [PubMed]
- 15.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, Godleski JJ, Coull BA. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang ST, Cao Y, Dong J, Li C, Qiu J, Li X. The association between urinary phthalate metabolites and serum thyroid function in US adolescents. Sci Rep. 2023;13(1):11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, Zhang L, Luo N, Wang M, Tang C, Jing J, Chen H, Hu Q, Tan L, Ma X, et al. Metal mixture exposure and the risk for immunoglobulin A nephropathy: evidence from weighted quantile sum regression. Environ Sci Pollut Res Int. 2023;30(37):87783–92. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Liu J, Ma Y, Li Y, Gao D, Chen L, Ma T, Dong Y, Ma J. Association between Body Fat and elevated blood pressure among children and adolescents aged 7–17 years: using dual-energy X-ray absorptiometry (DEXA) and Bioelectrical Impedance Analysis (BIA) from a cross-sectional study in China. Int J Environ Res Public Health. 2021;18(17):9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyan R. Body composition techniques. Indian J Med Res. 2018;148(5):648–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adolescents, NHBPEPWGoHBPiCa. FOURTH REPORT ON diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 22.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, et al. Clinical practice Guideline for Screening and Management of High Blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Chen M, Ma Y, Ma T, Gao D, Li Y, Wang X, Chen L, Ma Q, Zhang Y, et al. Habitual dairy consumption is inversely associated with depressive and social anxiety symptoms among children and adolescents aged 7–17 years: findings from a cross-sectional study in Beijing, China. J Affect Disord. 2022;319:309–17. [DOI] [PubMed] [Google Scholar]
- 24.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 25.Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peralta AA, Schwartz J, Gold DR, Coull B, Koutrakis P. Associations between PM(2.5) metal components and QT interval length in the normative aging study. Environ Res. 2021;195:110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodrich AJ, Kleeman MJ, Tancredi DJ, Ludeña YJ, Bennett DH, Hertz-Picciotto I, Schmidt RJ. Ultrafine particulate matter exposure during second year of life, but not before, associated with increased risk of autism spectrum disorder in BKMR mixtures model of multiple air pollutants. Environ Res. 2024;242:117624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Y, Fu Y, Yao H, Wu X, Yang Z, Zeng H, Zeng Z, Liang H, Li Y, Jing C. Relationship between phthalates exposures and hyperuricemia in U.S. general population, a multi-cycle study of NHANES 2007–2016. Sci Total Environ. 2023;859(Pt 1):160208. [DOI] [PubMed] [Google Scholar]
- 29.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2015;20(1):100–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu S, Gennings C, Wright RJ, Wilson A, Burris HH, Just AC, Braun JM, Svensson K, Zhong J, Brennan KJM, et al. Prenatal stress, methylation in inflammation-related genes, and adiposity measures in early childhood: the Programming Research in obesity, growth environment and social stress cohort study. Psychosom Med. 2018;80(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang W, Guo T, Guo H, Chen X, Ma Y, Deng H, Yu H, Chen Q, Li H, Liu Q, et al. Ambient particulate air pollution, blood cell parameters, and effect modification by psychosocial stress: findings from two studies in three major Chinese cities. Environ Res. 2022;210:112932. [DOI] [PubMed] [Google Scholar]
- 32.Day DB, Sathyanarayana S, LeWinn KZ, Karr CJ, Mason WA, Szpiro AA. A permutation test-based Approach to strengthening inference on the effects of Environmental mixtures: comparison between single-Index Analytic methods. Environ Health Perspect. 2022;130(8):87010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen GC, Arthur R, Iyengar NM, Kamensky V, Xue X, Wassertheil-Smoller S, Allison MA, Shadyab AH, Wild RA, Sun Y, et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur Heart J. 2019;40(34):2849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taksali SE, Caprio S, Dziura J, Dufour S, Calí AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57(2):367–71. [DOI] [PubMed] [Google Scholar]
- 35.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen MA, Uiterwaal CS, Visseren FL, van der Ent CK, Grobbee DE, Dalmeijer GW. Abdominal fat and blood pressure in healthy young children. J Hypertens. 2016;34(9):1796–803. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Deng H, Pan W, Huang X, Xu K, Zhang X, Hu X, Gu X. The Inverse Association of Leg Fat Mass and osteoporosis in individuals with type 2 diabetes independent of lean Mass. Diabetes Metab Syndr Obes. 2022;15:1321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Y, Wade H, Zhang B, Xu W, Wu R, Li S, Su Q. Polymorphisms of Fat Mass and obesity-Associated Gene in the Pathogenesis of child and adolescent metabolic syndrome. Nutrients. 2023;15(12):2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agbaje AO. Associations of accelerometer-based sedentary time, light physical activity and moderate-to-vigorous physical activity with resting cardiac structure and function in adolescents according to sex, fat mass, lean mass, BMI, and hypertensive status. Scand J Med Sci Sports. 2023;33(8):1399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JC, Carson TL, Thompson HJ, Agurs-Collins T. The Triple Health Threat of Diabetes, obesity, and Cancer-Epidemiology, disparities, mechanisms, and interventions. Obes (Silver Spring). 2021;29(6):954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thumann BF, Börnhorst C, Ahrens W, Arvidsson L, Gwozdz W, Iguacel I, Mårild S, Molnár D, Rach S, Russo P, et al. Cross-sectional and longitudinal associations between Psychosocial Well-being and cardiometabolic markers in European children and adolescents. Psychosom Med. 2020;82(8):764–73. [DOI] [PubMed] [Google Scholar]
- 42.Choi YJ, Lee YA, Hong YC, Cho J, Lee KS, Shin CH, Kim BN, Kim JI, Park SJ, Bisgaard H, et al. Effect of prenatal bisphenol A exposure on early childhood body mass index through epigenetic influence on the insulin-like growth factor 2 receptor (IGF2R) gene. Environ Int. 2020;143:105929. [DOI] [PubMed] [Google Scholar]
- 43.Viitasalo A, Schnurr TM, Pitkänen N, Hollensted M, Nielsen TRH, Pahkala K, Lintu N, Lind MV, Atalay M, Frithioff-Bøjsøe C, et al. Genetic predisposition to higher body fat yet lower cardiometabolic risk in children and adolescents. Int J Obes (Lond). 2019;43(10):2007–16. [DOI] [PubMed] [Google Scholar]
- 44.Mastorakos G, Valsamakis G, Paltoglou G, Creatsas G. Management of obesity in menopause: diet, exercise, pharmacotherapy and bariatric surgery. Maturitas. 2010;65(3):219–24. [DOI] [PubMed] [Google Scholar]
- 45.Velez LM, Van C, Moore T, Zhou Z, Johnson C, Hevener AL, Seldin MM. Genetic variation of putative myokine signaling is dominated by biological sex and sex hormones. Elife. 2022;11:e76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández-Alfonso MS, Somoza B, Tsvetkov D, Kuczmanski A, Dashwood M, Gil-Ortega M. Role of Perivascular Adipose Tissue in Health and Disease. Compr Physiol. 2017;8(1):23–59. [DOI] [PubMed] [Google Scholar]
- 47.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bendinelli B, Pastore E, Fontana M, Ermini I, Assedi M, Facchini L, Querci A, Caini S, Masala G. A priori dietary patterns, physical activity level, and body composition in Postmenopausal women: a cross-sectional study. Int J Environ Res Public Health. 2022;19(11):6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qahwaji DM. Impact of Dietary Intake and physical activity on metabolic syndrome in Saudi adults: an exploratory pilot study. Prev Nutr Food Sci. 2022;27(1):45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized for the analyses are presented within the paper, and upon reasonable request, the underlying data for this article will be made available by contacting the corresponding author.