Abstract
Background
The potential therapeutic role of magnesium (Mg) in type 2 diabetes mellitus (T2DM) remains insufficiently studied despite its known involvement in critical processes like lipid metabolism and insulin sensitivity. This study examines the impact of Mg-focused nutritional education on lipid profile parameters, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) in T2DM patients.
Methods
Thirty participants with T2DM were recruited for this within-subject experimental study. Participants underwent a three-month dietary intervention focused on increasing the intake of Mg-rich foods through nutritional education. Anthropometric measurements and lipids were assessed at baseline and after the intervention period, with the primary outcome variables including changes in lipid parameters.
Results
The findings showed a significant inverse association between dietary Mg intake and total cholesterol levels (r = − 0.36, p = 0.05). However, other parameters, TG, LDL-C, and HDL-C, were not found to be associated with Mg intake.
Conclusion
The study demonstrated an inverse association between Mg intake and cholesterol level. Providing nutritional education and guidance on incorporating Mg-rich foods into the diet may be a crucial strategy for improving the health and well-being of T2DM patients in Saudi Arabia. The feasibility and practicality of focused nutritional education as an intervention make it a low-cost, scalable, and sustainable approach that can be readily implemented in clinical and community settings. Further studies are needed to explore the long-term impact of dietary Mg interventions on a larger sample with longer education periods.
Keywords: Dietary magnesium intake, Lipid profile, Cholesterol, Magnesium food frequency questionnaire, Nutritional education
Background
Type 2 diabetes mellitus (T2DM) is a long-term condition characterized by inadequate insulin production, leading to elevated blood sugar levels and an increased risk of cardiovascular issues, particularly due to dyslipidemia and an atherogenic lipid profile [1]. Dyslipidemia is highly prevalent in T2DM: more than one-third of diabetic patients have atherogenic dyslipidemia [2]. This contributes to the development of cardiovascular diseases, the leading cause of mortality in diabetic patients [3].
In T2DM management, various nutrients have been recognized for their crucial significance, with magnesium (Mg) emerging as one of the key elements of interest [4]. Mg is involved in numerous enzymatic processes that are critical for proper glucose and lipid metabolism [5]. It plays a pivotal role in activating enzymes responsible for glucose transport and insulin receptor signaling, thereby promoting insulin sensitivity [6]. It also influences processes such as carbohydrate oxidation, insulin secretion, and insulin binding. Mg deficiency has been linked to insulin resistance, a hallmark of T2DM [6]. Furthermore, Mg influences lipid metabolism by regulating the activity of key enzymes, such as lipoprotein lipase, which impacts triglyceride levels, and lecithin-cholesterol acyltransferase, which affects cholesterol metabolism [7]. These processes make Mg an essential nutrient for mitigating both hyperglycemia and dyslipidemia in T2DM [7].
A systematic review of 41 studies involving diverse populations across different age groups revealed that individuals with the highest Mg consumption had a 22% reduced risk of developing T2DM compared with those with the lowest intake [8]. In addition, increasing Mg intake was associated with a 19% lower risk for every additional 100 mg consumed per day [9]. Studies have demonstrated that Mg deficiency is associated with insulin resistance [10] and atherogenic dyslipidemia, including increased triglycerides (TG) and decreased levels of high-density lipoprotein cholesterol (HDL-C) levels [11]. Another systematic review of 12 clinical trials specifically examined the association between Mg and dyslipidemia-related markers and found that Mg levels were positively correlated with HDL-C and inversely associated with TG in diabetic populations [12]. Jin and Nicodemus-Johnson analyzed data from 12,284 individuals involved in the National Health and Nutrition Examination Study (NHANES) from 2001 to 2013. Their results showed that in women, Mg intake positively correlated with HDL-C levels while being inversely related to the TC/HDL-C ratio. Furthermore, in both males and females, Mg intake exhibited a negative correlation with TG levels [13]. Other cross-sectional studies have explored the association between lipid profile parameters and dietary Mg intake, revealing a positive relationship [14]. These findings suggest that adequate Mg intake may be beneficial in preventing and managing lipid abnormalities and controlling T2DM.
Therapeutic Mg supplementation has been explored as a potential intervention in the management of T2DM. While some studies have reported no significant improvement in diabetic patients with Mg supplementation, multiple meta-analyses involving large sample sizes have shown promising outcomes, including lipid profile modification. One systematic review and meta-analysis revealed that Mg supplements did not significantly affect the plasma concentration of any lipid, including cholesterol [15]. However, meta-analyses pooling data from over 1500 participants have indicated that long-term (over 12 months) Mg supplementation significantly decreased TC levels and that supplementation with over 300 mg of Mg raised HDL-C levels significantly among T2DM patients [16]. These findings underline Mg’s therapeutic potential for managing lipid abnormalities in T2DM.
Nutritional education plays a critical role by empowering individuals with the knowledge and skills necessary to make sustainable dietary changes. Such programs are particularly effective in promoting adherence to dietary recommendations by enhancing individuals’ understanding of the health benefits associated with nutrient-rich foods. Studies have demonstrated that structured educational programs significantly improve dietary behavior and health outcomes in chronic conditions, including T2DM, by fostering goal setting, consistent follow-up, and personalized feedback [17]. Providing nutritional education for at least three months is a proven strategy to modify dietary behavior and improve health outcomes in T2DM patients [17]. Incorporating Mg-rich foods, such as leafy greens, nuts, and seeds, into the diet has the potential to improve lipid profiles and, by extension, cardiovascular health [18]. However, there is limited research on how educational interventions targeting Mg intake through the diet, rather than supplementation, specifically affect the lipid profile in people with T2DM. The role Mg plays in lipid metabolism and the management of T2DM is well-documented, with numerous studies highlighting its benefits in improving health outcomes for diabetic patients [13–16]. However, further research is necessary to clarify the direct effects of dietary Mg on the management of complications associated with T2DM. This study seeks to address this gap.
Recently, Saudi Arabia was ranked seventh highest in T2DM prevalence globally and the second highest in the Middle East [19]. Studies also indicate that Mg intake in the Saudi population is below recommended levels, further exacerbating the risk of metabolic disorders such as T2DM [20]. This underscores the urgent need for this research in this population.
This study aims to evaluate the impact of Mg-focused nutritional education on Mg intake, through the diet rather than supplementation, and lipids, including TC, TG, low-density lipoprotein cholesterol (LDL-C), and HDL-C, in patients with T2DM. The findings will not only contribute to the scientific understanding of Mg’s role in lipid metabolism but also offer practical strategies for improving T2DM management in populations with low Mg intake through the diet. By specifically targeting dietary behaviors in a high-risk population, the study introduces a novel and practical approach to improving health outcomes. Changes in dietary intake may support sustainable, long-term behavior modifications in T2DM patients better than supplementation can. The findings aim to provide valuable insights into managing T2DM complications, particularly dyslipidemia, within the context of Saudi Arabia and similar populations. Furthermore, this study, to our knowledge, is the first to address this issue in the Saudi population.
Methods
Experimental design
This study followed a quasi-experimental (within-subject) design to evaluate the effect of targeted educational intervention on the lipid profile. The primary outcome was changes in lipid parameters. The study involved two clinical visits spaced 3 months (12 weeks) apart. This approach was deemed the most feasible type for education-based interventions that often depend on patients' voluntary participation and compliance. Approval was granted by the Research and Ethics Committee at King Abdulaziz University Hospital (KAUH) (Reference no.: 361-21). Individuals, men and women aged 18 years and older, with a body mass index (BMI) of 18.5 kg/m2 or above and T2DM were enrolled in the study.
The endocrinologist at the outpatient clinics recruited eligible participants through convenience sampling. Potential study participants were interviewed face-to-face to gauge their interest. To assess potential eligibility, the potential participants were referred to the dietitian to complete checking their inclusion and exclusion criteria at the nutritional clinic. Exclusion criteria included a history of bariatric surgery or medical conditions that could interfere with study results, such as gastrointestinal disorders with malabsorption, renal diseases, and severe anemia. Additionally, women who were pregnant or had been lactating within the past 12 months were not eligible, as these conditions impact dietary intake, nutrient needs, and metabolic states, leading to variability in Mg intake [21]. Participants were also excluded if they reported taking medications such as antibiotics, diuretics, heartburn medications, laxatives in excess, or any supplements such as Mg, calcium, or fiber.
Following the informed consent process, demographic factors associated with intake, including educational level and marital status, were recorded as part of baseline data collection. In addition, medical history, onset of T2DM, and anthropometric measurements were collected. Lipid profiles were biochemically assessed, and a dietary evaluation was conducted. Nutrition counseling by a dietitian was provided to these participants. Each study visit lasted approximately 30 to 45 min.
Study procedure and measures
Participants had two visits with the dietitian, at the baseline visit (week 0) and the endpoint visit (week 12)—before and after the Mg-focused nutritional education—to complete the following study measures. BMI was determined based on the participant’s weight and height [22].
Dietary assessment
In this study, the term “Mg intake” refers exclusively to Mg intake through the diet without supplementation, as assessed through the Mg Food Frequency Questionnaire (MgFFQ). The MgFFQ was validated semiquantitatively against a 14-day food diary and found that the MgFFQ is a valid tool to assess long-term Mg intake [23]. The questionnaire asked participants about their consumption of certain foods and beverages and at what amount and frequency. Foods on the questionnaire included dark leafy greens, nuts and seeds, different types of fish, grains, and dairy products, among others. The MgFFQ was administered at baseline and re-administered in the follow-up (endpoint) visit. Caloric needs were calculated for the participants during the first visit using the Saudi Ministry of Health calorie calculator tool. Each participant was given a personalized meal plan designed to provide 420 mg of Mg for males and 320 mg for females. Participants were then informed about foods rich in Mg and instructed to follow lists of Mg-rich sources throughout the three-month study period. Magnesium intake was assessed during the study duration through 24-h dietary recall. Three 24-h recall sheets were collected and analyzed using the Automated Self-Administered Dietary Assessment (ASA24) tool at the beginning and a few days prior to the end of the interventional period, covering 3 weekday recalls. The ASA24 is a widely accessible dietary assessment tool, offering high feasibility for large-scale nutritional research [24]. To ensure compliance and engagement, participants received material and reminders about Mg-rich food sources every three weeks through WhatsApp messages throughout the period of the intervention. These follow-up messages served as regular reminders aimed at encouraging patients to incorporate Mg-rich foods into their diet and adhere to the recommendations for intervention. Participants were also invited to ask any questions they might have for clarification.
Blood sampling and analysis
Fasting blood samples were drawn from all participants by trained staff at KAUH immediately following the anthropometric and dietary assessments at both visits. The samples were collected in the morning following a 12-h overnight fast, which participants were instructed to do—to measure TC, LDL, HDL, and TG. Sample storage and analysis followed standard laboratory staff procedures at KAUH. Blood serum for lipid profile was collected using EDTA tubes. Samples were refrigerated at − 5 °C for a maximum of 7 days. All sample analyses were done by Atellica® Solution Auto analyzers (Siemens Healthineers).
Statistical analysis
The analysis was accomplished using IBM SPSS Statistics, Version 26.0 (Armonk, NY, USA: IBM Corp). Assumptions for statistical tests, including normality, were evaluated prior to analysis. In case of assumption violations, including baseline data, nonparametric tests were performed. The Wilcoxon signed-rank test was conducted to assess variations in Mg intake, as evaluated by the Mg food frequency questionnaire (MgFFQ), alongside lipid parameters including LDL-C, TC, HDL-C, and TG. Additionally, Pearson correlation analysis was employed to explore the strength and direction of the relationship between Mg intake and the lipid profile following the Mg-focused nutritional education. All tests were two tailed, with statistical significance set at P ≤ 0.05, representing the probability of observing the results by chance under the null hypothesis.
The aim was to recruit 85 participants based on power calculations (power of 0.80, with an effect size of 0.5, significance level of 0.05, two tailed) in order to detect a significant effect. However, due to challenges with participant recruitment, the resulting sample size was below the intended target. Factors that affected participation, as indicated by the patients, were logistical challenges such as transportation and scheduling. Other potential factors might include the targeted population's unfamiliarity with research practices or their cultural sensitivities and limited health literacy.
Results
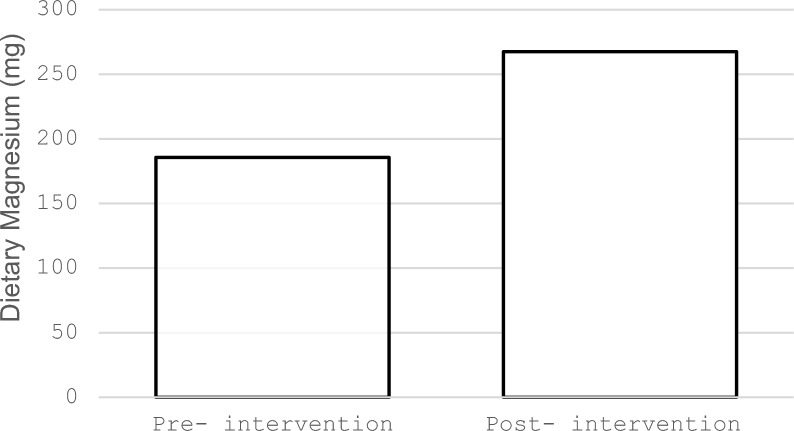
The study included 30 participants; 83% were female and 17% were male. The sociodemographic characteristics of the sample, including age, weight, BMI, sex, education, and marital status (see Table 1 for sociodemographic and macronutrient data). Participants’ mean age was 55.7 ± 9.8 years, the mean weight was 81.2 ± 15.89 kg, and the mean BMI was 33.44 ± 7.17 kg/m2. The sample was evenly divided between those with secondary education or less (50%, n = 15) and those with a university degree or higher (bachelor, 43.3%, n = 13; postgraduate degree, 6.6%, n = 2). Most of the participants were married (n = 29, 96.6%). Macronutrient intake values are presented in Table 1, expressed as mean ± standard deviation (SD) based on dietary recall data. Baseline energy intake was 1418.52 ± 557.24 kcal; carbohydrate intake was 159.67 ± 64.33 g; and fat intake was 58.11 ± 33.09 g. Table 2 shows participants' Mg intake and lipid profiles before and after the Mg-focused nutritional education intervention. The intake of Mg, as indicated by the MgFFQ, showed a statistically significant increase after the intervention (P < 0.001), approaching the recommended dietary guidelines (see Fig. 1). Triglycerides, TC, and LDL-C showed statistically significant reduction after the Mg-focused nutritional education (P < 0.001, P = 0.011, P = 0.027, respectively). HDL-C showed no statistically significant differences (P = 0.06).
Table 1.
Participants’ sociodemographic characteristics and macronutrient intake, N = 30
| Characteristic | n (%) |
|---|---|
| Age (years), mean ± SD | 55.7 ± 9.8 |
| Weight (kg), mean ± SD | 81.2 ± 15.89 |
| BMI (kg/m2), mean ± SD | 33.44 ± 7.17 |
| Sex | |
| Female | 25 (83.3) |
| Male | 5 (16.6) |
| Education | |
| Elementary & secondary | 15 (50) |
| Bachelor | 13 (43.3) |
| Postgraduate | 2 (6.6) |
| Marital status | |
| Single | 1 (3.3) |
| Married | 29 (96.6) |
| Macronutrient intake | |
| Energy (Kcal) | 1418.52 ± 557.24 |
| Carbohydrates (g) | 159.67 ± 64.33 |
| Fat (g) | 58.11 ± 33.09 |
BMI body mass index, Kcal Kilocalories, g grams
Table 2.
Magnesium intake and lipid profiles before and after the Mg-focused nutritional education intervention
| Variable | Pre-intervention (Mean ± SD) |
Post-intervention (Mean ± SD) |
P value |
|---|---|---|---|
| Magnesium intake as per the MgFFQ (mg) | 185.64 ± 150.98 | 267.45 ± 136.90 | < 0.001* |
| Triglycerides (mmol/L) | 1.55 ± 0.35 | 1.40 ± 0.48 | 0.001* |
| Total cholesterol (mmol/L) | 4.62 ± 1.06 | 4.35 ± 1.13 | 0.011* |
| Low density lipoprotein cholesterol(mmol/L) | 2.65 ± 1.12 | 2.59 ± 1.16 | 0.027* |
| High density lipoprotein cholesterol (mmol/L) | 1.35 ± 0.30 | 1.36 ± 0.29 | 0.066 |
SD standard deviation, MgFFQ Magnesium Food Frequency Questionnaire, mg milligrams, mmol/L millimoles per liter
*Statistically significant differences at P level of 0.05
Fig. 1.
Mean Mg intake before and after the intervention, as measured by the Mg Food Frequency Questionnaire, showing significant improvement
Table 3 and Fig. 2 illustrate the association between Mg intake and the lipid profile following the intervention. Pearson correlation revealed a significant moderate negative association between blood cholesterol level and Mg intake after the intervention (r = − 0.36, P = 0.05). However, no significant association was found between Mg intake and the other lipid parameters, LDL-C, HDL-C, and TG (r = − 0.18, P = 0.33; r = − 0.08, P = 0.66; r = − 0.09, P = 0.64, respectively).
Table 3.
Correlation between Mg intake and lipid profile parameters after the Mg-focused nutritional education intervention (N = 30)
| TC r (P value) |
LDL-C r (P value) |
HDL-C r (P value) |
TG r (P value) |
|
|---|---|---|---|---|
| Magnesium intake | − 0.36* (0.05) | − 0.18 (0.33) | − 0.08 (0.66) | − 0.09 (0.64) |
Analysis was performed using Pearson correlation. TC = total cholesterol; LDL-C = low-density lipoprotein cholesterol; HDL-C = high-density lipoprotein cholesterol; TG = triglycerides
*Significant at the 0.05 level (2-tailed), statistically significant correlation between Mg intake and TC level
Fig. 2.
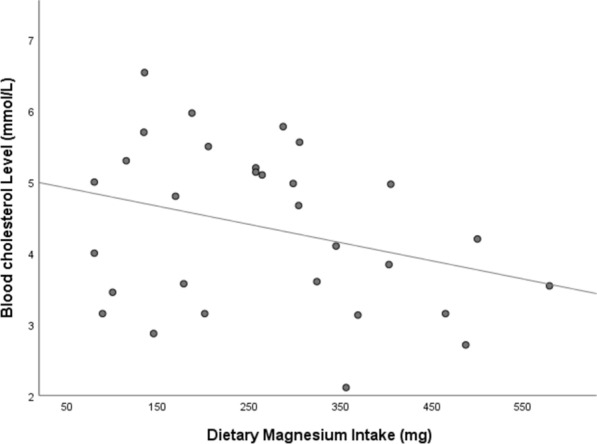
Inverse association between Mg intake and blood cholesterol level after the intervention
Discussion
This was the first study to examine the potential impact of a three-month Mg-focused nutritional education as a practical intervention on participants’ lipid profiles in Saudi Arabia [19]. Our research indicates that higher Mg intake correlates with better TC levels in patients with T2DM. However, TG, LDL, and HDL levels exhibited no significant changes. The findings highlight the critical role of Mg in lipid metabolism for this high-risk group. Considering the high prevalence of T2DM in Saudi Arabia, focusing on educating patients about Mg-rich foods provides a culturally tailored approach to addressing potential nutritional deficiencies. This strategy aligns with global efforts to integrate sustainable dietary modifications into diabetes management, emphasizing the broader relevance of such interventions for similar populations.
Our findings are consistent with previous work showing that low Mg intake is linked to high TC levels [25]. Numerous studies have suggested that serum Mg is linked to the lipid profile; specifically, patients with elevated levels of TC, TG, and LDL and reduced levels of HDL often exhibit lower Mg concentrations [16, 26, 27]. A systematic review of 12 randomized controlled trials demonstrated that Mg supplementation significantly reduced serum LDL levels (P = 0.006) but resulted in no significant improvement in TC, TG, or HDL levels among T2DM patients compared with controls. However, subgroup analysis showed that after receiving over 12 weeks of Mg supplementation, participants exhibited significantly lower serum TC levels. Additionally, supplementation doses below 300 mg significantly reduced serum LDL levels (P = 0.001). Doses over 300 mg were associated with increased serum HDL levels (P = 0.026), indicating that the effect of Mg treatment might be influenced by dose and duration [16]. Furthermore, a systematic review by Rodrigues et al. found that low serum Mg levels were linked to higher TC, TG, and LDL levels, as well as lower HDL levels in patients with diabetes [26].
According to Xu et al., the presence of T2DM appears to modify the association between the lipid profile and serum Mg, especially for LDL-C [28]. A study involving 8,163 Chinese adults, exploring the complex interplay between serum Mg, blood lipids, and T2DM found that blood lipid levels, excluding HDL-C, were generally correlated with serum Mg levels, with T2DM significantly influencing this relationship, particularly concerning LDL-C. Additionally, a recent study in American adults found that Mg-rich foods may prevent hyperlipidemia and diabetes [14].
It is difficult to reach a definite understanding of the nature of the association between Mg and lipid parameters due to the heterogeneity of the studies and the need to account for factors such as age, sex, BMI, glucose levels, and kidney function. Magnesium plays a crucial role in various cellular functions, influencing numerous aspects of the endocrine system. It also has a regulatory role on the activity of several enzymes involved in lipid metabolism, including lecithin-cholesterol acyl transferase, lipoprotein lipase, and desaturase. When individuals experience Mg deficiency, the activity of these enzymes is suppressed, resulting in undesirable changes in TG, LDL, HDL, and very low-density lipoprotein (VLDL) levels [29]. Low Mg levels may also increase the activity of HMG-CoA reductase, an enzyme responsible for cholesterol synthesis, resulting in hypercholesterolemia [7, 29]. A study emphasizing Mg’s potential role in influencing the connection between lipid profiles and cardiovascular risk revealed that Mg levels can impact the relationship between lipid parameters and a marker of atherosclerosis, specifically, the carotid intima media thickness (cIMT). When Mg levels were low, higher TG and LDL levels were linked to increased cIMT, and this association weakened when Mg levels were higher. These findings suggest that Mg may have a protective effect against the detrimental impact of dyslipidemia on cardiovascular health. Therefore, sufficient Mg levels may help mitigate the adverse effects of elevated TG and LDL on the development of atherosclerosis, as measured by cIMT [30].
Our study revealed that HDL-C was less responsive to the intervention. Indeed, raising HDL-C through dietary interventions is often challenging, because HDL-C levels are influenced by complex metabolic pathways [31]. Additionally, dietary limitations or individual variations in how the body responds to dietary components might have influenced the HDL-C response. For example, the specific types of fats and carbohydrates consumed can significantly impact HDL-C levels. Diets high in refined carbohydrates may counteract the benefits of healthy fats [32]. In addition, individual genetic predispositions can affect HDL-C metabolism, making it less responsive to dietary changes [31].
On the other hand, the observed changes in TG, TC, and LDL-C suggest a favorable shift in the lipid profile and have important clinical implications for cardiovascular risk in patients with T2DM. These alterations are associated with a decreased risk of atherosclerotic cardiovascular disease, which is prevalent in T2DM due to dyslipidemia and insulin resistance [28]. Research indicates that dietary Mg derived from whole food sources demonstrates superior health outcomes compared with supplemental forms, particularly in cardiovascular health management and hypertension control [33]. The advantages of food-based Mg are several, including enhanced bioavailability and natural nutrient synergy with compounds such as fiber and antioxidants, which are absent in supplemental forms [33]. Studies have shown that regular consumption of Mg-rich foods promotes sustainable long-term health benefits while minimizing the risk of excessive intake that can occur with supplementation [34].
Certainly, a significant contributor to dyslipidemia is an unhealthy diet, especially one rich in sugar, fat, and calories while lacking in vegetables, fruits, and whole grains [35]. Fruits, whole grains, and vegetables are excellent dietary sources of Mg. Therefore, it is plausible to hypothesize that the Mg content in the diet may be a potential risk factor for the development of dyslipidemia. However, more research is needed to establish a clear causal relationship between the amount of Mg consumed in the diet and lipid profile disturbances.
Additionally, it is crucial to recognize that much of the evidence linking Mg to serum lipid profiles comes from clinical studies involving oral Mg supplements, and that there is limited direct evidence on the efficacy of a Mg-focused diet in improving Mg intake. We conclude from our study that a Mg-focused educational program is effective as a standalone intervention. A systematic review found that structured nutrition-focused programs significantly improved dietary habits, including increased intake of essential nutrients, among specific populations. These programs can lead to measurable improvements in nutrient consumption, including Mg-rich foods [36].
There are several important limitations to consider. Firstly, the study's relatively small sample size and the female predominance may impact the generalizability of the findings. Secondly, the reliance on the MgFFQ and self-reported adherence for data on Mg intake may introduce limitations regarding data reliability. Recall could introduce bias and inaccuracies, which may impact the perceived effectiveness of the intervention. Future studies should focus on tackling factors hindering participant recruitment to increase sample size and gender diversity. In addition, repeated administration of the MgFFQ should be considered to enhance data accuracy and provide a clearer picture of adherence. Several strengths deserve attention, including the intervention's low cost and educational aspect, which significantly enhance its feasibility for wider implementation, especially in resource-constrained environments. Focusing on dietary intake through tailored education offers a sustainable and accessible solution for improving the intake of specific nutrients and, thus, nutritional habits in T2DM. Also, the study used the MgFFQ, which is a valid tool for measuring Mg intake, and followed up with patients every three weeks, which can help improve their adherence to consuming Mg-rich foods. The participants recruited in our sample were similar to individuals who may benefit from the study’s results. Furthermore, this study included a low-cost intervention involving nutritional education to enhance dietary Mg. It also incorporated repeated laboratory assessments, before and after the intervention, as an integral part of the within-subject design.
Conclusion
The provision of Mg-focused nutritional education for a duration of three months showed a positive impact on the lipid profile, particularly TC level, among diabetic patients. This study highlights nutritional education about Mg as a feasible and cost-effective strategy to improve the health of diabetic patients. Future studies are needed to validate our promising findings on a larger scale.
In regions with high diabetes prevalence, more research is needed to evaluate the long-term impact of a Mg-rich diet on lipid metabolism and cardiovascular health, investigate the impacts of Mg intake on glucose control, and compare the efficacy of dietary Mg intake with supplementary sources for a better understanding of their respective benefits. Additional studies should also explore the effect of varying doses of Mg to better understand the sustainability and broader implications of Mg-based dietary interventions.
Abbreviations
- Mg
Magnesium
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
- TG
Triglycerides
- LDL-C
Low-density lipoprotein-cholesterol
- HDL-C
High-density lipoprotein-cholesterol
- NHANES
National Health and Nutrition Examination Study
- KAUH
King Abdulaziz University Hospital
- MgFFQ
Magnesium Food Frequency Questionnaire
- ASA24
Automated Self-Administered Dietary Assessment
- BMI
Body mass index
- HMG-CoA
β-Hydroxy β-methylglutaryl-CoA
- cIMT
Carotid intima media thickness
Author contributions
Conceptualization: M.N. and E.A.; Data curation: M.N., E.A and H.M.; Formal analysis: E.A, M.N.; Investigation: M.N.; Methodology: M.N. and E.A.; Project administration: M.N. and E.A.; Resources: M.N.; Software: E.A.; Writing—original draft: M.N., E.A., A.A., N.H., & R.B; Writing—review & editing: M.N. E.A and H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no.: GPIP:1771–290-2024. The authors, therefore, acknowledge with thanks DSR for the technical and financial support.
Data availability
The data sets used and analyzed for the current study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study adhered to the Helsinki Declaration and was approved by the Research and Ethics Committee at King Abdulaziz University Hospital (Reference No.: 361–21). All patients gave written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banach M, Surma S, Reiner Z, Katsiki N, Penson PE, Fras Z, et al. Personalized management of dyslipidemias in patients with diabetes-it is time for a new approach. Cardiovasc Diabetol. 2022;21(1):263. 10.1186/s12933-022-01684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246–58. 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minari TP, Tácito LHB, Yugar LBT, Ferreira-Melo SE, Manzano CF, Pires AC, et al. Nutritional strategies for the management of type 2 diabetes mellitus: a narrative review. Nutrients. 2023;15(24):5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbagallo M, Dominguez L. Magnesium and aging. Curr Pharm Des. 2010;16(7):832–9. [DOI] [PubMed] [Google Scholar]
- 6.Kostov K. Effects of magnesium deficiency on mechanisms of insulin resistance in type 2 diabetes: focusing on the processes of insulin secretion and signaling. Int J Mol Sci. 2019;20(6):1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue I. Lipid metabolism and magnesium. Clin Calcium. 2005;15(11):65–76. [PubMed] [Google Scholar]
- 8.Zhao B, Zeng L, Zhao J, Wu Q, Dong Y, Zou F, et al. Association of magnesium intake with type 2 diabetes and total stroke: an updated systematic review and meta-analysis [published correction appears in BMJ Open. 2020 Apr 16;10(4):e032240corr1. 10.1136/bmjopen-2019-032240corr1]. BMJ Open. 2020;10(3): e032240. 10.1136/bmjopen-2019-032240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruby A, Guasch-Ferré M, Bhupathiraju SN, Manson JAE, Willett WC, McKeown NM, et al. Magnesium intake, quality of carbohydrates, and risk of type 2 diabetes: results from three US cohorts. Diabetes Care. 2017;40(12):1695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005;28(5):1175–81. [DOI] [PubMed] [Google Scholar]
- 11.Liu A, Xu P, Gong C, Zhu Y, Zhang H, Nie W, et al. High serum concentration of selenium, but not calcium, cobalt, copper, iron, and magnesium, increased the risk of both hyperglycemia and dyslipidemia in adults: a health examination center based cross-sectional study. J Trace Elem Med Biol. 2020;59: 126470. [DOI] [PubMed] [Google Scholar]
- 12.Găman MA, Dobrică EC, Cozma MA, Antonie NI, Stănescu AMA, Găman AM, et al. Crosstalk of magnesium and serum lipids in dyslipidemia and associated disorders: a systematic review. Nutrients. 2021;13(5):1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin H, Nicodemus-Johnson J. Gender and age stratified analyses of nutrient and dietary pattern associations with circulating lipid levels identify novel gender and age-specific correlations. Nutrients. 2018;10(11):1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han M, Zhang Y, Fang J, Sun M, Liu Q, Ma Z, et al. Associations between dietary magnesium intake and hypertension, diabetes, and hyperlipidemia. Hypertension Res. 2024;47(2):331–41. [DOI] [PubMed] [Google Scholar]
- 15.Simental-Mendía LE, Simental-Mendía M, Sahebkar A, Rodríguez-Morán M, Guerrero-Romero F. Effect of magnesium supplementation on lipid profile: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2017;73(5):525–36. [DOI] [PubMed] [Google Scholar]
- 16.Asbaghi O, Moradi S, Nezamoleslami S, Moosavian SP, Hojjati Kermani MA, Lazaridi AV, et al. The effects of magnesium supplementation on lipid profile among type 2 diabetes patients: a systematic review and meta-analysis of randomized controlled trials. Biol Trace Elem Res. 2021;199(3):861–73. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Hur MH. The effects of dietary education interventions on individuals with type 2 diabetes: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(16):8439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation. 2013;128(5):553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA, et al. Diabetes mellitus in Saudi Arabia: a review of the recent literature. Curr Diabetes Rev. 2016;12(4):359–68. [DOI] [PubMed] [Google Scholar]
- 20.Abualrahi AM, Alhanabi FH, Alalloush RS, Alsalman ZH, Albaker WI, AlSheikh MH, et al. Assessment of dietary magnesium intake in the Eastern Province of Saudi Arabia. J Med Life. 2023;16(12):1789–95. 10.25122/jml-2023-0279.PMID:38585527;PMCID:PMC10994627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouanne M, Oddoux S, Noël A, Voisin-Chiret AS. Nutrient requirements during pregnancy and lactation. Nutrients. 2021;13(2):692. 10.3390/nu13020692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16(3):177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukumar D, DeLuccia R, Cheung M, Ramadoss R, Ng T, Lamoureux A. Validation of a newly developed food frequency questionnaire to assess dietary intakes of magnesium. Nutrients. 2019;11(11):2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subar AF, Potischman N, Dodd KW, Thompson FE, Baer DJ, Schoeller DA, et al. Performance and feasibility of recalls completed using the automated self-administered 24-hour dietary assessment tool in relation to other self-report tools and biomarkers in the interactive diet and activity tracking in AARP (IDATA) study. J Acad Nutr Diet. 2020;120(11):1805–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bain LKM, Myint PK, Jennings A, Lentjes MAH, Luben RN, Khaw KT, et al. The relationship between dietary magnesium intake, stroke and its major risk factors, blood pressure and cholesterol, in the EPIC-Norfolk cohort. Int J Cardiol. 2015;196:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues AK, Melo AE, Domingueti CP. Association between reduced serum levels of magnesium and the presence of poor glycemic control and complications in type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(2):127–34. [DOI] [PubMed] [Google Scholar]
- 27.Rasheed H, Elahi S, Ajaz H. Serum magnesium and atherogenic lipid fractions in type II diabetic patients of Lahore, Pakistan. Biol Trace Elem Res. 2012;148(2):165–9. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Xu W, Yao H, Sun W, Zhou Q, Cai L. Associations of serum and urinary magnesium with the pre-diabetes, diabetes and diabetic complications in the Chinese Northeast population. PLoS ONE. 2013;8(2): e56750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelczyńska M, Moszak M, Bogdański P. The role of magnesium in the pathogenesis of metabolic disorders. Nutrients. 2022;14(9):1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cambray S, Ibarz M, Bermudez-Lopez M, Marti-Antonio M, Bozic M, Fernandez E, et al. Magnesium levels modify the effect of lipid parameters on carotid intima media thickness. Nutrients. 2020;12(9):2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannon BA, Edwards CG, Thompson SV, Burke SK, Burd NA, Holscher HD, et al. Genetic variants in lipid metabolism pathways interact with diet to influence blood lipid concentrations in adults with overweight and obesity. Lifestyle Genom. 2020;13(6):155–63. 10.1159/000507021. [DOI] [PubMed] [Google Scholar]
- 32.Yanai H, Tada N. Effects of consumption of various fatty acids on serum HDL-cholesterol levels. J Endocrinol Metab. 2018;8(5):94–9. 10.14740/JEM.V8I5.534. [Google Scholar]
- 33.Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome, and other conditions: a review. Nutr Clin Pract. 2008;23(2):142–51. 10.1177/0884533608314533. [DOI] [PubMed] [Google Scholar]
- 34.Leenders NHJ, Vervloet MG. Magnesium: a magic bullet for cardiovascular disease in chronic kidney disease? Nutrients. 2019;11(2):455. 10.3390/nu11020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gommers LMM, Hoenderop JGJ, Bindels RJM, De Baaij JHF. Hypomagnesemia in type 2 diabetes: a vicious circle? Diabetes. 2016;65(1):3–13. [DOI] [PubMed] [Google Scholar]
- 36.Boidin A, Tam R, Mitchell L, Cox GR, O’Connor H. The effectiveness of nutrition education programmes on improving dietary intake in athletes: a systematic review. Br J Nutr. 2021;125(12):1359–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and analyzed for the current study are available from the corresponding author upon reasonable request.