Abstract
STUDY QUESTION
To what extent can hypertensive disorders in pregnancy (HDP) explain the higher risk of preterm birth following frozen embryo transfer (frozen-ET) and fresh embryo transfer (fresh-ET) in ART compared with naturally conceived pregnancies?
SUMMARY ANSWER
HDP did not contribute to the higher risk of preterm birth in pregnancies after fresh-ET but mediated 20.7% of the association between frozen-ET and preterm birth.
WHAT IS KNOWN ALREADY
Risk of preterm birth is higher after ART compared to natural conception. However, there is also a higher risk of HDP in pregnancies after ART compared to natural conception, in particular after frozen-ET. HDP increases the risk of both spontaneous and medically indicated preterm birth. It is not known to what extent the higher risk of preterm birth in ART-conceived pregnancies is mediated through HDP.
STUDY DESIGN, SIZE, DURATION
This registry-based cohort study included singleton pregnancies from the Committee of Nordic ART and Safety (CoNARTaS) cohort from Denmark (1994–2014), Norway (1988–2015), and Sweden (1988–2015). The analysis included 78 300 singletons born after fresh-ET, 18 037 after frozen-ET, and 4 426 682 after natural conception. The exposure was ART conception with either frozen-ET or fresh-ET versus natural conception. The main mediator of interest was any of the following HDP: gestational hypertension, preeclampsia, eclampsia, or chronic hypertension with superimposed preeclampsia. The main outcome was any preterm birth, defined as delivery <37 weeks of gestation. Secondary outcomes were spontaneous and medically indicated preterm birth, and different severities of preterm birth based on the gestational age threshold.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We linked data from the national Medical Birth Registries, ART registries/databases, and the National Patient Registries in each country using the unique national identity number of the mother. Criteria for inclusion were singleton pregnancies with birth order 1–4 in women aged ≥20 years at delivery. We used logistic regression to estimate odds ratios (ORs) with 95% CIs of preterm birth and decomposed the total effect into direct and mediated (indirect) effects to estimate the proportion mediated by HDP. Main models included adjustment for the year of delivery, maternal age, parity, and country.
MAIN RESULTS AND THE ROLE OF CHANCE
Pregnancies following frozen-ET had a higher risk of any preterm birth compared to natural conception (occurrence 6.6% vs 5.0%, total effect OR 1.29, 95% CI 1.21–1.37) and 20.7% of the association was mediated by HDP (mediated effect OR 1.05, 95% CI 1.04–1.05). The mediation occurred primarily in medically indicated preterm births. Pregnancies following fresh-ET also had a higher risk of any preterm birth compared to naturally conceived pregnancies (occurrence 8.1% vs 5.0%, total effect OR 1.49, 95% CI: 1.45–1.53), but none of this could be mediated by HDP (mediated effect OR 1.00, 95%CI 1.00–1.00, proportion mediated 0.5%). Sensitivity analyses with extra confounder adjustment for body mass index and smoking, and restriction to primiparous women, were consistent with our main findings. Furthermore, the results were not driven by differences in ART procedures (intracytoplasmic sperm injection, culture duration, or the number of embryos transferred).
LIMITATIONS, REASONS FOR CAUTION
Although we could adjust for some important confounders, we cannot exclude residual confounding, particularly from factors associated with infertility.
WIDER IMPLICATIONS OF THE FINDINGS
This population-based mediation analysis suggests that some of the higher risk of preterm birth after ART treatment may be explained by the higher risk of HDP after frozen-ET. If causality is established, investigations into preventive strategies such as prophylactic aspirin in pregnancies after frozen-ET may be warranted.
STUDY FUNDING/COMPETING INTEREST(S)
Funding was provided by NordForsk (project number: 71450), the Nordic Federation of Obstetrics and Gynaecology (project numbers NF13041, NF15058, NF16026, and NF17043), the Norwegian University of Science and Technology (project number 81850092), an ESHRE Grant for research in reproductive medicine (grant number 2022-2), and the Research Council of Norway’s Centres of Excellence funding scheme (project number 262700). D.A.L.’s and A.E.’s contribution to this work was supported by the European Research Council under the European Union’s Horizon 2020 research and innovation program (grant agreements No 101021566) and the UK Medical Research Council (MC_UU_00032/05). D.A.L. has received support from Roche Diagnostics and Medtronic Ltd for research unrelated to that presented here. Pinborg declares grants from Gedeon Richter, Ferring, Cryos, and Merck, consulting fees from IBSA, Ferring, Gedeon Richter, Cryos, and Merck, payments from Gedeon Richter, Ferring, Merck, and Organon,travel support from Gedeon Richter. All other authors declare no conflicts of interest related to this work.
TRIAL REGISTRATION NUMBER
ISRCTN 35879.
Keywords: ART, infertility, embryo transfer, hypertensive disorders in pregnancy, hypertension, pre-eclampsia, preterm birth, frozen embryo transfer, fresh embryo transfer, mediation analysis
Introduction
In ART, frozen embryo transfer (frozen-ET) is increasingly common due to improved cryopreservation methods, the use of blastocyst culture, and the ‘freeze-all’ approach, in which all good-quality embryos are frozen for transfer in later cycles and no fresh embryo transfer (fresh-ET) takes place (Thurin et al., 2004; Devroey et al., 2011; Maheshwari et al., 2016; Rienzi et al., 2017; Wyns et al., 2021; Zaat et al., 2021). Important advantages of frozen-ET include a lower risk of ovarian hyperstimulation syndrome and facilitation of single ET. With the use of new cryopreservation methods, such as vitrification, an improvement in cumulative delivery rates has occurred (Saket et al., 2021; Zaat et al., 2021).
Meta-analyses show that compared to natural conception, frozen-ET and fresh-ET are associated with a higher risk of preterm birth (Elias et al., 2020). A Nordic population-based cohort study showed that these associations persisted, though were attenuated, in within sibship analyses, suggesting that ART treatment factors (as opposed to parental factors like genetics, underlying health, and health behaviours) could be responsible for at least some of the excess risk (Westvik-Johari et al., 2021). Furthermore, in the same cohort, a higher risk of hypertensive disorders in pregnancy (HDP) after frozen-ET compared to natural conception was found, also within sibships, but there was no association between fresh-ET and HDP (Petersen et al., 2023). Studies have also shown that in ART-conceived pregnancies compared to naturally conceived pregnancies (Vermey et al., 2019; Petersen et al., 2020), there is a higher risk of placental abruption, a condition also related to HDP (Tikkanen, 2011). HDP and placental abruption increase the risk of preterm birth, because they may require immediate delivery through induction of labour or caesarean section (i.e. medically indicated preterm birth), but also because these complications are associated with spontaneous preterm birth (Salihu et al., 2003; Downes et al., 2017; Oberg et al., 2018; Chappell et al., 2019).
Preterm birth has severe implications for both the short- and long-term health of the child but is challenging to predict and prevent (Slattery and Morrison, 2002; Moster et al., 2008; Hack et al., 2011; Costeloe et al., 2012; Meertens et al., 2018). Given the higher risk of HDP after ART conception, exploring the contribution of HDP to the excess of preterm births following ART could increase our understanding of ART treatment safety, and guide preventive measures during pregnancy. Mediation analysis (VanderWeele, 2016), which has been used rarely in studies of ART conception (Stern et al., 2020, 2021), provides a framework for quantifying the proportion mediated while accounting for potential sources of bias.
The aim of this study was to investigate to what extent the previously reported higher risk of HDP after ART can explain the higher risk of preterm birth in ART-conceived pregnancies.
Materials and methods
The study methods were pre-specified in an analysis plan published on the Open Science Framework website on 05 August 2022 (Petersen et al., 2022).
Data sources
The Committee of Nordic ART and Safety (CoNARTaS) cohort includes all deliveries registered in the Medical Birth Registries in four Nordic countries, described in detail elsewhere (Opdahl et al., 2020). For this study, we included data from Denmark (1994–2014), Norway (1988–2015), and Sweden (1988–2015), but not from Finland, where we did not have the necessary details on ART treatment. The national identity number allocated to each resident in the Nordic countries was used to link data from the Medical Birth Registries to data from the national ART registries and databases, and the Danish National Patient Registry.
In Denmark, all ART cycles in public and private clinics have been recorded in their national ART quality registry since 1994. In Norway, public and private ART clinics provide information to the Medical Birth Registry on all ART cycles that result in an ultrasound-verified pregnancy (Weeks 6–7). In Sweden, information on the conception method was collected by the National Board of Health and Welfare until 2006, and from 2007 all ART cycles have been registered in the National Quality Registry of Assisted Reproduction.
Medical conditions during pregnancy were registered in the Danish National Patient Registry and the Norwegian and Swedish Medical Birth Registries according to national adaptations of the 8th, 9th, and 10th versions of International Statistical Classifications of Diseases and Related Health Problems, as defined in Supplementary Table S1.
Exposure assessment
The exposures were frozen-ET or fresh-ET versus natural conception (reference group). Natural conception comprised all pregnancies with no registration of ART conception. Pregnancies after ovulation induction and insemination were coded as natural conceptions.
Outcome assessment
In Danish and Norwegian data, gestational age was estimated from ultrasound scans in the first or second trimester, respectively. If these estimates were unavailable, we used the transfer date for ART-conceived pregnancies and the last menstrual period for naturally conceived pregnancies. In Sweden, gestational age was based on the transfer date for ART-conceived pregnancies, and second-trimester ultrasound scans for naturally conceived pregnancies, and if these were unavailable, the date of the last menstrual period was used. The main outcome was any preterm birth, defined as birth between 22 + 0 and 36 + 6 weeks of gestation. Secondary outcomes were: (i) spontaneous preterm birth, (ii) medically indicated preterm birth, (iii) extremely or very preterm birth (<32 + 0 weeks of gestation), and (iv) extremely preterm birth only (<28 + 0 weeks of gestation).
Mediator assessment
The main mediator was HDP, defined as a combined variable including gestational hypertension, preeclampsia, eclampsia, and chronic hypertension with superimposed preeclampsia. Secondary mediators were: (i) restriction to preeclampsia, superimposed preeclampsia, and eclampsia (i.e. not including pregnancies with isolated gestational hypertension), (ii) placental abruption, and (iii) HDP+placental abruption.
Covariate assessment
Smoking status was self-reported and registered throughout the whole study period in Denmark and Sweden, and since 1999 in Norway. Harmonization across the countries was only possible as no smoking versus any smoking during pregnancy. In Sweden, maternal height and weight were registered from 1988 to 1989, and from 1992 to 2015. In Denmark and Norway, maternal height and weight were registered since 2004 and 2007, respectively. In all countries, the proportion of observations with missing data on these variables was high during the first period of registration, as shown in Supplementary Figs S1 and S2.
Study population
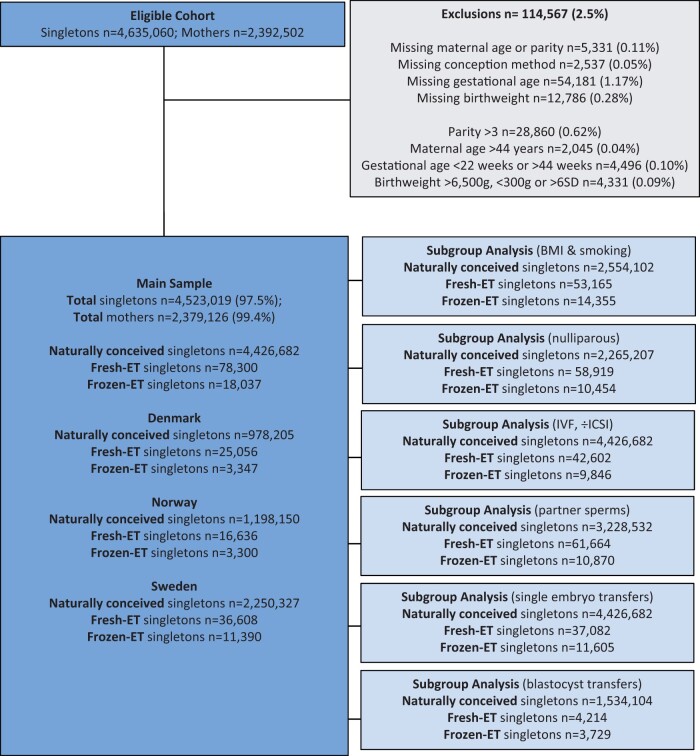
We defined the study period from 1988 (the first year with a registered delivery after frozen-ET) to 2015 in Norway and Sweden, and from 1994 to 2014 in Denmark. The eligible cohort was defined as all singleton deliveries with mothers who were ≥20 years and had their first delivery during the study period, comprising 4 635 060 deliveries by 2 392 502 women (Fig. 1). We excluded observations with missing parity, maternal age, birthweight, or gestational age. Next, we excluded deliveries with birth order higher than 4, and deliveries at maternal age >44 years as there were few or no ART conceptions in these groups. We also excluded observations with extreme values on birthweight (<300 or >6500 g, >6 SDs above the expected value (Marsál et al., 1996)), or gestational age (<22 or >44 weeks). Our main sample then comprised 4 523 019 deliveries among 2 379 126 mothers, including 78 300 deliveries after fresh-ET and 18 037 after frozen-ET.
Figure 1.
Flowchart of study population. The subgroup with only blastocyst transfers was restricted to birth years 2005–2015. Fresh-ET, fresh embryo transfer; Frozen-ET, frozen embryo transfer.
Statistical analysis
Exposure-outcome confounders represent common causes of exposure (ART treatment) and outcome (preterm birth). Both ART treatment and preterm birth may also share common causes with the mediator, since other known risk factors for developing HDP, e.g. maternal age, parity, and body mass index (BMI) could affect the probability of both ART treatment and preterm birth, constituting exposure-mediator confounding and mediator-outcome confounding, respectively.
We used mediation analysis (Vanderweele and Vansteelandt, 2010; Valeri and Vanderweele, 2013; VanderWeele, 2016; Valente et al., 2020), similar to other research in reproductive medicine and perinatal epidemiology (Oberg et al., 2018; Ananth and Brandt, 2022). We used logistic regression to estimate odds ratios (ORs) of preterm birth with 95% CIs, comparing frozen-ET and fresh-ET to natural conception. In the following, we describe how we estimated the total, direct, and mediated effects for frozen-ET and fresh-ET compared to natural conception. First, the total effect is the overall association between the exposure and the outcome, in our case the overall effect of frozen-ET/fresh-ET on preterm birth, regardless of any downstream mediating variables. A valid estimate of this total effect requires control for exposure-outcome confounding. The natural direct effect of frozen-ET/fresh-ET provides an estimate of the influence of frozen-ET/fresh-ET on preterm birth that is independent of HDP (i.e. that not mediated by HDP). Conversely, the mediated (or natural indirect) effect represents the influence of frozen-ET/fresh-ET on preterm birth that can be attributed solely to the effect of frozen-ET/fresh-ET on HDP (i.e. some treatment factor increases the risk of HDP; Petersen et al., 2022). The total effect is the product of the ORs for the direct and mediated effects. For an easier interpretation, we calculated the proportion mediated on the risk difference scale (Vanderweele and Vansteelandt, 2010), where 0% corresponds to no mediation by HDP, and 100% corresponds to the entire excess risk being mediated by HDP (no direct effect). The proportion mediated thus gives a measure of what would happen to the excess risk of preterm birth after frozen-ET/fresh-ET if we somehow intervened on the causal pathway between frozen-ET/fresh-ET and HDP (VanderWeele, 2013). We estimated this by the formula (Vanderweele and Vansteelandt, 2010):
Valid direct and mediated effect estimates require that baseline covariates control for exposure-outcome, mediator-outcome, and exposure-mediator confounding (Robins and Greenland, 1992; VanderWeele, 2010). In the main models, we adjusted for year of delivery in categories to take into account a combination of laboratory changes and availability of treatment (1988–1996, 1997–2001, 2002–2006, 2007–2012, or 2013–2015), maternal age (20–24, 25–29, 30–34, 35–39, or 40–44 years), parity (1st, 2nd, or 3rd–4th birth), and country, which were assumed to be both exposure-mediator confounders and mediator-outcome confounders.
We performed several sensitivity analyses to investigate the robustness of our results. Firstly, in a subgroup with available information, we further adjusted for the potential confounding from maternal smoking during pregnancy (yes/no), and BMI (<18.5, 18.5–24.9, 25–29.9, or ≥30 kg/m2). BMI was included as a categorical variable because of its J-shaped association with preterm birth in our data. Secondly, to limit the impact of prior pregnancy experiences (Skjaerven et al., 1988), we restricted the analysis to primiparous women only. Thirdly, we restricted the ART-conceived pregnancies in different ways to explore the impact of ART treatment factors: we restricted to fertilization by IVF (i.e. we excluded fertilization by ICSI, which is used mainly for male infertility in the Nordic countries (Nyboe Andersen et al., 2008)), to fertilization with partner sperm to limit the potential impact of donor sperm (González-Comadran et al., 2014; Dunietz et al., 2017), to single ETs to limit the potential impact of vanishing twins (Magnus et al., 2017; Harris et al., 2020), and to blastocyst transfers to take into account the prolonged exposure to culture media and differences in duration of other in vitro exposures (Ginström Ernstad et al., 2019a). During the study period, most frozen blastocysts were vitrified whereas most cleavage stage embryos were slow-frozen (Ginström Ernstad et al., 2019a). We also explored the potential mediator-outcome confounding from any diabetes (pregestational or gestational), which is a risk factor for developing HDP (Bryson et al., 2003; Hauth et al., 2011), noting that the quality of gestational diabetes diagnoses was poor in our data (Lindqvist et al., 2014). Finally, we repeated the main analyses using transfer date and culture duration to estimate gestational age in ART-conceived pregnancies from Denmark and Norway, to explore whether the results were different from the standard gestational age calculation.
We also explored the role of our secondary mediators, i.e. preeclampsia, placental abruption, and the combination of placental abruption and HDP. In analyses with placental abruption as the mediator, we included HDP as a mediator-outcome confounder, because HDP is a risk factor for developing placental abruption (Tikkanen, 2011).
Ethics
In Denmark and Finland, ethical approval is not required for scientific projects solely based on registry data. In Norway, ethical approval was given by the Regional Committee for Medical and Health Research Ethics (REK-Nord, 2010/1909-1-24, 14398). In Sweden approval was obtained from the Ethical committee in Gothenburg, Dnr 214–12, T422-12, T516-15, T233-16, T300-17, T1144-17, and T121-18.
Results
Descriptive results
Baseline characteristics of all pregnancies in our study population (4 426 682 singletons after natural conception, 78 300 singletons after fresh-ET and 18 037 singletons after frozen-ET) are presented in Table 1, according to conception method and HDP diagnosis. ART-conceived singletons comprised increasingly larger proportions of the total birth cohorts throughout the study period, and more than 80% of the included ART-conceived singletons were born after 2002. Mothers conceiving by frozen-ET and fresh-ET were older (mean age 34.3 and 33.8 years, respectively) than naturally conceiving mothers (mean age 29.6 years). Mothers conceiving by fresh-ET were more commonly primiparous (75.3%) than mothers conceiving naturally or by frozen-ET (58.0% and 51.2%, respectively). A lower proportion of ART-conceiving mothers smoked during pregnancy, while the mean BMI was similar in all conception groups.
Table 1.
Baseline characteristics of the study population (main sample) according to conception method and HDP.a,b
| Frozen-ET |
Fresh-ET |
Natural conception |
||||
|---|---|---|---|---|---|---|
| HDP | Non-HDP | HDP | Non-HDP | HDP | Non-HDP | |
| N = 1326 | N = 16 711 | N = 4600 | N = 73 700 | N = 191 288 | N = 4 235 394 | |
| Country, No. (%) | ||||||
| Denmark | 195 (14.7) | 3152 (18.9) | 1376 (29.9) | 23 680 (32.1) | 40 816 (21.3) | 937 389 (22.1) |
| Norway | 325 (24.5) | 2975 (17.8) | 1099 (23.9) | 15 537 (21.1) | 61 375 (32.1) | 1 136 775 (26.8) |
| Sweden | 806 (60.8) | 10 583 (63.3) | 2125 (46.2) | 34 483 (46.8) | 89 097 (46.6) | 2 161 230 (51.0) |
| Year of delivery, No. (%) | ||||||
| 1988–1996 | 33 (2.5) | 465 (2.8) | 387 (8.4) | 5415 (7.4) | 43 970 (23.0) | 980 189 (23.1) |
| 1997–2001 | 68 (5.1) | 1033 (6.2) | 661 (14.4) | 10 547 (14.3) | 33 744 (17.6) | 774 900 (18.3) |
| 2002–2006 | 176 (13.3) | 2369 (14.2) | 1013 (22.0) | 16 753 (22.7) | 38 872 (20.3) | 873 091 (20.6) |
| 2007–2011 | 472 (35.6) | 6045 (36.2) | 1429 (31.1) | 22 976 (31.2) | 42 871 (22.4) | 924 424 (21.8) |
| 2012–2015 | 577 (43.5) | 6799 (40.7) | 1110 (24.1) | 18 009 (24.4) | 31 831 (16.6) | 682 790 (16.1) |
| Parity, No. (%) | ||||||
| 0 | 965 (72.8) | 9489 (56.8) | 3892 (84.6) | 55 027 (74.7) | 132 489 (69.3) | 2 132 718 (50.4) |
| 1 | 315 (23.8) | 6230 (37.3) | 616 (13.4) | 16 383 (22.2) | 43 001 (22.5) | 1 546 041 (36.5) |
| 2 or 3 | 46 (3.5) | 992 (5.9) | 92 (2.0) | 2290 (3.1) | 15 798 (8.3) | 556 635 (13.1) |
| Maternal age, mean (SD), years | 34.4 (4.4) | 34.3 (4.1) | 33.9 (4.3) | 33.7 (4.2) | 29.6 (5.0) | 29.6 (4.8) |
| Pregestational hypertension, No. (%) | 52 (3.9) | 107 (0.6) | 223 (4.9) | 484 (0.7) | 7879 (4.1) | 19 057 (0.5) |
| Any diabetes, No. (%) | 75 (5.7) | 402 (2.4) | 272 (5.9) | 2036 (2.8) | 8560 (4.5) | 66 813 (1.6) |
| Maternal BMI, mean (SD), kg/m2 | 25.8 (4.7) | 24.1 (3.9) | 26.2 (4.8) | 24.1 (4.0) | 26.3 (5.6) | 24.1 (4.4) |
| Missing, outside registration period, (%) | 80 (6.0) | 1116 (6.7) | 914 (20.0) | 14 269 (19.4) | 61 271 (32.0) | 1 253 555 (29.6) |
| Missing, during registration period, (%) | 207 (15.6) | 2083 (12.5) | 594 (12.9) | 8356 (11.3) | 23 189 (12.1) | 479 286 (11.3) |
| Maternal smoking in pregnancy, No. (%) | 32 (2.6) | 508 (3.2) | 226 (5.5) | 3829 (5.7) | 14 894 (9.4) | 434 643 (12.1) |
| Missing, outside registration period, (%) | 75 (5.7) | 921 (5.5) | 351 (7.6) | 5006 (6.8) | 15 610 (8.2) | 289 833 (6.8) |
| Missing, during registration period, (%) | 9 (0.7) | 106 (0.6) | 131 (2.9) | 1875 (2.5) | 17 858 (9.3) | 360 726 (8.5) |
| Caesarean section, No. (%) | 594 (44.8) | 4539 (27.2) | 1998 (43.4) | 17 912 (24.3) | 63 658 (33.3) | 607 288 (14.3) |
| Induction of labour, No. (%) | 771 (58.1) | 3729 (22.3) | 2249 (49.5) | 12 441 (16.9) | 86 764 (45.4) | 480 308 (11.3) |
| Sex, No. (%) | ||||||
| Boys | 677 (51.1) | 8538 (51.1) | 2320 (50.4) | 37 699 (51.2) | 100 062 (52.3) | 2 175 081 (51.4) |
| Girls | 649 (48.9) | 8173 (48.9) | 2279 (49.5) | 35 988 (48.8) | 91 196 (47.7) | 2 059 885 (48.6) |
| Birthweight, mean (SD), grams | 3283 (783) | 3601 (593) | 3110 (840) | 3425 (600) | 3209 (816) | 3552 (547) |
| ART fertilization method, No. (%) | ||||||
| IVF | 765 (57.7) | 9081 (54.3) | 2587 (56.2) | 42 015 (57.0) | – | – |
| ICSI | 482 (36.4) | 6134 (36.7) | 1914 (41.6) | 30 324 (41.2) | – | – |
| Unknown | 79 (6.0) | 1496 (9.0) | 96 (2.1) | 1361 (1.9) | – | – |
| Embryos transferred, No. (%) | ||||||
| 1 | 860 (64.9) | 10 745 (64.3) | 2100 (45.6) | 34 982 (47.5) | – | – |
| 2 | 332 (25.0) | 3877 (23.2) | 1773 (38.5) | 28 214 (38.3) | – | – |
| 3 | 8 (0.6) | 123 (0.7) | 121 (2.6) | 1770 (2.4) | – | – |
| Unknown | 126 (9.5) | 1996 (11.8) | 606 (13.2) | 8734 (11.9) | – | – |
| Embryo culture, No. (%), days | ||||||
| 2–3 (Cleavage) | 815 (61.5) | 10 892 (65.2) | 3471 (75.5) | 58 201 (79.0) | – | – |
| 5–6 (Blastocyst) | 280 (21.1) | 3476 (20.8) | 237 (5.2) | 4213 (5.7) | – | – |
| Unknown | 231 (14.4) | 2343 (14.0) | 892 (19.4) | 11 286 (15.3) | – | – |
Hypertensive disorders in pregnancy, i.e. gestational hypertension, preeclampsia, eclampsia, or chronic hypertension with superimposed preeclampsia.
Percentages may not total to 100% on account of rounding.
BMI, body mass index (calculated as weight in kilograms divided by height in metres squared).
Pregnancies after frozen and fresh-ET were more frequently induced and/or delivered by caesarean section. Among frozen-ET pregnancies, 36.7% were fertilized by ICSI, 64.3% were single ETs, and 20.8% were blastocyst transfers. Fresh-ET pregnancies had similar proportions of ICSI and single ET, but only 5.7% were after blastocyst transfer.
Main results
Preterm birth was more common in pregnancies with HDP (17.6% of pregnancies diagnosed with HDP, compared to 4.4% of pregnancies with no HDP), even after adjusting for differences in maternal preconception characteristics (Supplementary Table S2). This association between HDP and preterm birth was prominent after all three conception methods.
Figure 2 describes the distribution of gestational age according to hypertension status. While normotensive pregnancies and pregnancies diagnosed with gestational hypertension had similar gestational age distributions, preeclamptic pregnancies tended to be delivered earlier, a pattern which applied to all three conception groups.
Figure 2.
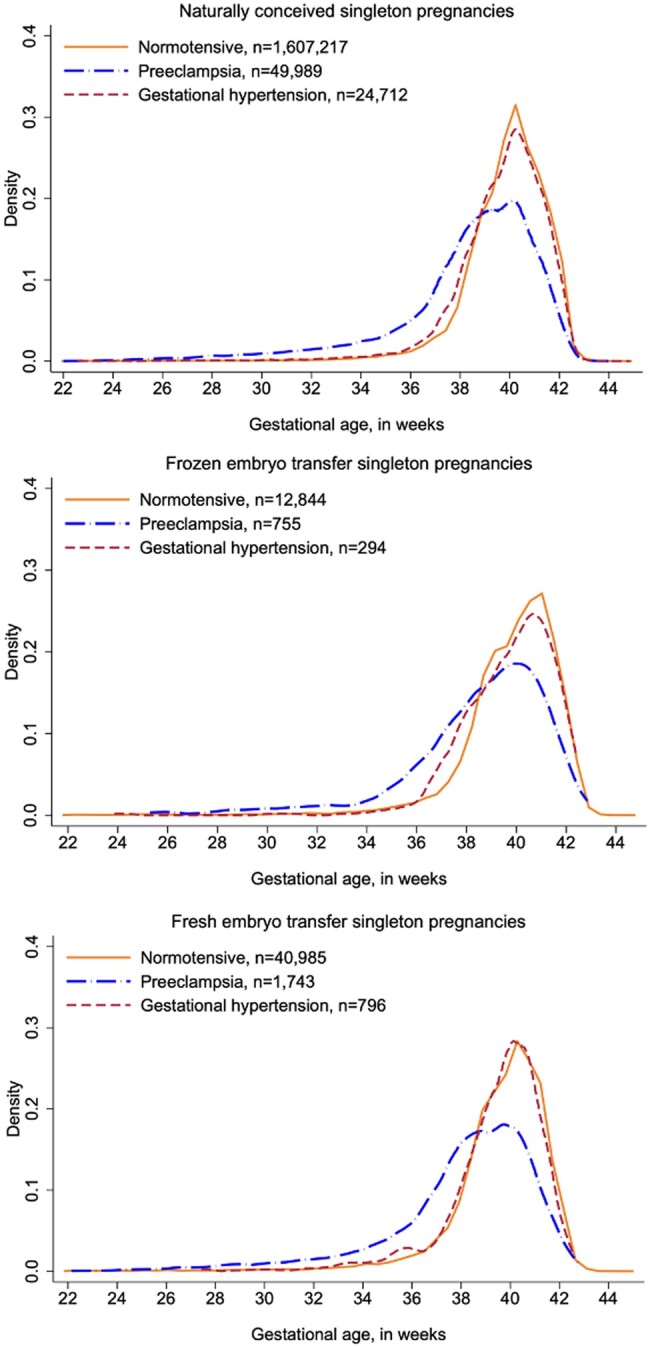
Distributions of gestational age. Distributions of gestational age at delivery according to conception method and occurrence of hypertensive disorders in pregnancy, among singleton pregnancies in Denmark (2007–2014), Norway, and Sweden (2007–2015).
In Table 2, we present the mediation analysis with the decomposition of the total effect into the direct effect and the mediated effects, as well as estimates of the proportion mediated. After adjustments for year of delivery, maternal age, parity, and country, frozen-ET pregnancies had higher odds of preterm birth compared to naturally conceived pregnancies, with a total effect OR of 1.29 (95% CI 1.21–1.37). HDP mediated some of this association, with a mediated effect OR of 1.05 (95% CI 1.04–1.05), and an estimated proportion mediated of 20.7%. Frozen-ET pregnancies also had higher adjusted odds of spontaneous preterm birth compared to naturally conceived pregnancies, with a total effect OR 1.19 (95% CI 1.11–1.29), which was not mediated by HDP (mediated effect OR 1.00, 95% CI 1.00–1.00). The observed excess risk of medically indicated preterm birth, extremely or very preterm birth (<32 + 0 weeks), and extremely preterm birth only (<28 + 0 weeks), in those conceived by frozen-ET ART compared with natural conception, was mediated by HDP to some extent, with a proportion mediated of 38.2%, 21.2%, and 10.6%, respectively.
Table 2.
Main results. Effect of frozen-ET and fresh-ET on preterm birth overall (total), through HDPa (mediated) and independent of HDPa (direct). Odds ratios (OR) with 95% confidence intervals.
| Cases/deliveries (%) | Total effect OR b | Direct effect OR b | Mediated OR b | % Mediated c | |
|---|---|---|---|---|---|
| Any preterm birth (<37 weeks) | |||||
| Natural conception | 219 454/4 426 682 (5.0%) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | – |
| Frozen-ET | 1198/18 037 (6.6%) | 1.29 (1.21 to 1.37) | 1.23 (1.16 to 1.30) | 1.045 (1.044 to 1.046) | 20.7 |
| Fresh-ET | 6351/78 300 (8.1%) | 1.49 (1.45 to 1.53) | 1.48 (1.44 to 1.52) | 1.002 (1.000 to 1.003) | 0.5 |
| Spontaneous preterm birth (<37 weeks)d | |||||
| Natural conception | 139 894/4 267 470 (3.3%) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | – |
| Frozen-ET | 709/18 033 (3.9%) | 1.19 (1.11 to 1.29) | 1.19 (1.17 to 1.26) | 1.003 (1.001 to 1.005) | 2.0 |
| Fresh-ET | 3667/78 003 (4.7%) | 1.34 (1.30 to 1.39) | 1.34 (1.30 to 1.39) | 1.000 (0.999 to 1.001) | 0.4 |
| Medically indicated preterm birth (<37 weeks)d | |||||
| Natural conception | 69 767/4 267 470 (1.6%) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | – |
| Frozen-ET | 488/18 033 (2.7%) | 1.45 (1.33 to 1.60) | 1.28 (1.22 to 1.34) | 1.136 (1.133 to 1.138) | 38.2 |
| Fresh-ET | 2633/78 003 (3.4%) | 1.71 (1.64 to 1.78) | 1.70 (1.63 to 1.78) | 1.005 (1.003 to 1.006) | 1.1 |
| Extremely or very preterm birth (<32 weeks) | |||||
| Natural conception | 31 634/4 426 682 (0.7%) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | – |
| Frozen-ET | 224/18 037 (1.2%) | 1.49 (1.30 to 1.70) | 1.38 (1.29 to 1.48) | 1.075 (1.073 to 1.077) | 21.2 |
| Fresh-ET | 1250/78 300 (1.6%) | 1.83 (1.72 to 1.94) | 1.82 (1.72 to 1.94) | 1.002 (1.001 to 1.004) | 0.5 |
| Extremely preterm birth (<28 weeks) | |||||
| Natural conception | 10 423/4 426 682 (0.2%) | 1.00 (Ref.) | 1.00 (Ref.) | 1.00 (Ref.) | – |
| Frozen-ET | 93/18 037 (0.5%) | 1.74 (1.42 to 2.14) | 1.66 (1.50 to 1.84) | 1.047 (1.044 to 1.050) | 10.6 |
| Fresh-ET | 457/78 300 (0.6%) | 1.98 (1.80 to 2.18) | 1.98 (1.79 to 2.18) | 1.002 (1.000 to 1.003) | 0.3 |
Hypertensive disorders in pregnancy, i.e. gestational hypertension, preeclampsia, eclampsia, or chronic hypertension with superimposed preeclampsia.
The total effect decomposes into the product of the direct effect odds ratio and the mediated effect odds ratio.
Proportion mediated = DE × (ME − 1)/(DE × ME − 1), where DE is direct effect and ME is mediated effect.
Models are adjusted for year of delivery, maternal age, parity, and country.
For Sweden, the study population was restricted to deliveries 1991–2015.
ET, embryo transfer. Ref, reference.
Fresh-ET pregnancies also had higher odds of preterm birth compared to naturally conceived pregnancies after adjustments, total effect OR 1.49 (95% CI 1.45–1.53). This positive association was independent of HDP (mediated effect OR 1.00, 95% CI 1.00–1.00), with only 0.5% of the total effect mediated. Fresh-ET pregnancies had higher adjusted odds of spontaneous preterm birth (total effect OR 1.34, 95% CI 1.30–1.39) and medically indicated preterm birth (total effect OR 1.71, 95% CI 1.64–1.78) compared to natural conception, but none of these associations were mediated by HDP. The excess risk of extremely or very preterm birth and extremely preterm birth only after fresh-ET was also not mediated by HDP.
Additional analyses
Sensitivity analyses (Supplementary Table S3) with extra confounder control for BMI, smoking in pregnancy and diabetes mellitus, restriction to primiparous women, exclusion of ICSI conception, restriction to conceptions with partner sperm, restriction to single ETs, and restriction to blastocyst transfers yielded results consistent with our main findings, as between 14.3% and 28.0% of the association between frozen-ET and preterm birth was mediated by HDP, and the excess risk of preterm birth in fresh-ET continued to be independent of HDP. Distributions of gestational age based on ET date versus ultrasound examination were similar (Supplementary Fig. S3), and analyses using transfer date as the basis for estimating gestational age in ART-conceived pregnancies gave identical results as the main analyses (Supplementary Table S4).
In analyses (Table 3) exploring the effect of other mediator variables, preeclampsia mediated 26.0% of the association with preterm birth after frozen-ET, whereas placental abruption mediated around 8%. For fresh-ET, 14.3% of the association with preterm birth could be explained by placental abruption (mediated OR 1.05, 95% CI 1.05–1.05), with no evidence of mediation by preeclampsia in these analyses.
Table 3.
Secondary mediators. Effect of frozen-ET and fresh-ET on preterm birth overall (total), through HDPa or secondary mediators (mediated) and independent of HDPa or secondary mediators(direct). Odds ratios (OR) with 95% confidence intervals.
| Frozen-ET versus natural conception | Total effect OR b | Direct effect OR b | Mediated OR b | % Mediated c |
|---|---|---|---|---|
| HDPa as mediator | 1.29 (1.21 to 1.37) | 1.23 (1.16 to 1.30) | 1.045 (1.044 to 1.046) | 20.7 |
| Preeclampsia as mediator | 1.30 (1.22 to 1.38) | 1.22 (1.18 to 1.26) | 1.063 (1.062 to 1.065) | 26.0 |
| Placental abruption as mediator | 1.31 (1.23 to 1.39) | 1.29 (1.25 to 1.32) | 1.020 (1.017 to 1.023) | 8.2 |
| Placental abruption as mediator, adjustment for HDPa | 1.24 (1.17 to 1.32) | 1.22 (1.15 to 1.30) | 1.017 (1.014 to 1.019) | 8.3 |
| Placental abruption+HDPa as mediator | 1.30 (1.22 to 1.37) | 1.21 (1.18 to 1.25) | 1.064 (1.064 to 1.065) | 26.7 |
| Frozen-ET versus natural conception | Total effect OR b | Direct effect OR b | Mediated OR b | % Mediated c |
|---|---|---|---|---|
| HDPa as mediator | 1.49 (1.45 to 1.53) | 1.48 (1.44 to 1.52) | 1.002 (1.000 to 1.003) | 0.5 |
| Preeclampsia as mediator | 1.49 (1.45 to 1.53) | 1.48 (1.44 to 1.52) | 1.006 (1.005 to 1.007) | 1.9 |
| Placental abruption as mediator | 1.53 (1.49 to 1.57) | 1.45 (1.41 to 1.49) | 1.052 (1.048 to 1.056) | 14.3 |
| Placental abruption as mediator, adjustment for HDPa | 1.53 (1.49 to 1.57) | 1.46 (1.42 to 1.50) | 1.049 (1.046 to 1.053) | 13.8 |
| Placental abruption+HDPa as mediator | 1.49 (1.45 to 1.53) | 1.48 (1.44 to 1.52) | 1.011 (1.010 to 1.012) | 3.2 |
Hypertensive disorders in pregnancy, i.e. gestational hypertension, preeclampsia, eclampsia, or chronic hypertension with superimposed preeclampsia.
The total effect decomposes into the product of the direct effect odds ratio and the mediated effect odds ratio.
Proportion mediated = DE × (ME − 1)/(DE × ME − 1), where DE is direct effect and ME is mediated effect.
Models are adjusted for year of delivery, maternal age, parity, and country.
ET, embryo transfer.
Discussion
Main findings
In this population-based cohort study with nationwide data from three countries, we found that HDP explained around 1/5 of the excess risk of any preterm birth after frozen-ET compared to natural conception, primarily by affecting medically indicated preterm birth. There was no association between HDP and fresh-ET compared with natural conception, and hence HDP did not contribute to the excess risk of preterm birth in this group.
Comparison to earlier research
Few studies have investigated the relationship between ART, HDP, and preterm birth using mediation analysis, but several studies have estimated the associations between ART and preterm birth, and between ART and HDP. In recent meta-analyses investigating the association between ART and preterm birth, the authors reported a higher risk of both spontaneous and medically indicated preterm birth after ART conception (Cavoretto et al., 2018, 2022). In sibling studies in the CoNARTaS cohort, fresh-ET and frozen-ET were both associated with a higher risk of preterm birth (Westvik-Johari et al., 2021), and frozen-ET was associated with a substantially higher risk of HDP (Petersen et al., 2022), suggesting that treatment factors could be responsible for some of the excess risk. A population-based US study used meditation analysis to investigate the influence of placental abnormalities and HDP on the risk of preterm birth after various ART treatments (Stern et al., 2021). The authors reported that compared to a fertile control group, fresh-ET pregnancies had 39% (95% CI 28–51) higher odds of late preterm birth (34–36 weeks), of which 4.1% (proportion mediated) could be explained by HDP. Corresponding estimates for frozen-ET pregnancies were 42% (95% CI 21–62) higher odds of late preterm birth, of which 25.9% (proportion mediated) could be explained by HDP. Our findings are in line with this study.
Strengths and limitations
Key strengths of this study include the population-based design with high-quality data on all deliveries in three Nordic countries over three decades of ART treatment (Ros et al., 1998; Thomsen et al., 2013; Schmidt et al., 2015). In the Nordic countries, ART treatment is strongly subsidized, ensuring that the couple’s financial situation should not be a major determinant of ART conception. Our data enabled precisely estimated associations in most analyses. However, statistical power was limited in some of the subgroups and sensitivity analyses, mainly due to few events in the frozen-ET group. Of note, the mediation analyses did not allow for robust standard errors to account for the clustering of pregnancies within each woman, resulting in narrower CIs. Still, in sensitivity analyses restricted to primiparous women (i.e. only one pregnancy per women), the results remained similar as in the main analyses.
We could adjust for several potential confounders. Nevertheless, confounder control for BMI and smoking during pregnancy was limited by a large degree of missingness, and confounder control for gestational diabetes was limited by poor quality of the registry data (Lindqvist et al., 2014). We thus cannot exclude residual confounding and the mediation analyses being biased by mediator-outcome confounding which can result in important bias in either direction (known as collider bias (Pearce and Lawlor, 2016)). The factors contributing to infertility were largely unknown in our data and may also have influenced our results. First, the cause of infertility may account for some of the positive associations we found between ART conception and preterm birth, and ART conception and HDP (Messerlian et al., 2013; Palomba et al., 2015; Luke et al., 2017; Sunkara et al., 2021). Second, it seems plausible that the causes and severity of infertility may have influenced the couple’s probability of having surplus embryos eligible for freezing in the first place. Unfortunately, we had no data on either number of embryos obtained from the ART cycle, nor whether the frozen-ET pregnancy was after an initial, failed fresh-ET or from a freeze-all approach. However, the freeze-all approach was still uncommon during the study period, and most frozen-ET conceptions in our data will have been proceeded by a fresh-ET. Thus, because most frozen-ET pregnancies in our data are conditional on having surplus embryos for freezing, women conceiving after frozen-ET in our data likely constitute a group with less severe infertility than women conceiving after fresh-ET. Furthermore, we had no data on medications used during ART treatment or pregnancy, such as ovarian stimulation, luteal phase support with progesterone, type of frozen cycle, or aspirin. The type of frozen-ET cycle (programmed vs stimulated vs natural) has been reported to be associated with pregnancy outcomes, including preterm birth and HDP (Ginström Ernstad et al., 2019b; Asserhøj et al., 2021). Prophylactic aspirin treatment was uncommon in the Nordic countries during the study period and should have little impact on the results (Sverre et al., 2022; Riishede et al., 2023).
Although ART-conceiving women may be more inclined to seek medical attention, practically all pregnant women in the Nordic countries (ART and non-ART) attend the publicly financed antenatal care program with screening for pregnancy complications. Hence, we expect any differential misclassification of HDP through increased detection in ART-conceived pregnancies to be small, and also unlikely to differ between fresh-ET and frozen-ET pregnancies. Furthermore, the separation of spontaneous preterm birth from medically indicated preterm birth was not completely accurate without very detailed clinical data, and there may be some misclassification of these. Next, pregnancies with ovulation induction or intrauterine insemination were coded as natural conceptions, but given the large number of completely unassisted conceptions, we expect any resulting information bias to be small. Finally, in our main analyses, the preferred gestational age estimate in ART-conceived pregnancies was the one used for clinical decision-making (i.e. ultrasound in Denmark and Norway, and transfer date in Sweden). Although it is not clear which method is the most clinically relevant for ART conception, it seems plausible that early deviations in foetal growth could have resulted in misclassification of gestational age and in turn preterm birth (Ginod et al., 2018). Reassuringly, analyses using transfer date for all countries showed very similar results.
Interpretation and implications
Preterm birth is a complex condition which likely has a multifactorial aetiology, spontaneous preterm birth, and medically indicated preterm birth alike (Goldenberg et al., 2008; Romero et al., 2014). Maternal and foetal conditions other than HDP and placental abruption, such as foetal growth restriction, other causes of antepartum haemorrhage and congenital anomalies are more common in ART-conceived pregnancies, and might increase the risk of both spontaneous and medically indicated preterm birth in this group (Romero et al., 2014). Causes of infertility might be involved in these associations, because several preconception characteristics of the ART conceiving women are associated with a higher risk of preterm birth, making it difficult to disentangle the exact role of ART treatment factors (Declercq et al., 2015). We explored some of these issues by separating spontaneous preterm birth and medically indicated preterm birth, and found that both frozen-ET and fresh-ET pregnancies had higher risks of both types of preterm birth, but also that the contribution from HDP to these excess risks was limited to a relatively small proportion of medically indicated preterm birth in frozen-ET. Preeclampsia seemed to be a slightly stronger mediator of preterm birth than HDP, which might be attributed to its higher severity more often leading to medically indicated preterm birth.
The high risk of a range of adverse short- and long-term health outcomes for children born preterm (Moster et al., 2008; Hack et al., 2011; Costeloe et al., 2012) stresses the need for improved prevention of preterm birth. Our results raise the question of whether preventive strategies targeted towards HDP, such as prophylactic aspirin, might help prevent medically indicated preterm birth in pregnancies after frozen-ET. However, our results also indicate that most of the excess risk of preterm birth in ART-conceived pregnancies overall was independent of HDP, and may require other prevention targets. In particular, the strong association of fresh-ET with medically indicated preterm birth calls for further investigation to identify the responsible foetal or maternal complications, and whether these can be attributed to modifiable treatment factors. Some studies show a possible reduction of preterm birth with prophylactic prescription of aspirin (Andrikopoulou et al., 2018; Landman et al., 2022), but this is not currently recommended in clinical guidelines for this indication alone (American College of Obstetricians and Gynecologists, 2021; Wennerholm et al., 2023). Progesterone, which is frequently administered to support the luteal phase after ET in ART, might be an alternative preventive treatment, but for which the optimal timing, duration, and preventive potential are yet to be established (Di Guardo et al., 2020; Shoham et al., 2021; Stewart et al., 2021).
Conclusions
This population-based study with mediation analysis suggests that some of the higher risk of preterm birth after ART treatment can be explained by the higher risk of hypertensive disorders in frozen-ET pregnancies. Further investigations to ascertain whether these associations are causal and, if so, how this knowledge may be translated into preventive strategies for preterm birth are warranted.
Supplementary Material
Acknowledgements
We thank all staff in the ART clinics and labour departments in the three contributing countries, for taking time to complete the ART registration forms and birth notifications in their busy working day. The details and completeness provide a solid foundation for our study.
Contributor Information
Sindre Hoff Petersen, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Trondheim, Norway.
Bjørn Olav Åsvold, HUNT Center for Molecular and Clinical Epidemiology, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Trondheim, Norway; HUNT Research Centre, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Levanger, Norway; Department of Endocrinology, Clinic of Medicine, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway.
Deborah A Lawlor, MRC Integrative Epidemiology Unit at the University of Bristol, Bristol, UK; Population Health Science, Bristol Medical School, University of Bristol, Bristol, UK.
Anja Pinborg, Fertility Clinic, Department of Gynecology, Fertility and Obstetrics, Rigshospitalet—Copenhagen University Hospital, Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark.
Anne Lærke Spangmose, Fertility Clinic, Department of Gynecology, Fertility and Obstetrics, Rigshospitalet—Copenhagen University Hospital, Copenhagen, Denmark.
Liv Bente Romundstad, Spiren Fertility Clinic, Trondheim, Norway; Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Christina Bergh, Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital, Gothenburg, Sweden.
Ulla-Britt Wennerholm, Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sahlgrenska University Hospital, Gothenburg, Sweden.
Mika Gissler, Department of Data and Analytics, THL Finnish Institute for Health and Welfare, Helsinki, Finland; Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden.
Aila Tiitinen, Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Hospital, Helsinki, Finland.
Ahmed Elhakeem, MRC Integrative Epidemiology Unit at the University of Bristol, Bristol, UK; Population Health Science, Bristol Medical School, University of Bristol, Bristol, UK.
Signe Opdahl, Department of Public Health and Nursing, Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology, Trondheim, Norway; Centre for Big Data Research in Health, University of New South Wales, Sydney, Australia.
Supplementary data
Supplementary data are available at Human Reproduction online.
Data availability
The research data cannot be shared publicly due to national data protection regulations but may be accessed from a server at Statistics Denmark, after approval by the relevant Ethics Committees, the responsible research institutions, and registry keeping authorities in each country.
Authors’ roles
S.H.P. and S.O. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design of the study: All authors. Statistical analysis: S.H.P., A.E., B.O.Å., S.O. Interpretation of results: All authors. Drafting of the manuscript: S.H.P. Critical revision of the manuscript for important intellectual content and final approval of the version to be published: All authors.
Funding
NordForsk (project number: 71450); Nordic Federation of Obstetrics and Gynaecology (project numbers NF13041, NF15058, NF16026 and NF17043); Norwegian University of Science and Technology (project number 81850092); ESHRE Grant for Research in Reproductive Medicine (grant number 2022-2); Research Council of Norway’s Centres of Excellence Funding Scheme (project number 262700). D.A.L.’s and A.E.’s contribution to this work was supported by the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (grant agreement number 101021566) and the UK Medical Research Council (MC_UU_00011/1).
Conflict of interest
D.A.L. has received support from Roche Diagnostics and Medtronic Ltd for research unrelated to that presented here. Pinborg declares grants from Gedeon Richter, Ferring, Cryos, and Merck, consulting fees from IBSA, Ferring, Gedeon Richter, Cryos, and Merck, payments from Gedeon Richter, Ferring, Merck, and Organon,travel support from Gedeon Richter. All other authors of this paper have no conflicts of interest to declare.
References
- American College of Obstetricians and Gynecologists. Low-dose aspirin use for the prevention of preeclampsia and related morbidity and mortality. Practice Advisory December 2021. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/12/low-dose-aspirin-use-for-the-prevention-of-preeclampsia-and-related-morbidity-and-mortality (13 December 2022, date last accessed).
- Ananth CV, Brandt JS. A principled approach to mediation analysis in perinatal epidemiology. Am J Obstet Gynecol 2022;226:24–32.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrikopoulou M, Purisch SE, Handal-Orefice R, Gyamfi-Bannerman C. Low-dose aspirin is associated with reduced spontaneous preterm birth in nulliparous women. Am J Obstet Gynecol 2018;219:399.e1–399.e6. [DOI] [PubMed] [Google Scholar]
- Asserhøj LL, Spangmose AL, Aaris Henningsen AK, Clausen TD, Ziebe S, Jensen RB, Pinborg A. Adverse obstetric and perinatal outcomes in 1,136 singleton pregnancies conceived after programmed frozen embryo transfer (FET) compared with natural cycle FET. Fertil Steril 2021;115:947–956. [DOI] [PubMed] [Google Scholar]
- Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 2003;158:1148–1153. [DOI] [PubMed] [Google Scholar]
- Cavoretto P, Candiani M, Giorgione V, Inversetti A, Abu-Saba MM, Tiberio F, Sigismondi C, Farina A. Risk of spontaneous preterm birth in singleton pregnancies conceived after IVF/ICSI treatment: meta-analysis of cohort studies. Ultrasound Obstet Gynecol 2018;51:43–53. [DOI] [PubMed] [Google Scholar]
- Cavoretto PI, Giorgione V, Sotiriadis A, Viganò P, Papaleo E, Galdini A, Gaeta G, Candiani M. IVF/ICSI treatment and the risk of iatrogenic preterm birth in singleton pregnancies: systematic review and meta-analysis of cohort studies. J Matern Fetal Neonatal Med 2022;35:1987–1996. [DOI] [PubMed] [Google Scholar]
- Chappell LC, Brocklehurst P, Green ME, Hunter R, Hardy P, Juszczak E, Linsell L, Chiocchia V, Greenland M, Placzek A et al. ; PHOENIX Study Group. Planned early delivery or expectant management for late preterm pre-eclampsia (PHOENIX): a randomised controlled trial. Lancet 2019;394:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345:e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declercq E, Luke B, Belanoff C, Cabral H, Diop H, Gopal D, Hoang L, Kotelchuck M, Stern JE, Hornstein MD. Perinatal outcomes associated with assisted reproductive technology: the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART). Fertil Steril 2015;103:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devroey P, Polyzos NP, Blockeel C. An OHSS-Free Clinic by segmentation of IVF treatment. Hum Reprod 2011;26:2593–2597. [DOI] [PubMed] [Google Scholar]
- Di Guardo F, Midassi H, Racca A, Tournaye H, De Vos M, Blockeel C. Luteal phase support in IVF: comparison between evidence-based medicine and real-life practices. Front Endocrinol (Lausanne) 2020;11:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes KL, Shenassa ED, Grantz KL. Neonatal outcomes associated with placental abruption. Am J Epidemiol 2017;186:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunietz GL, Holzman C, Zhang Y, Li C, Todem D, Boulet SL, McKane P, Kissin DM, Copeland G, Bernson D et al. Assisted reproduction and risk of preterm birth in singletons by infertility diagnoses and treatment modalities: a population-based study. J Assist Reprod Genet 2017;34:1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias FTS, Weber-Adrian D, Pudwell J, Carter J, Walker M, Gaudet L, Smith G, Velez MP. Neonatal outcomes in singleton pregnancies conceived by fresh or frozen embryo transfer compared to spontaneous conceptions: a systematic review and meta-analysis. Arch Gynecol Obstet 2020;302:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginod P, Choux C, Barberet J, Rousseau T, Bruno C, Khallouk B, Sagot P, Astruc K, Fauque P. Singleton fetal growth kinetics depend on the mode of conception. Fertil Steril 2018;110:1109–1117.e2. [DOI] [PubMed] [Google Scholar]
- Ginström Ernstad E, Spangmose AL, Opdahl S, Henningsen AA, Romundstad LB, Tiitinen A, Gissler M, Wennerholm UB, Pinborg A, Bergh C et al. Perinatal and maternal outcome after vitrification of blastocysts: a Nordic study in singletons from the CoNARTaS group. Hum Reprod 2019a;34:2282–2289. [DOI] [PubMed] [Google Scholar]
- Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol 2019b;221:126.e1–126.e18. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Comadran M, Urresta Avila J, Saavedra Tascón A, Jimenéz R, Solà I, Brassesco M, Carreras R, Checa M. The impact of donor insemination on the risk of preeclampsia: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2014;182:160–166. [DOI] [PubMed] [Google Scholar]
- Hack M, Schluchter M, Andreias L, Margevicius S, Taylor HG, Drotar D, Cuttler L. Change in prevalence of chronic conditions between childhood and adolescence among extremely low-birth-weight children. Jama 2011;306:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL, Sacha CR, Basnet KM, James KE, Freret TS, Kaimal AJ, Yeh J, Souter I, Roberts DJ, Toth TL. Vanishing twins conceived through fresh in vitro fertilization: obstetric outcomes and placental pathology. Obstet Gynecol 2020;135:1426–1433. [DOI] [PubMed] [Google Scholar]
- Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ, Varner MW, Wapner RJ, Thorp JM Jr, Mercer BM et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol 2011;204:327.e1–327.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landman A, de Boer MA, Visser L, Nijman TAJ, Hemels MAC, Naaktgeboren CN, van der Weide MC, Mol BW, van Laar J, Papatsonis DNM et al. Evaluation of low-dose aspirin in the prevention of recurrent spontaneous preterm labour (the APRIL study): a multicentre, randomised, double-blinded, placebo-controlled trial. PLoS Med 2022;19:e1003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist M, Persson M, Lindkvist M, Mogren I. No consensus on gestational diabetes mellitus screening regimes in Sweden: pregnancy outcomes in relation to different screening regimes 2011 to 2012, a cross-sectional study. BMC Pregnancy Childbirth 2014;14:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol 2017;217:327.e1–327.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus MC, Ghaderi S, Morken N-H, Magnus P, Bente Romundstad L, Skjærven R, Wilcox AJ, Eldevik Håberg S. Vanishing twin syndrome among ART singletons and pregnancy outcomes. Hum Reprod 2017;32:2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil Steril 2016;106:1703–1708. [DOI] [PubMed] [Google Scholar]
- Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996;85:843–848. [DOI] [PubMed] [Google Scholar]
- Meertens LJE, van Montfort P, Scheepers HCJ, van Kuijk SMJ, Aardenburg R, Langenveld J, van Dooren IMA, Zwaan IM, Spaanderman MEA, Smits LJM. Prediction models for the risk of spontaneous preterm birth based on maternal characteristics: a systematic review and independent external validation. Acta Obstet Gynecol Scand 2018;97:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerlian C, Maclagan L, Basso O. Infertility and the risk of adverse pregnancy outcomes: a systematic review and meta-analysis. Hum Reprod 2013;28:125–137. [DOI] [PubMed] [Google Scholar]
- Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008;359:262–273. [DOI] [PubMed] [Google Scholar]
- Nyboe Andersen A, Carlsen E, Loft A. Trends in the use of intracytoplasmatic sperm injection marked variability between countries. Hum Reprod Update 2008;14:593–604. [DOI] [PubMed] [Google Scholar]
- Oberg AS, VanderWeele TJ, Almqvist C, Hernandez-Diaz S. Pregnancy complications following fertility treatment-disentangling the role of multiple gestation. Int J Epidemiol 2018;47:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdahl S, Henningsen AA, Bergh C, Gissler M, Romundstad LB, Petzold M, Tiitinen A, Wennerholm UB, Pinborg AB. Data resource profile: Committee of Nordic Assisted Reproductive Technology and Safety (CoNARTaS) cohort. Int J Epidemiol 2020;49:365–366f. [DOI] [PubMed] [Google Scholar]
- Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–592. [DOI] [PubMed] [Google Scholar]
- Pearce N, Lawlor DA. Causal inference-so much more than statistics. Int J Epidemiol 2016;45:1895–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Elhakeem A, Lawlor D, Åsvold B, Opdahl S. Analysis plan: preterm birth after fresh and frozen embryo transfer in assisted reproduction: disentangling the role of hypertensive disorders in pregnancy using causal mediation analysis. 2022. https://osf.io/bmzr7 (13 December 2022, date last accessed).
- Petersen S, Westvik-Johari K, Spangmose AL, Pinborg A, Romundstad LB, Bergh C, Åsvold BO, Gissler M, Tiitinen A, Wennerholm UB et al. Risk of hypertensive disorders in pregnancy after fresh and frozen embryo transfer in assisted reproduction: a population-based cohort study with within-sibship analysis. Hypertension 2023;80:e6–e16. [DOI] [PubMed] [Google Scholar]
- Petersen SH, Bergh C, Gissler M, Åsvold BO, Romundstad LB, Tiitinen A, Spangmose AL, Pinborg A, Wennerholm UB, Henningsen AA et al. Time trends in placenta-mediated pregnancy complications after assisted reproductive technology in the Nordic countries. Am J Obstet Gynecol 2020;223:226.e1–226.e9. [DOI] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riishede I, Rode L, Sperling L, Overgaard M, Ravn JD, Sandager P, Skov H, Wagner SR, Nørgaard P, Clausen TD et al. Pre-eclampsia screening in Denmark (PRESIDE): national validation study. Ultrasound Obstet Gynecol 2023;61:682–690. [DOI] [PubMed] [Google Scholar]
- Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992;3:143–155. [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol 1998;147:1062–1070. [DOI] [PubMed] [Google Scholar]
- Saket Z, Källén K, Lundin K, Magnusson Å, Bergh C. Cumulative live birth rate after IVF: trend over time and the impact of blastocyst culture and vitrification. Hum Reprod Open 2021;2021:hoab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu HM, Li Q, Rouse DJ, Alexander GR. Placenta previa: neonatal death after live births in the United States. Am J Obstet Gynecol 2003;188:1305–1309. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham G, Leong M, Weissman A. A 10-year follow-up on the practice of luteal phase support using worldwide web-based surveys. Reprod Biol Endocrinol 2021;19:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjaerven R, Wilcox AJ, Lie RT, Irgens LM. Selective fertility and the distortion of perinatal mortality. Am J Epidemiol 1988;128:1352–1363. [DOI] [PubMed] [Google Scholar]
- Slattery MM, Morrison JJ. Preterm delivery. Lancet 2002;360:1489–1497. [DOI] [PubMed] [Google Scholar]
- Stern JE, Liu CL, Hwang SS, Dukhovny D, Diop H, Cabral H. Contributions to prematurity of maternal health conditions, subfertility, and assisted reproductive technology. Fertil Steril 2020;114:828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Liu CL, Hwang SS, Dukhovny D, Farland LV, Diop H, Coddington CC, Cabral H. Influence of placental abnormalities and pregnancy-induced hypertension in prematurity associated with various assisted reproductive technology techniques. J Clin Med 2021;10:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LA, Simmonds M, Duley L, Llewellyn A, Sharif S, Walker RAE, Beresford L, Wright K, Aboulghar MM, Alfirevic Z et al. Evaluating Progestogens for Preventing Preterm birth International Collaborative (EPPPIC): meta-analysis of individual participant data from randomised controlled trials. The Lancet 2021;397:1183–1194. [DOI] [PubMed] [Google Scholar]
- Sunkara SK, Antonisamy B, Redla AC, Kamath MS. Female causes of infertility are associated with higher risk of preterm birth and low birth weight: analysis of 117 401 singleton live births following IVF. Hum Reprod 2021;36:676–682. [DOI] [PubMed] [Google Scholar]
- Sverre J, Smedslund G, Stoinska-Schneider A, Kucuk B, Castaneda M, Refsdal T, Brurberg K. First-Trimester Screening for Preeclampsia with the Use of an Algorithm: A Health Technology Assessment. Norwegian Institute of Public Health, 2022. https://www.fhi.no/en/publ/25ampsia-with-the-use-of-an-algorithm/ (13 December 2022, date last accessed). [Google Scholar]
- Thomsen LC, Klungsøyr K, Roten LT, Tappert C, Araya E, Baerheim G, Tollaksen K, Fenstad MH, Macsali F, Austgulen R et al. Validity of the diagnosis of pre-eclampsia in the Medical Birth Registry of Norway. Acta Obstet Gynecol Scand 2013;92:943–950. [DOI] [PubMed] [Google Scholar]
- Thurin A, Hausken J, Hillensjö T, Jablonowska B, Pinborg A, Strandell A, Bergh C. Elective single-embryo transfer versus double-embryo transfer in in vitro fertilization. N Engl J Med 2004;351:2392–2402. [DOI] [PubMed] [Google Scholar]
- Tikkanen M. Placental abruption: epidemiology, risk factors and consequences. Acta Obstet Gynecol Scand 2011;90:140–149. [DOI] [PubMed] [Google Scholar]
- Valente MJ, Rijnhart JJM, Smyth HL, Muniz FB, MacKinnon DP. Causal mediation programs in R, Mplus, SAS, SPSS, and Stata. Struct Equ Modeling 2020;27:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods 2013;18:137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology 2010;21:540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. Policy-relevant proportions for direct effects. Epidemiology 2013;24:175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol 2010;172:1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermey BG, Buchanan A, Chambers GM, Kolibianakis EM, Bosdou J, Chapman MG, Venetis CA. Are singleton pregnancies after assisted reproduction technology (ART) associated with a higher risk of placental anomalies compared with non-ART singleton pregnancies? A systematic review and meta-analysis. BJOG 2019;126:209–218. [DOI] [PubMed] [Google Scholar]
- Wennerholm UB, Bergman L, Kuusela P, Ljungström E, Möller AC, Hongslo Vala C, Ekelund AC, Liljegren A, Petzold M, Sjögren P et al Progesterone, cerclage, pessary, or acetylsalicylic acid for prevention of preterm birth in singleton and multifetal pregnancies—a systematic review and meta-analyses. Front Med (Lausanne) 2023;10:1111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westvik-Johari K, Romundstad LB, Lawlor DA, Bergh C, Gissler M, Henningsen AA, Håberg SE, Wennerholm UB, Tiitinen A, Pinborg A et al. Separating parental and treatment contributions to perinatal health after fresh and frozen embryo transfer in assisted reproduction: a cohort study with within-sibship analysis. PLoS Med 2021;18:e1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyns C, De Geyter C, Calhaz-Jorge C, Kupka MS, Motrenko T, Smeenk J, Bergh C, Tandler-Schneider A, Rugescu IA, Vidakovic S et al. ; European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE). ART in Europe, 2017: results generated from European registries by ESHRE. Hum Reprod Open 2021;2021:hoab026.34377841 [Google Scholar]
- Zaat T, Zagers M, Mol F, Goddijn M, van Wely M, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev 2021;2:CD011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The research data cannot be shared publicly due to national data protection regulations but may be accessed from a server at Statistics Denmark, after approval by the relevant Ethics Committees, the responsible research institutions, and registry keeping authorities in each country.