Abstract
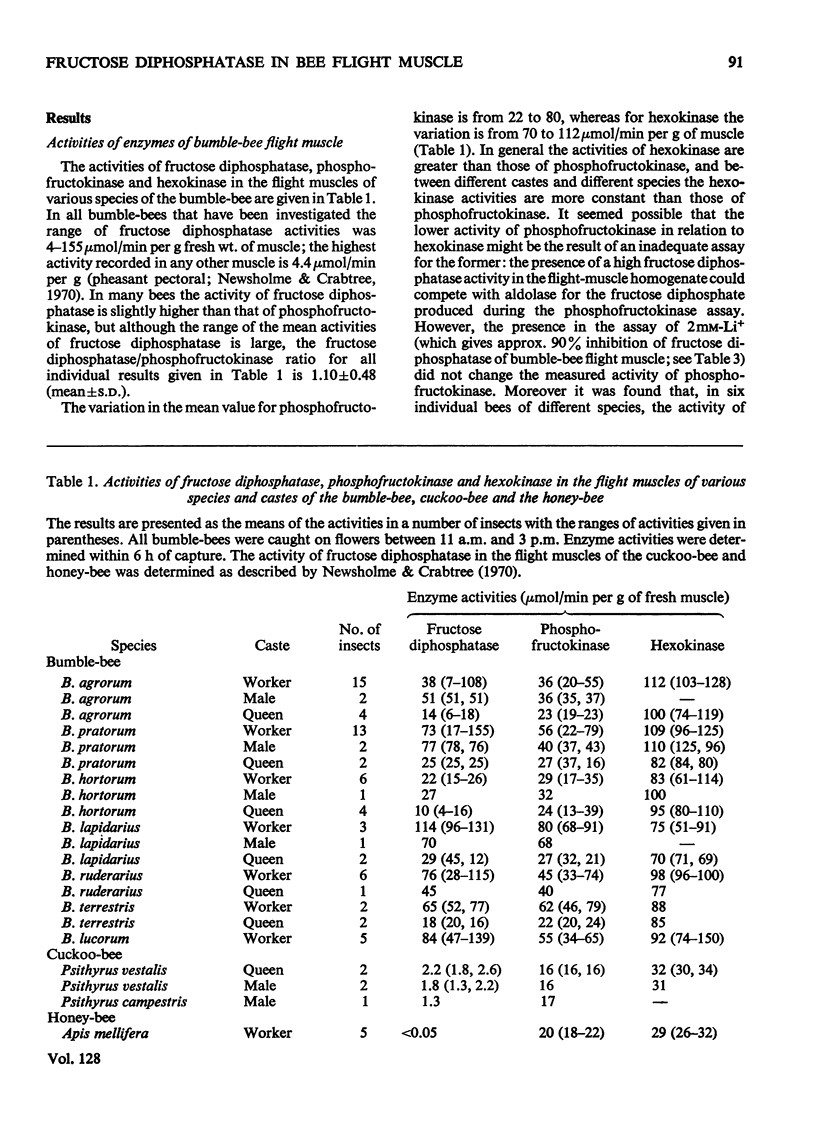
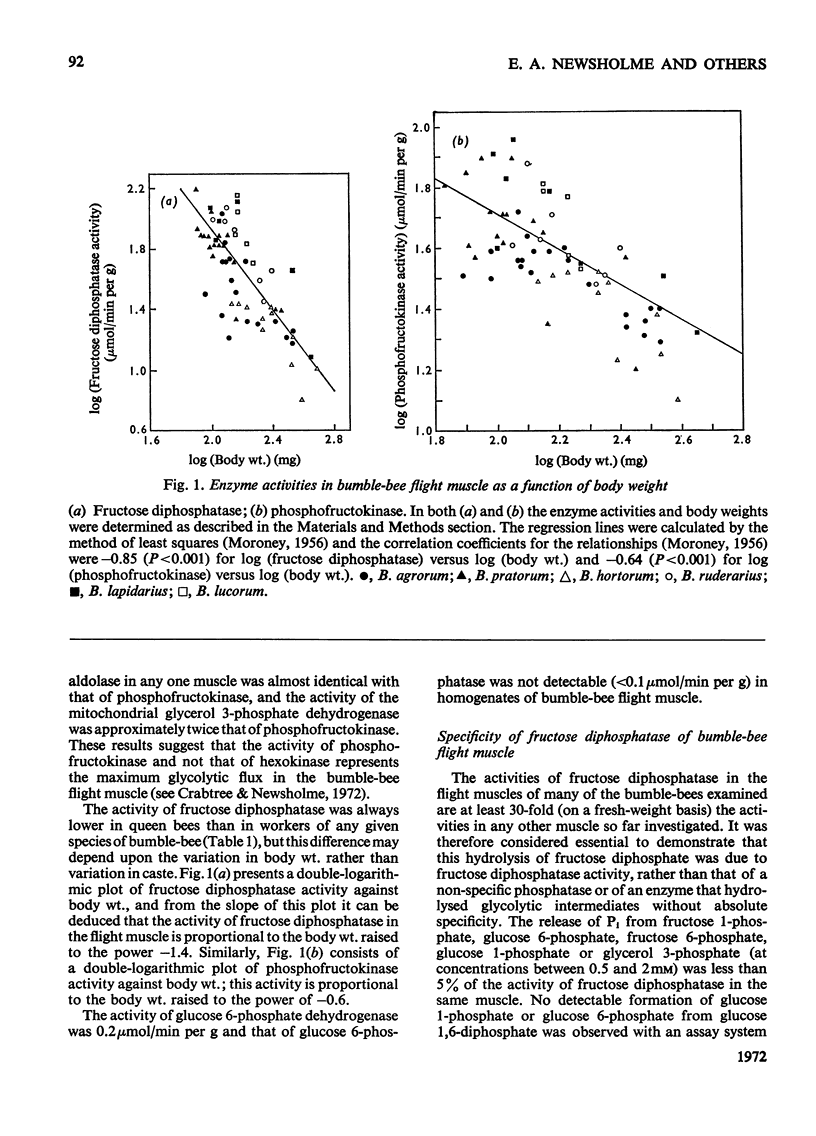
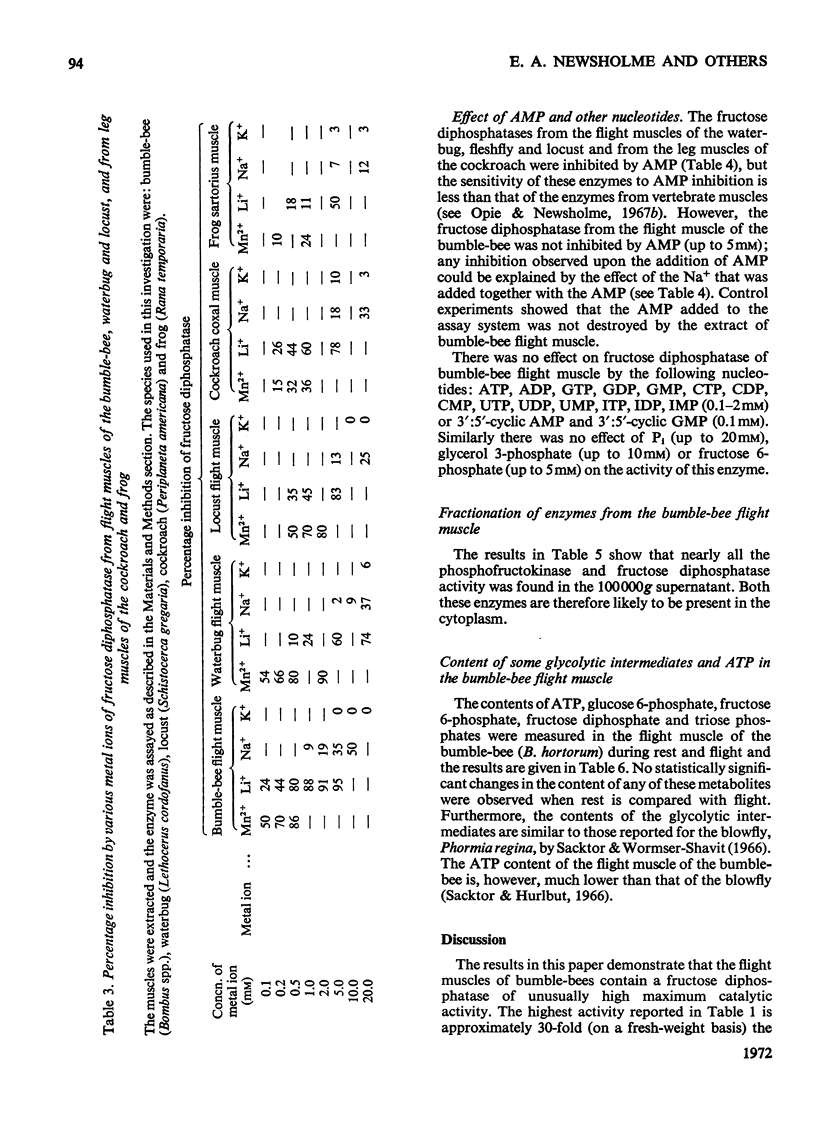
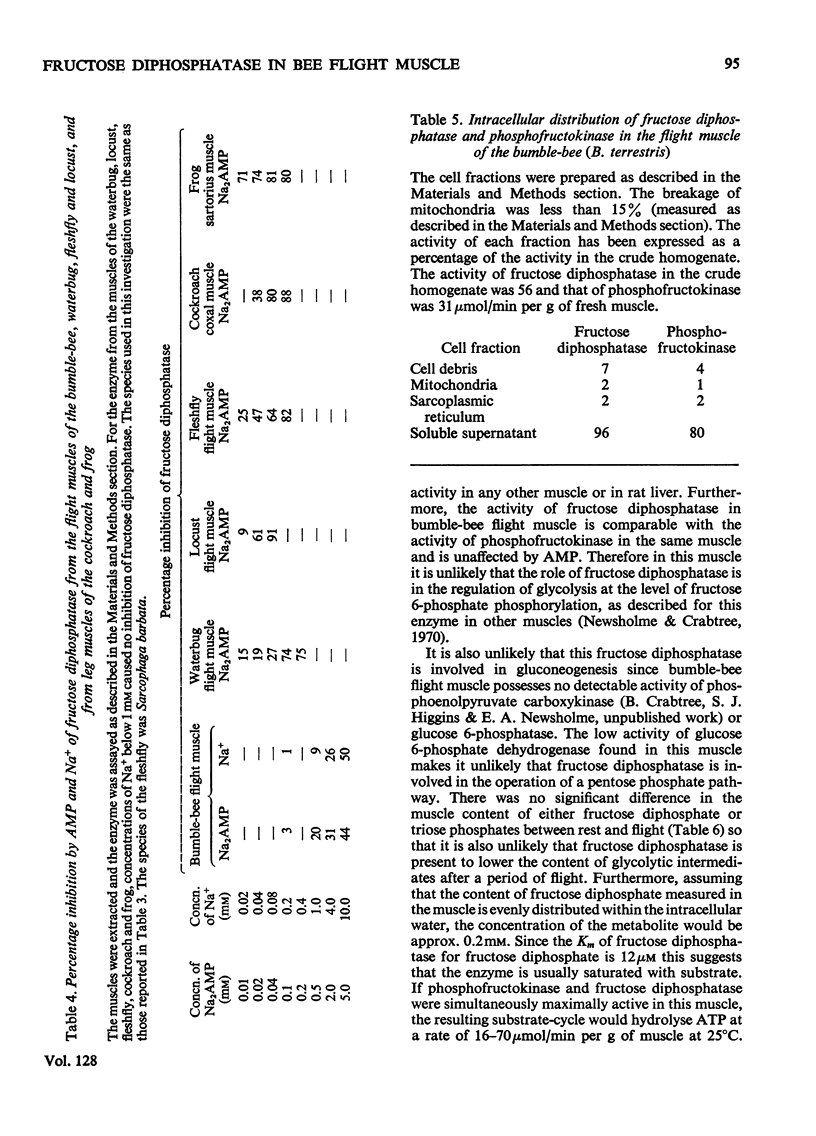
1. The maximum catalytic activities of fructose diphosphatase from flight muscles of bumble-bees (Bombus spp.) are at least 30-fold those reported for the enzyme from other tissues. The maximum activity of fructose diphosphatase in the flight muscle of any particular bee is similar to that of phosphofructokinase in the same muscle, and the activity of hexokinase is similar to or greater than the activity of phosphofructokinase. There is no detectable activity of glucose 6-phosphatase and only a very low activity of glucose 6-phosphate dehydrogenase in these muscles. The activities of both fructose diphosphatase and phosphofructokinase vary inversely with the body weight of the bee, whereas that of hexokinase is relatively constant. 2. There is no significant hydrolysis of fructose 1-phosphate, fructose 6-phosphate, glucose 1,6-diphosphate and glycerol 3-phosphate by extracts of bumble-bee flight muscle. 3. Fructose 1,6-diphosphatase from bumble-bee flight muscle and from other muscles is inhibited by Mn2+ and univalent cations; the potency of inhibition by the latter varies in the order Li+>Na+>K+. However, the fructose diphosphatase from bumble-bee flight muscle is different from the enzyme from other tissues in that it is not inhibited by AMP. 4. The contents of ATP, hexose monophosphates, fructose diphosphate and triose phosphates in bumble-bee flight muscle showed no significant changes between rest and flight. 5. It is proposed that both fructose diphosphatase and phosphofructokinase are simultaneously active and catalyse a cycle between fructose 6-phosphate and fructose diphosphate in resting bumble-bee flight muscle. Such a cycle would produce continuous hydrolysis of ATP, with the release of energy as heat, which would help to maintain the thoracic temperature during rest periods at a level adequate for flight.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree B., Newsholme E. A. The activities of phosphorylase, hexokinase, phosphofructokinase, lactate dehydrogenase and the glycerol 3-phosphate dehydrogenases in muscles from vertebrates and invertebrates. Biochem J. 1972 Jan;126(1):49–58. doi: 10.1042/bj1260049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., WOODFORD M. FRUCTOSE 1, 6-DIPHOSPHATASE IN STRIATED MUSCLE. Biochem J. 1965 Feb;94:436–445. doi: 10.1042/bj0940436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. The role of fructose-1,6-diphosphatase in the regulation of glycolysis in skeletal muscle. FEBS Lett. 1970 Apr 2;7(2):195–198. doi: 10.1016/0014-5793(70)80155-7. [DOI] [PubMed] [Google Scholar]
- Newsholme E. A., Robinson J., Taylor K. A radiochemical enzymatic activity assay for glycerol kinase and hexokinase. Biochim Biophys Acta. 1967 Mar 15;132(2):338–346. doi: 10.1016/0005-2744(67)90153-2. [DOI] [PubMed] [Google Scholar]
- Opie L. H., Newsholme E. A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem J. 1967 May;103(2):391–399. doi: 10.1042/bj1030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie L. H., Newsholme E. A. The inhibition of skeletal-muscle fructose 1,6-diphosphatase by adenosine monophosphate. Biochem J. 1967 Aug;104(2):353–360. doi: 10.1042/bj1040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor B., Hurlbut E. C. Regulation of metabolism in working muscle in vivo. II. Concentrations of adenine nucleotides, arginine phosphate, and inorganic phosphate in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):632–634. [PubMed] [Google Scholar]
- Sacktor B., Wormser-Shavit E. Regulation of metabolism in working muscle in vivo. I. Concentrations of some glycolytic, tricarboxylic acid cycle, and amino acid intermediates in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):624–631. [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wallace J. C., Newsholme E. A. A comparison of the properties of fructose 1,6-diphosphatase, and the activities of other key enzymes of carbohydrate metabolism, in the livers of embryonic and adult rat, sheep and domestic fowl. Biochem J. 1967 Aug;104(2):378–384. doi: 10.1042/bj1040378. [DOI] [PMC free article] [PubMed] [Google Scholar]


