Abstract
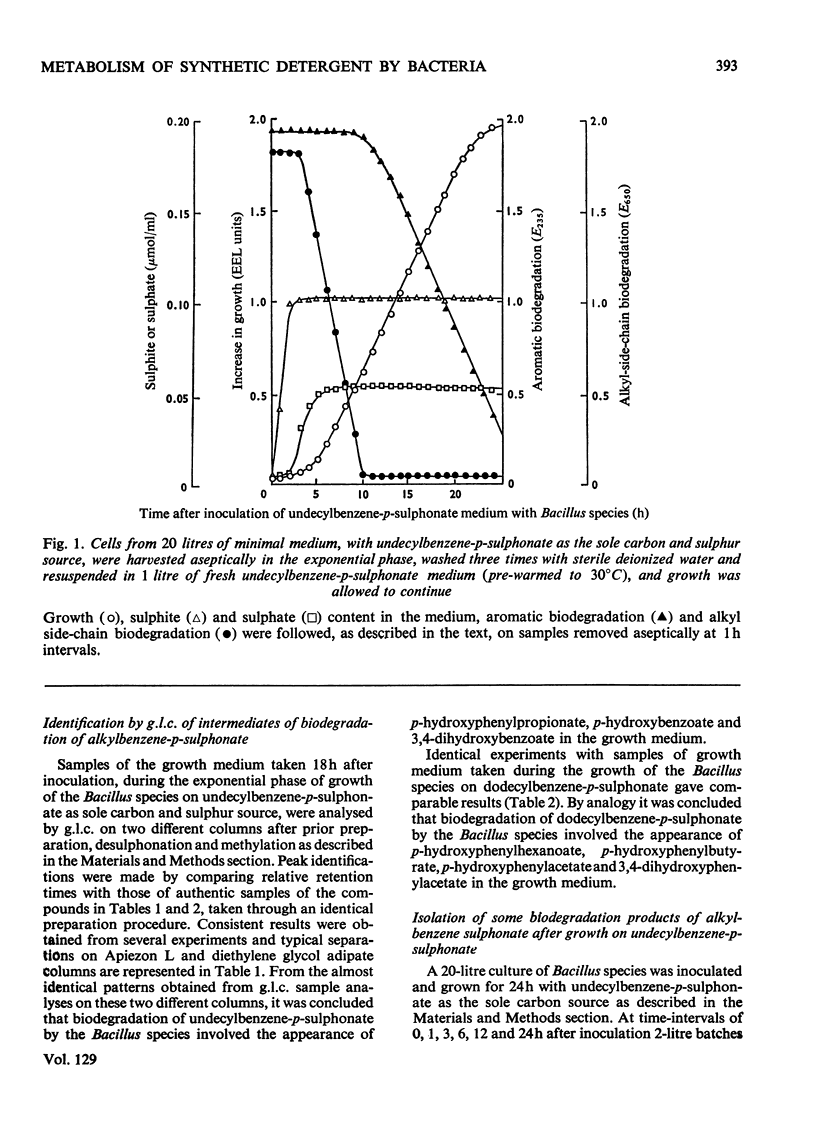
1. A study was made of the biodegradation of alkylbenzene sulphonate homologues, one of the major components of commercially marketed detergents. A Bacillus species was elected for growth on alkylbenzene sulphonate homologues as the sole source of carbon and sulphur. 2. The results from both whole-cell and cell-free systems indicated that the alkyl, aryl and sulphonate moieties of alkylbenzene sulphonate homologues were all further metabolized by the Bacillus species. 3. The alkyl side chain, after a presumed initial oxidation of the terminal methyl group, was subsequently oxidized by a β-oxidation pathway. Three enzymes of the β-oxidation pathway, i.e. acyl-CoA synthetase, acyl-CoA dehydrogenase and β-hydroxyacyl-CoA dehydrogenase, were identified in cell-free extracts of the detergent-grown Bacillus species. The substrate specificity of acyl-CoA synthetase indicated activity towards several alkylbenzene sulphonate homologues. 4. The sulphonate moiety was released as sulphite by a desulphonating enzyme. Some kinetic properties of this enzyme were determined. The sulphite was subsequently metabolized to either sulphate or adenosine 5′-sulphatophosphate. Two enzymes involved in sulphite metabolism, i.e. sulphite–cytochrome c reductase and adenosine 5′-sulphatophosphate–cytochrome c reductase were detected in cell-free extracts of undecylbenzene-p-sulphonate-grown Bacillus species. 5. The combined results of continuous sampling programmes monitored by both t.l.c. and sulphite appearance in the growth medium indicated that desulphonation of the aromatic moiety was the likely first step in the overall biodegradation of several alkylbenzene sulphonate homologues. 6. The presence of p-hydroxyphenylpropionate, p-hydroxybenzoate and 3,4-dihydroxybenzoate in cells after growth on several alkylbenzene sulphonate homologues containing an odd number of carbon atoms in the side chain was confirmed by g.l.c. and t.l.c. analysis. Cells grown on several homologues containing an even number of carbon atoms in the side chain were shown to contain p-hydroxyphenylacetate and 3,4-dihydroxyphenylacetate. 7. The aromatic nucleus obtained from undecylbenzene-p-sulphonate was further metabolized by an oxidation sequence involving an `ortho-cleavage' route. 8. An overall metabolic pathway for the biodegradation of various alkylbenzene sulphonate homologues by this Bacillus species is proposed.
Full text
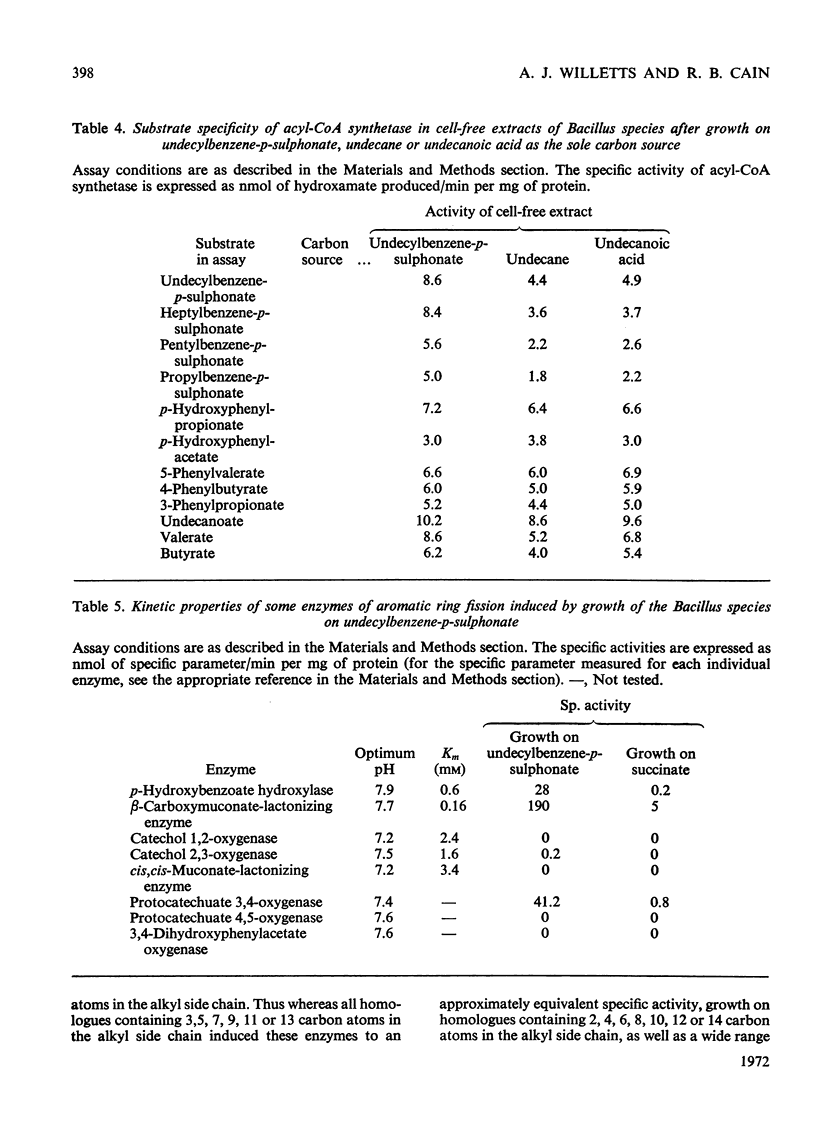
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
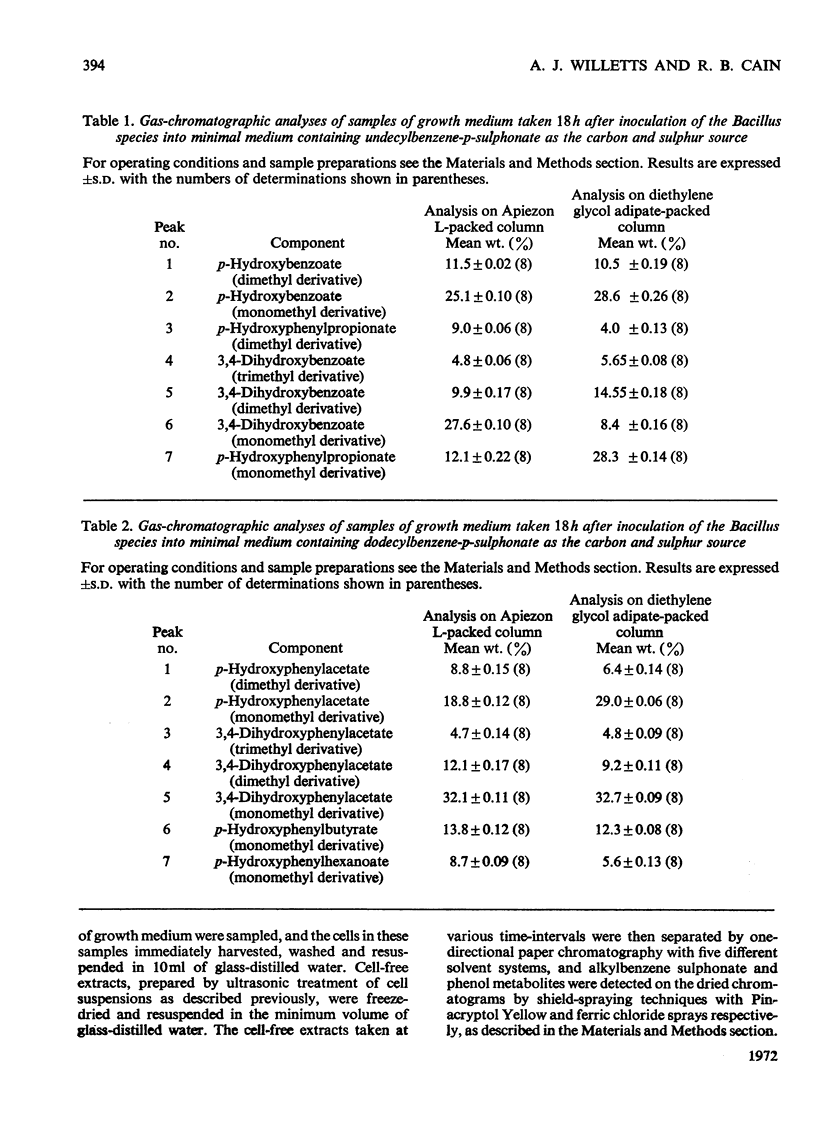
- ADACHI K., TAKEDA Y., SENOH S., KITA H. METABOLISM OF P-HYDROXYPHENYLACETIC ACID IN PSEUDOMONAS OVALIS. Biochim Biophys Acta. 1964 Dec 9;93:483–493. doi: 10.1016/0304-4165(64)90332-0. [DOI] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
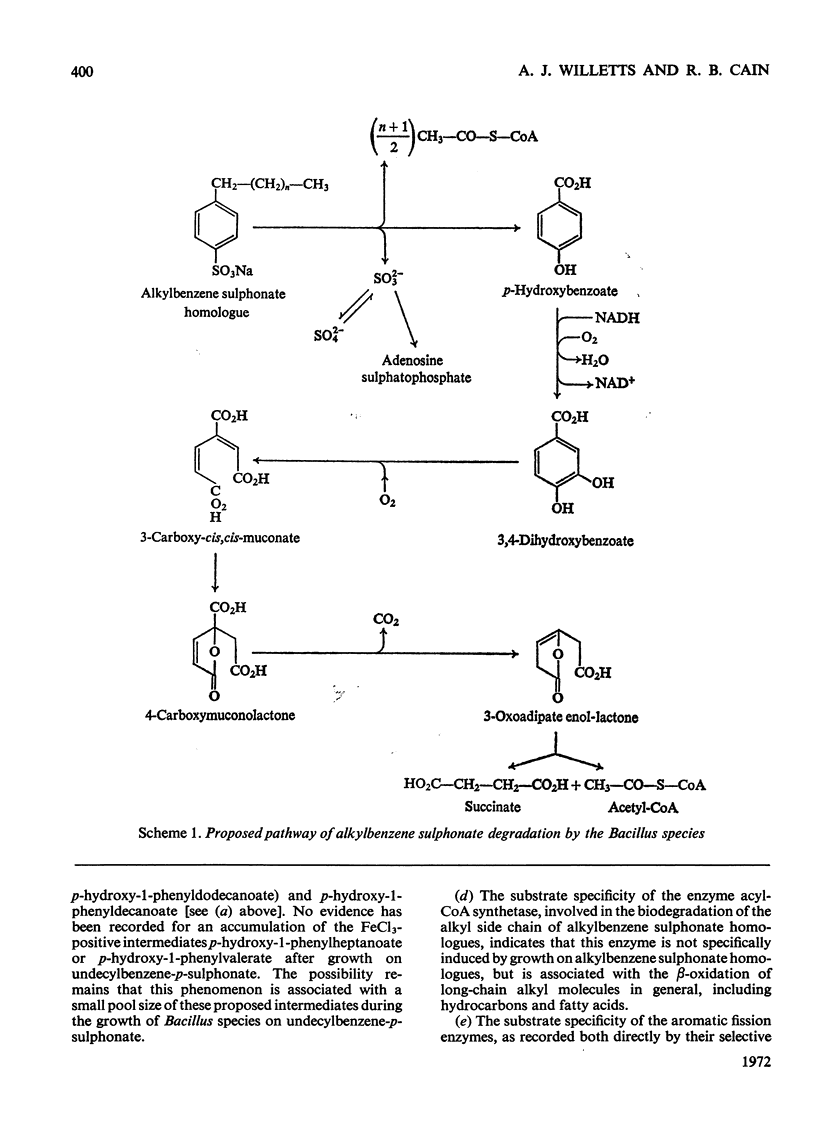
- CAIN R. B. The metabolism of protocatechuic acid by a vibrio. Biochem J. 1961 May;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain R. B., Bilton R. F., Darrah J. A. The metabolism of aromatic acids by micro-organisms. Metabolic pathways in the fungi. Biochem J. 1968 Aug;108(5):797–828. doi: 10.1042/bj1080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain R. B., Farr D. R. Metabolism of arylsulphonates by micro-organisms. Biochem J. 1968 Feb;106(4):859–877. doi: 10.1042/bj1060859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., GIBSON D. T. THE BACTERIAL DEGRADATION OF CATECHOL. Biochem J. 1965 May;95:466–474. doi: 10.1042/bj0950466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Evans W. C. The metabolism of aromatic compounds by Rhodopseudomonas palustris. A new, reductive, method of aromatic ring metabolism. Biochem J. 1969 Jul;113(3):525–536. doi: 10.1042/bj1130525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. E., MII S., MAHLER H. R., BOCK R. M. Studies on the fatty acid oxidizing system of animal tissues. III. Butyryl coenzyme A dehydrogenase. J Biol Chem. 1954 Jan;206(1):1–12. [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K., Stanier R. Y. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966 May 25;241(10):2453–2460. [PubMed] [Google Scholar]
- LYNEN F. Lipide metabolism. Annu Rev Biochem. 1955;24:653–688. doi: 10.1146/annurev.bi.24.070155.003253. [DOI] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. I. Survey of enzymes and properties of sulfite: cytochrome c oxidoreductase. Can J Biochem. 1970 Mar;48(3):334–343. doi: 10.1139/o70-056. [DOI] [PubMed] [Google Scholar]
- MARTIN R. O., STUMPF P. K. Fat metabolism in higher plants. XII. alpha-Oxidation of long chain fatty acids. J Biol Chem. 1959 Oct;234:2548–2554. [PubMed] [Google Scholar]
- McKenna E. J., Kallio R. E. The biology of hydrocarbons. Annu Rev Microbiol. 1965;19:183–208. doi: 10.1146/annurev.mi.19.100165.001151. [DOI] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Peterson J. A., Kusunose M., Kusunose E., Coon M. J. Enzymatic omega-oxidation. II. Function of rubredoxin as the electron carrier in omega-hydroxylation. J Biol Chem. 1967 Oct 10;242(19):4334–4340. [PubMed] [Google Scholar]
- ROBINSON H. C. THE REDUCTION OF INORGANIC SULPHATE TO INORGANIC SULPHITE IN THE SMALL INTESTINE OF THE RAT. Biochem J. 1965 Mar;94:687–691. doi: 10.1042/bj0940687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripin M. J., Noon K. F., Cook T. M. Bacterial metabolism of arylsulfonates. I. Benzene sulfonate as growth substrate for Pseudomonas testosteroni H-8. Appl Microbiol. 1971 Mar;21(3):495–499. doi: 10.1128/am.21.3.495-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tuner J. M. Microbial metabolism of amino ketones. Aminoacetone formation from 1-aminopropan-2-ol by a dehydrgenase in Escerichia coli. Biochem J. 1966 May;99(2):427–433. doi: 10.1042/bj0990427. [DOI] [PMC free article] [PubMed] [Google Scholar]


