Abstract
1. The main purpose of this study was to quantify the adaptation of spinal motoneurons to sustained and intermittent activation, using an extracellular route of stimulating current application to single test cells, in contrast to an intracellular route, as has been used previously. In addition, associations were tested between firing rate properties of the tested cells and other type (size)-related properties of these cells and their motor units. 2. Motoneurons supplying the medial gastrocnemius muscle of the deeply anaesthetized cat were stimulated for 240 s with microelectrodes which passed sustained extracellular current at 1.25 times the threshold for repetitive firing. Many cells were also tested following a rest period with intermittent 1 s current pulses (duration 600 ms) at the same relative stimulus strength. Cell discharge was assessed from the EMG of the motor unit innervated by the test neuron. The motoneurons and their motor units were assigned to four categories (i.e. types FF, FR, S and F; where F = FF + FR) based on conventional criteria. In all, twenty F (16 FF, 4 FR) and fourteen S cells were studied with sustained stimulation. Thirty of these cells (17 F, 13 S) and an additional two cells (1 F, 1 S) were studied with intermittent stimulation. 3. The mean threshold current required for sustained firing for a period of > or = 2 s was not significantly different for F and S cells. However, most of the other measured parameters of motoneuron firing differed significantly for these two cell groups. For example, at 1.25 times the threshold current for repetitive firing, the mean firing duration in response to 240 s of sustained activation was 123 +/- 88 s (+/- S.D.) for F cells vs. 233 +/- 19 s for S cells. These values were significantly longer than those from a comparable, previously reported study that employed intracellular stimulation. With intermittent stimulation, the firing durations of F and S cells were not significantly different from each other. 4. All cells exhibited a delay from the onset of current to the first spike, followed by a brief accelerating discharge that was followed by a slower drop in firing rate. Some cells (21 of 34 with sustained activation; 20 of 32 with intermittent) exhibited doublet discharges (interspike intervals < or = 10 ms) that were intermingled with the more predominant singlet discharges. Doublets were more common in the S cell type.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




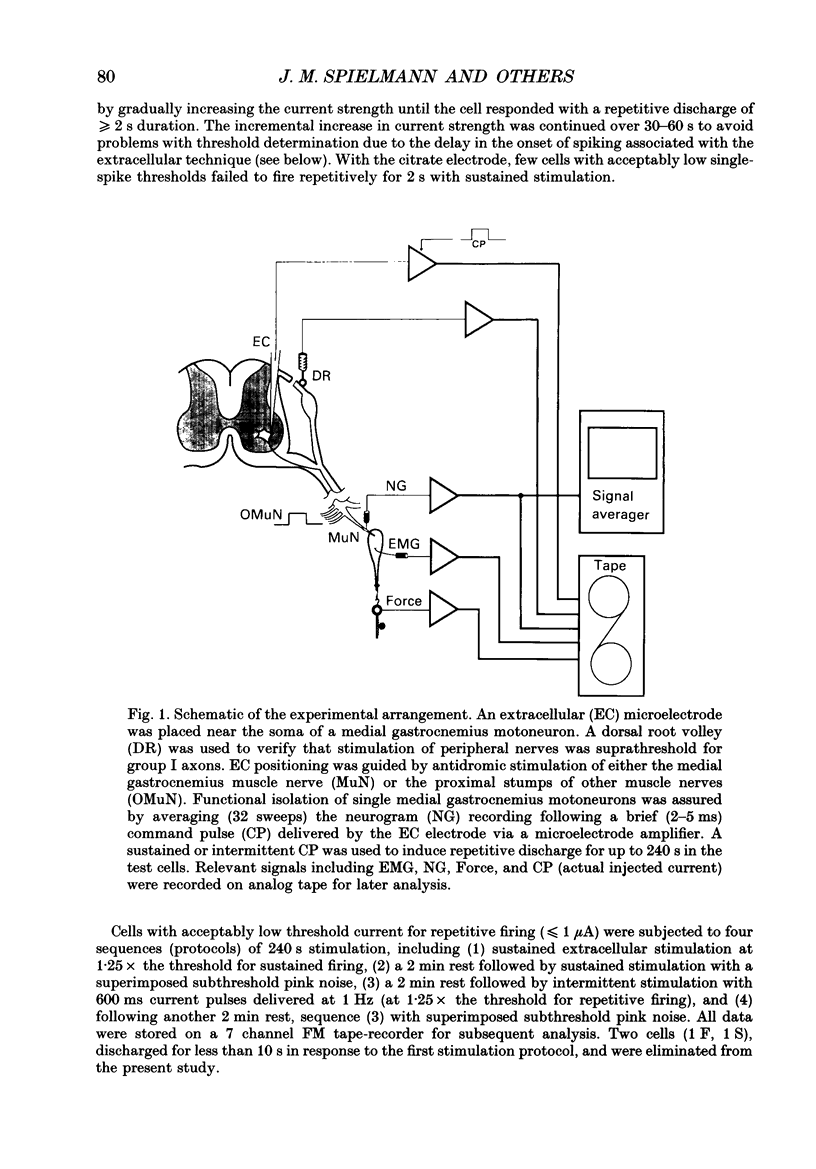
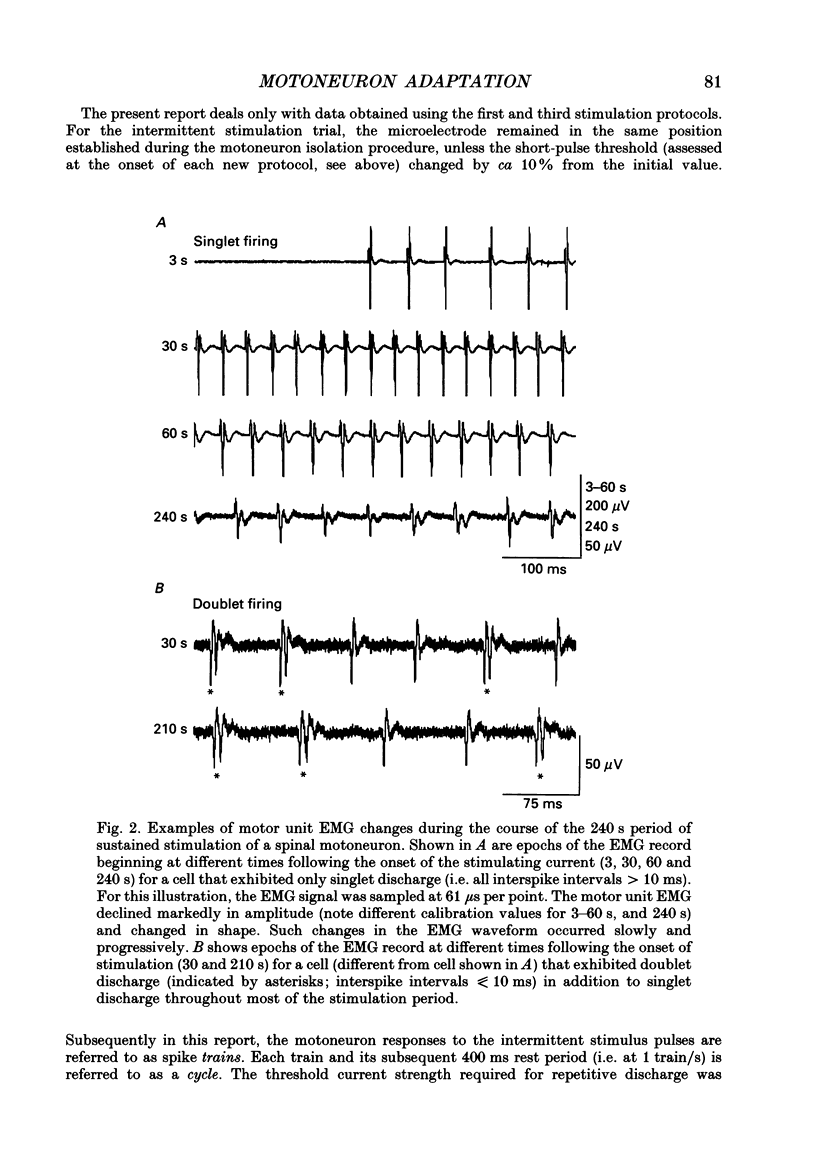
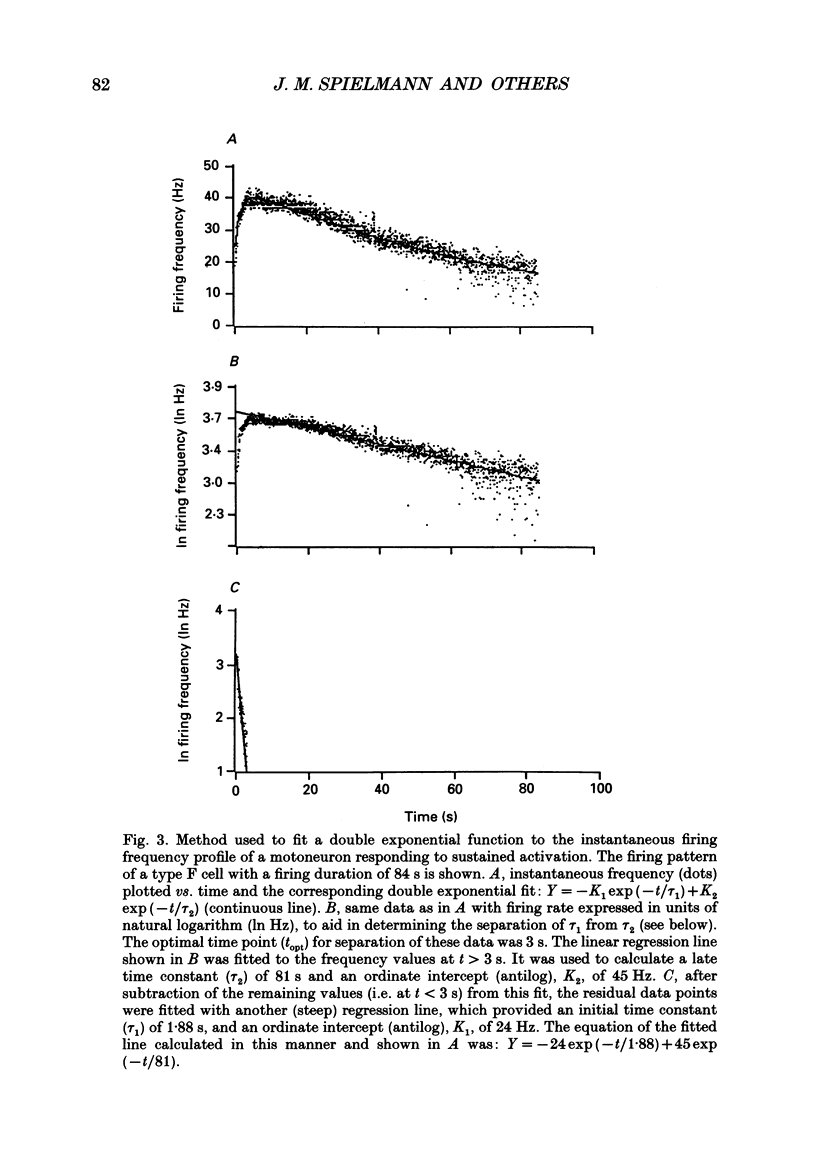



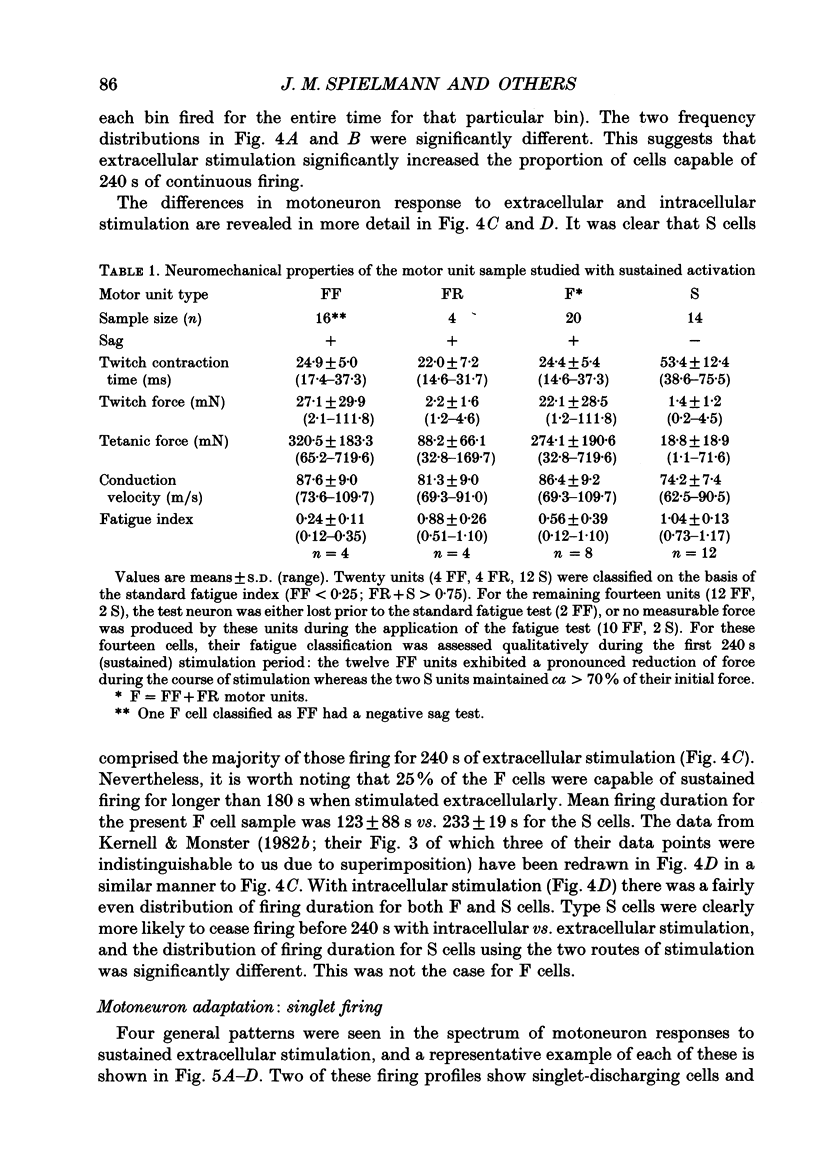
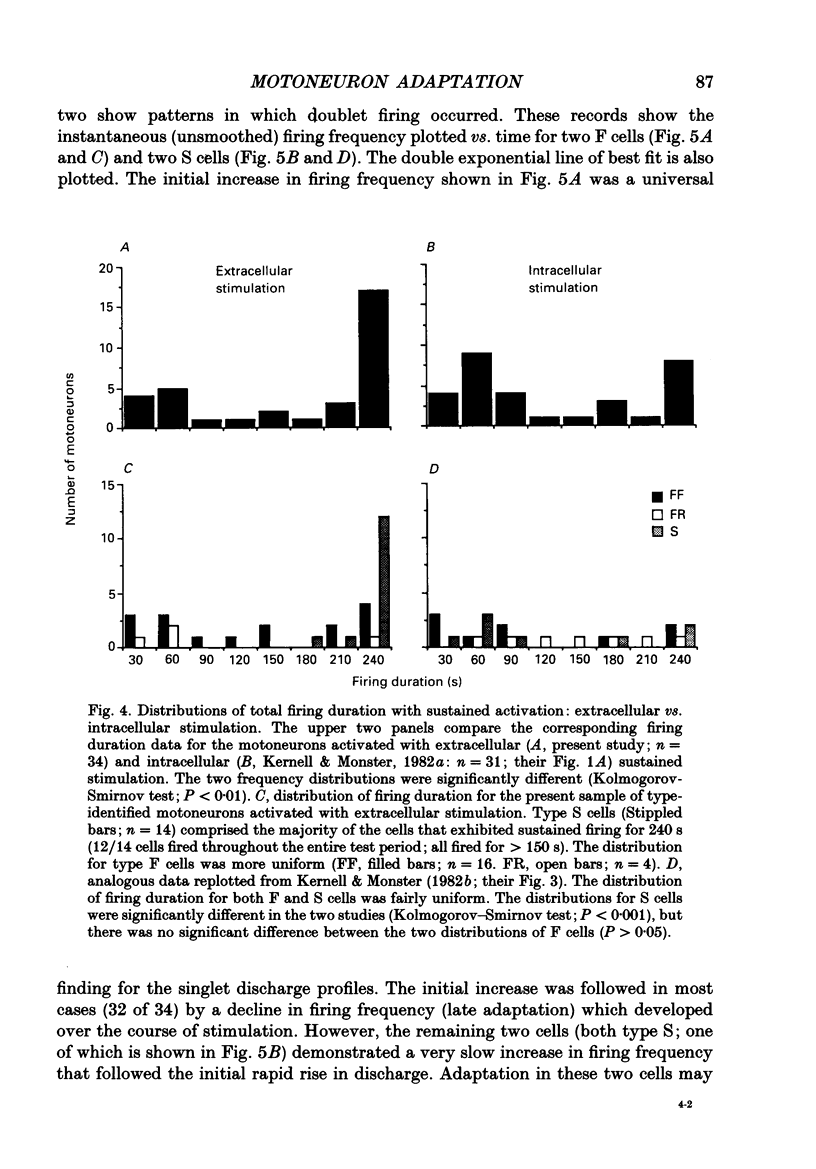
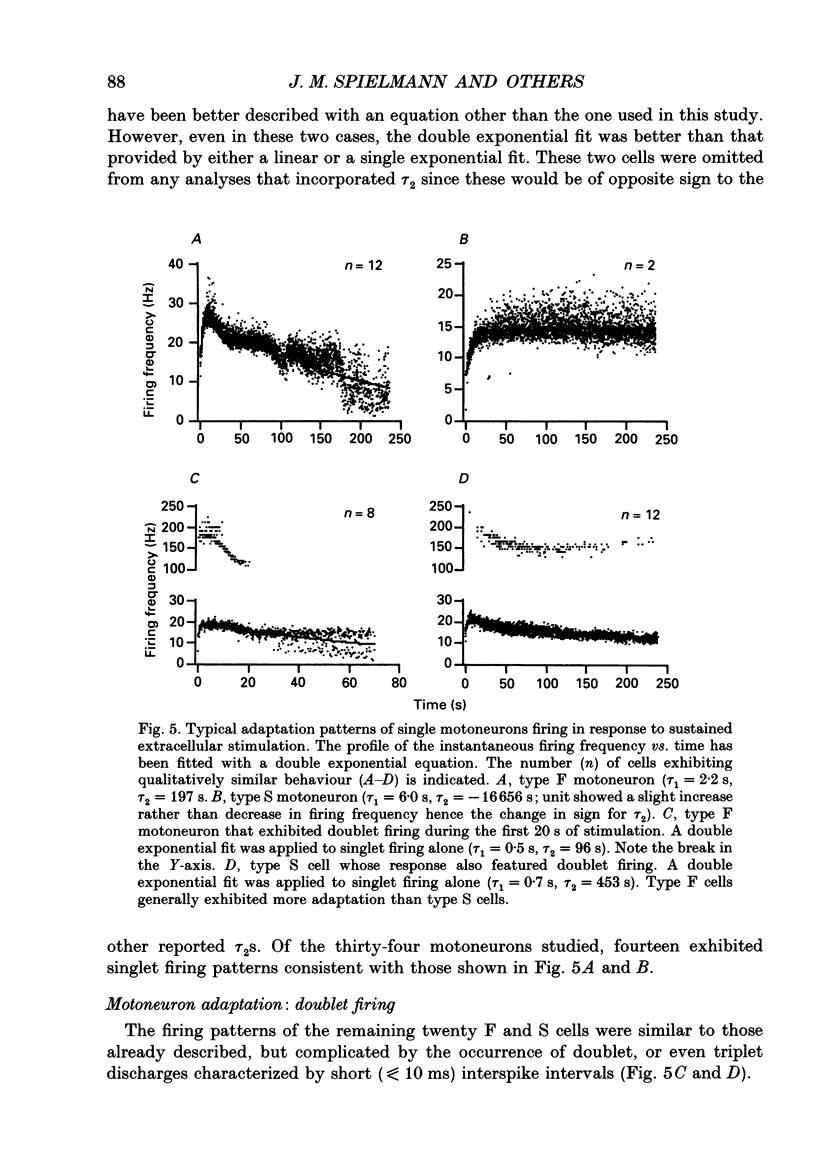


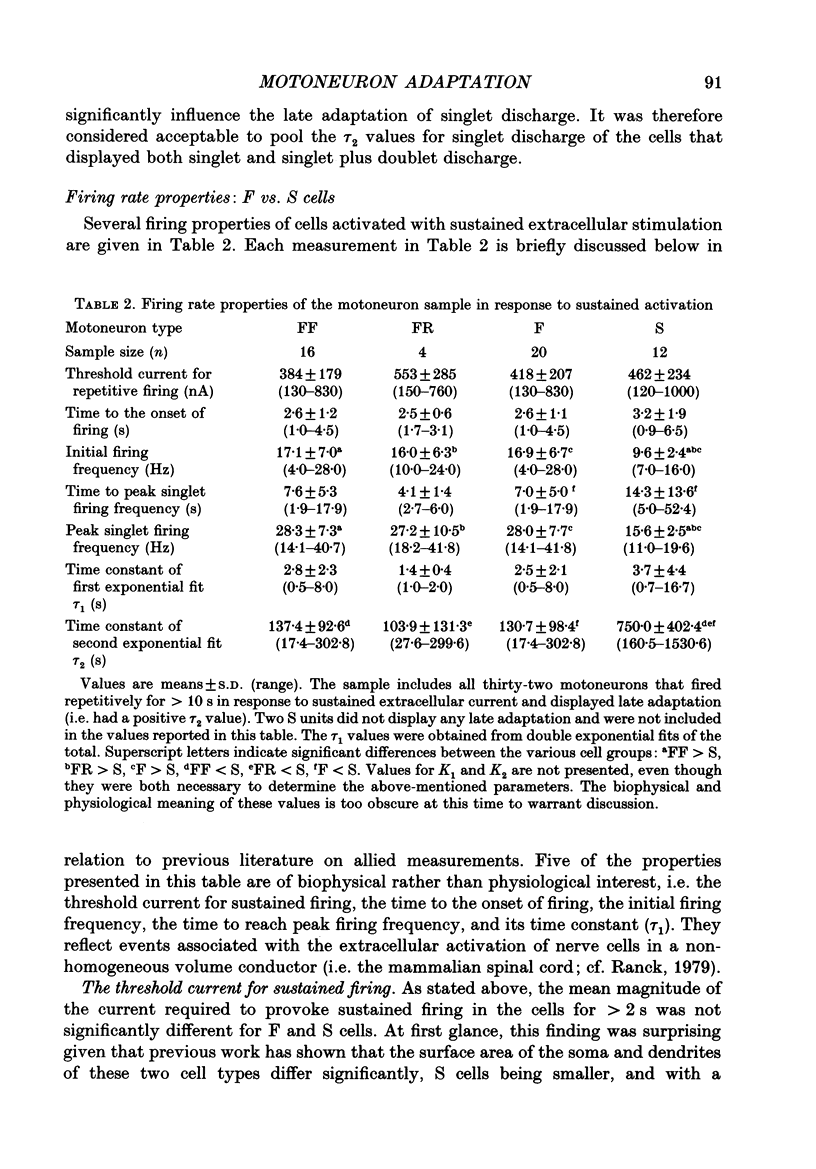








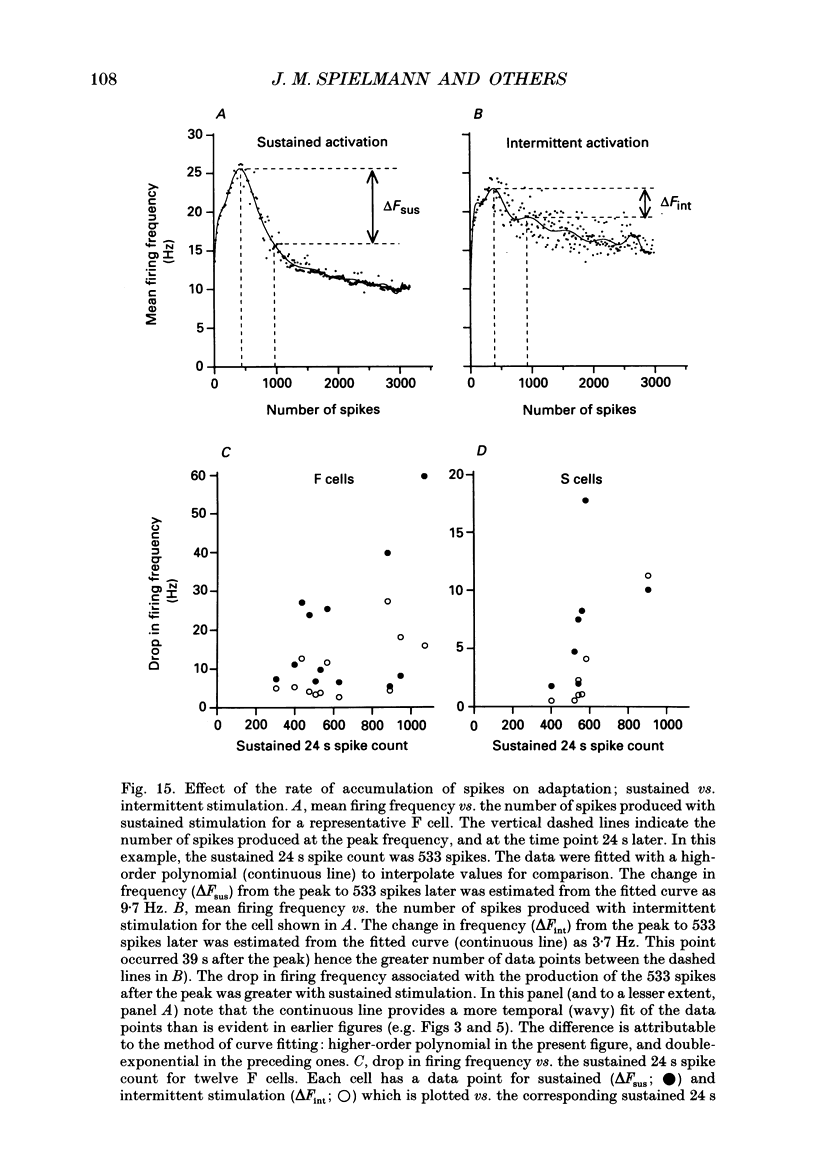












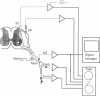
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., BRIDGER J. E. Neuron size and neuron population density in the lumbosacral region of the cat's spinal cord. J Anat. 1961 Jan;95:38–53. [PMC free article] [PubMed] [Google Scholar]
- Baldissera F., Gustafsson B. Firing behaviour of a neurone model based on the afterhyperpolarization conductance time course and algebraical summation. Adaptation and steady state firing. Acta Physiol Scand. 1974 Sep;92(1):27–47. doi: 10.1111/j.1748-1716.1974.tb05720.x. [DOI] [PubMed] [Google Scholar]
- Baldissera F. Relationships between the spike components and the delayed depolarization in cat spinal neurones. J Physiol. 1976 Jul;259(2):325–338. doi: 10.1113/jphysiol.1976.sp011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Barrett J. N., Crill W. E. Voltage-sensitive outward currents in cat motoneurones. J Physiol. 1980 Jul;304:251–276. doi: 10.1113/jphysiol.1980.sp013323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron D. H., Matthews B. H. The interpretation of potential changes in the spinal cord. J Physiol. 1938 Apr 14;92(3):276–321. doi: 10.1113/jphysiol.1938.sp003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P., Calancie B. Repetitive doublets in human flexor carpi radialis muscle. J Physiol. 1983 Jun;339:123–132. doi: 10.1113/jphysiol.1983.sp014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B. R., Dawson N. J., Johansson R. S., Lippold O. C. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986 Oct;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigland-Ritchie B., Johansson R., Lippold O. C., Woods J. J. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983 Jul;50(1):313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Botterman B. R., Cope T. C. Maximum tension predicts relative endurance of fast-twitch motor units in the cat. J Neurophysiol. 1988 Oct;60(4):1215–1226. doi: 10.1152/jn.1988.60.4.1215. [DOI] [PubMed] [Google Scholar]
- Brownstone R. M., Jordan L. M., Kriellaars D. J., Noga B. R., Shefchyk S. J. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res. 1992;90(3):441–455. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H. Generation of spike trains in CNS neurons. Brain Res. 1975 Jan 24;84(1):1–22. doi: 10.1016/0006-8993(75)90796-9. [DOI] [PubMed] [Google Scholar]
- Chan C. Y., Hounsgaard J., Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol. 1988 Aug;402:751–771. doi: 10.1113/jphysiol.1988.sp017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka R. M., Stuart D. G. Neurobiology of muscle fatigue. J Appl Physiol (1985) 1992 May;72(5):1631–1648. doi: 10.1152/jappl.1992.72.5.1631. [DOI] [PubMed] [Google Scholar]
- Gogan P., Gustafsson B., Jankowska E., Tyc-Dumont S. On re-excitation of feline motoneurones: its mechanism and consequences. J Physiol. 1984 May;350:81–91. doi: 10.1113/jphysiol.1984.sp015189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. J., Clamann H. P. Effect of microelectrode impalement on the reflex discharge of motoneurons. Exp Neurol. 1977 Nov;57(2):451–458. doi: 10.1016/0014-4886(77)90081-4. [DOI] [PubMed] [Google Scholar]
- Grafe P., Rimpel J., Reddy M. M., ten Bruggencate G. Changes of intracellular sodium and potassium ion concentrations in frog spinal motoneurons induced by repetitive synaptic stimulation. Neuroscience. 1982;7(12):3213–3220. doi: 10.1016/0306-4522(82)90243-3. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976 Jun;258(1):33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. An investigation of threshold properties among cat spinal alpha-motoneurones. J Physiol. 1984 Dec;357:453–483. doi: 10.1113/jphysiol.1984.sp015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J Physiol. 1984 Nov;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm T. M., Sasaki S., Stuart D. G., Windhorst U., Yuan C. S. The measurement of single motor-axon recurrent inhibitory post-synaptic potentials in the cat. J Physiol. 1987 Jul;388:631–651. doi: 10.1113/jphysiol.1987.sp016635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer J. A., O'Donovan M. J., Pratt C. A., Loeb G. E. Discharge patterns of hindlimb motoneurons during normal cat locomotion. Science. 1981 Jul 24;213(4506):466–467. doi: 10.1126/science.7244644. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Sugano N., Loeb G. E., Marks W. B., O'Donovan M. J., Pratt C. A. Cat hindlimb motoneurons during locomotion. II. Normal activity patterns. J Neurophysiol. 1987 Feb;57(2):530–553. doi: 10.1152/jn.1987.57.2.530. [DOI] [PubMed] [Google Scholar]
- Kernell D., Monster A. W. Motoneurone properties and motor fatigue. An intracellular study of gastrocnemius motoneurones of the cat. Exp Brain Res. 1982;46(2):197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Kernell D., Monster A. W. Threshold current for repetitive impulse firing in motoneurones innervating muscle fibres of different fatigue sensitivity in the cat. Brain Res. 1981 Dec 14;229(1):193–196. doi: 10.1016/0006-8993(81)90756-3. [DOI] [PubMed] [Google Scholar]
- Kernell D., Monster A. W. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46(2):191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kernell D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res. 1979 Jan 5;160(1):159–162. doi: 10.1016/0006-8993(79)90612-7. [DOI] [PubMed] [Google Scholar]
- Koenig E., Stoehr M. The characteristics of double discharges in electromyography during steady weak contraction. Electromyogr Clin Neurophysiol. 1986 May;26(3):169–179. [PubMed] [Google Scholar]
- Llinás R., Lopez-Barneo J. Electrophysiology of mammalian tectal neurons in vitro. II. Long-term adaptation. J Neurophysiol. 1988 Sep;60(3):869–878. doi: 10.1152/jn.1988.60.3.869. [DOI] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. A commentary on muscle unit properties in cat hindlimb muscles. J Morphol. 1980 Nov;166(2):217–230. doi: 10.1002/jmor.1051660208. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- Palmer S. S., Fetz E. E. Discharge properties of primate forearm motor units during isometric muscle activity. J Neurophysiol. 1985 Nov;54(5):1178–1193. doi: 10.1152/jn.1985.54.5.1178. [DOI] [PubMed] [Google Scholar]
- Ranck J. B., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 1975 Nov 21;98(3):417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol. 1982 Oct;48(4):875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Zajac F. E., Young J. L. Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J Neurophysiol. 1980 May;43(5):1221–1235. doi: 10.1152/jn.1980.43.5.1221. [DOI] [PubMed] [Google Scholar]