Abstract
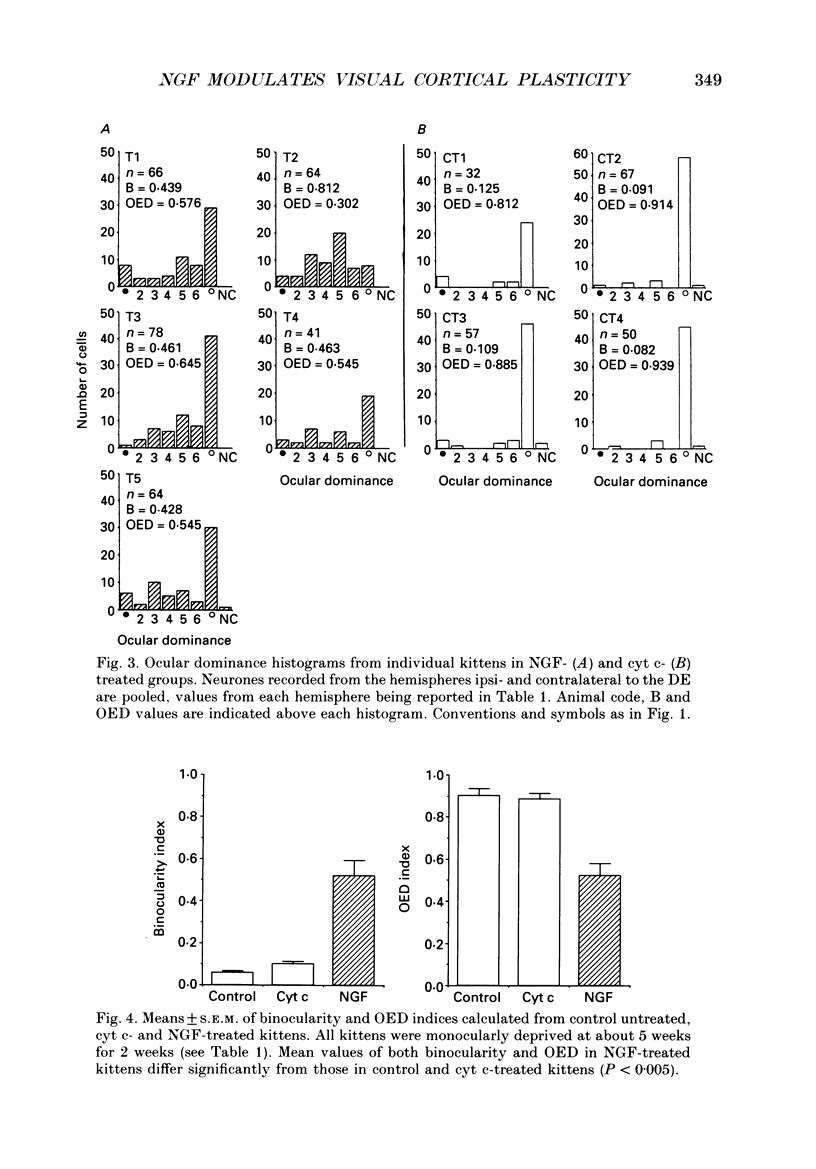
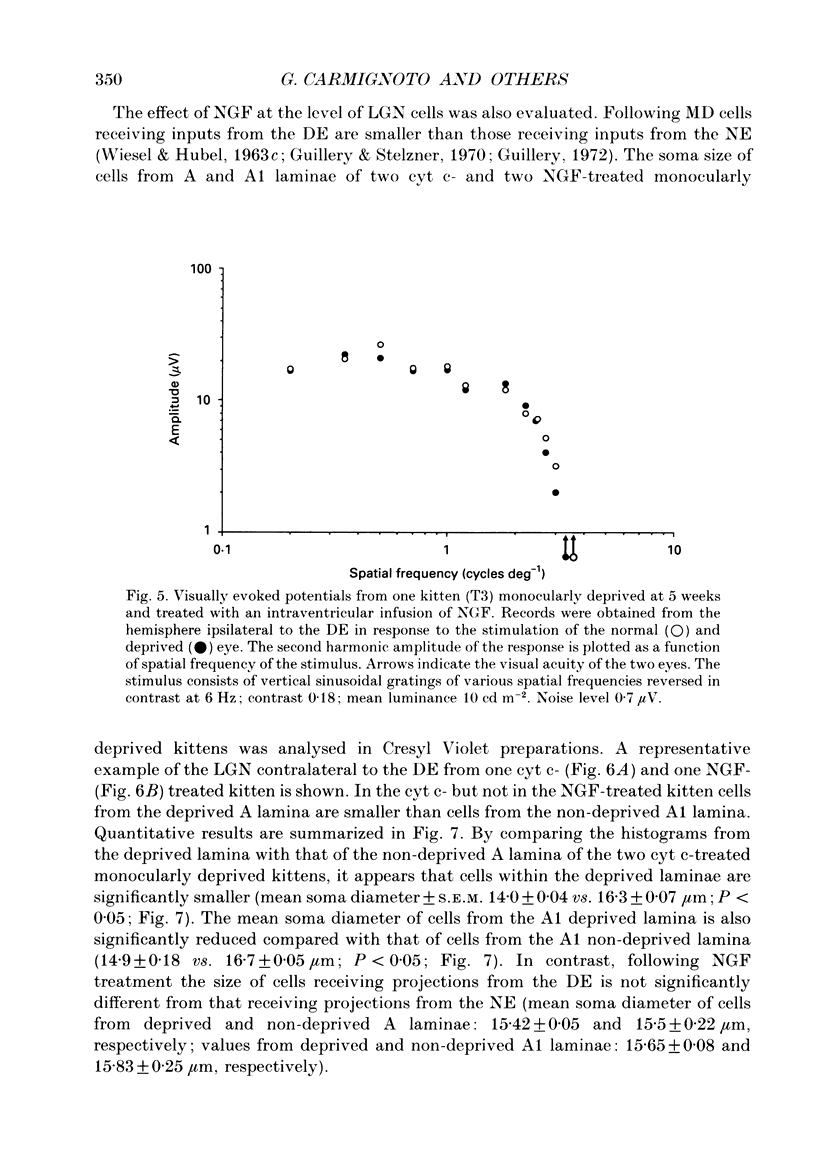
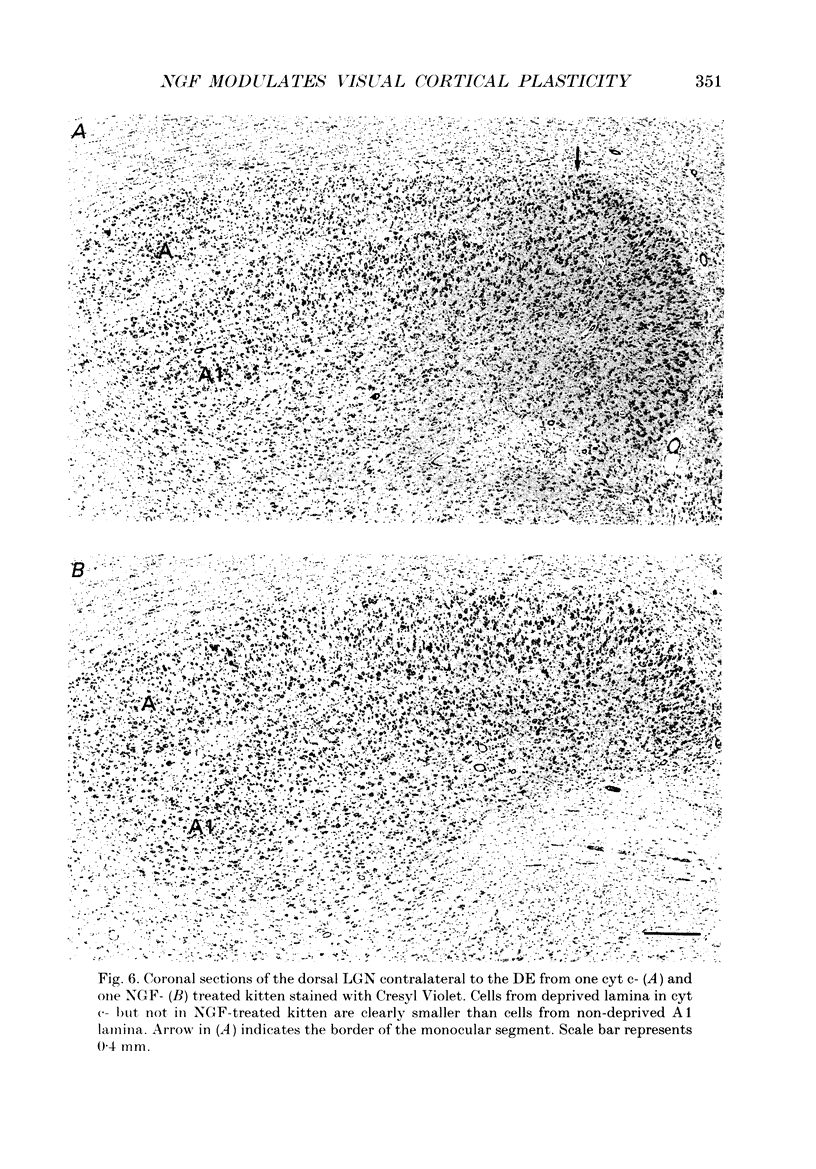
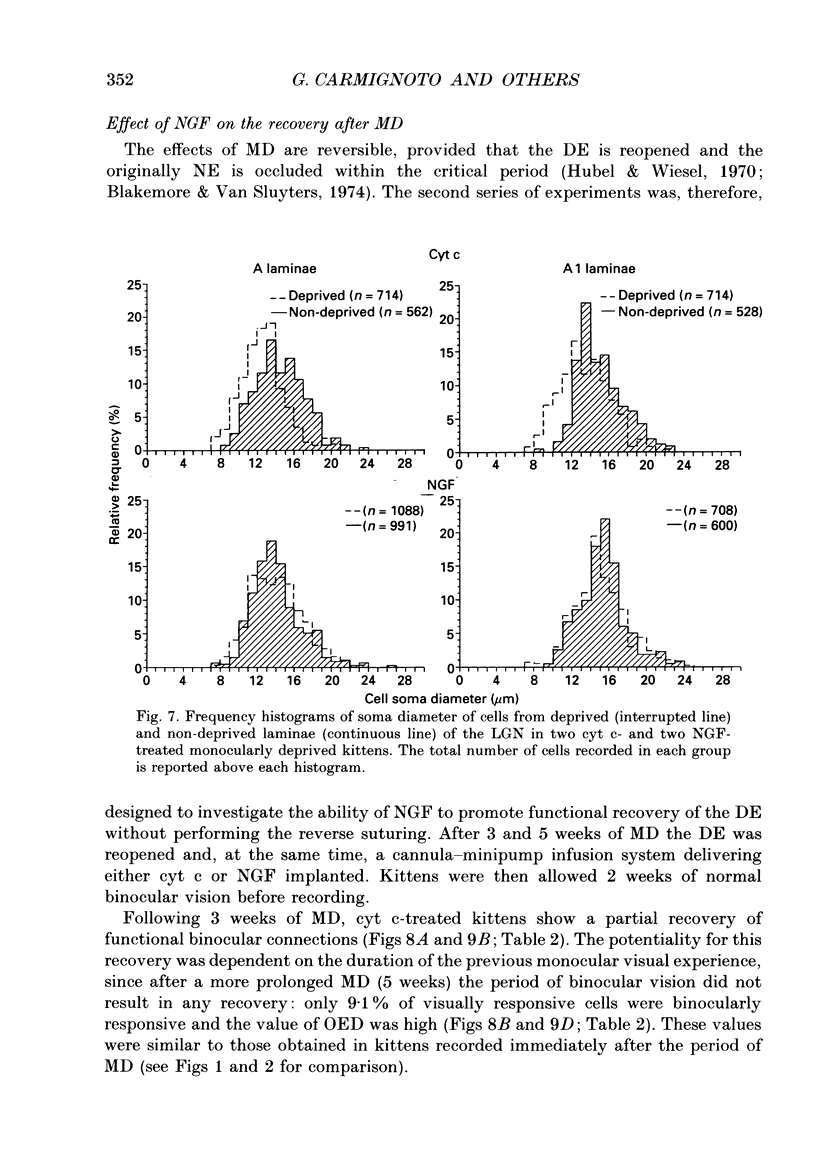
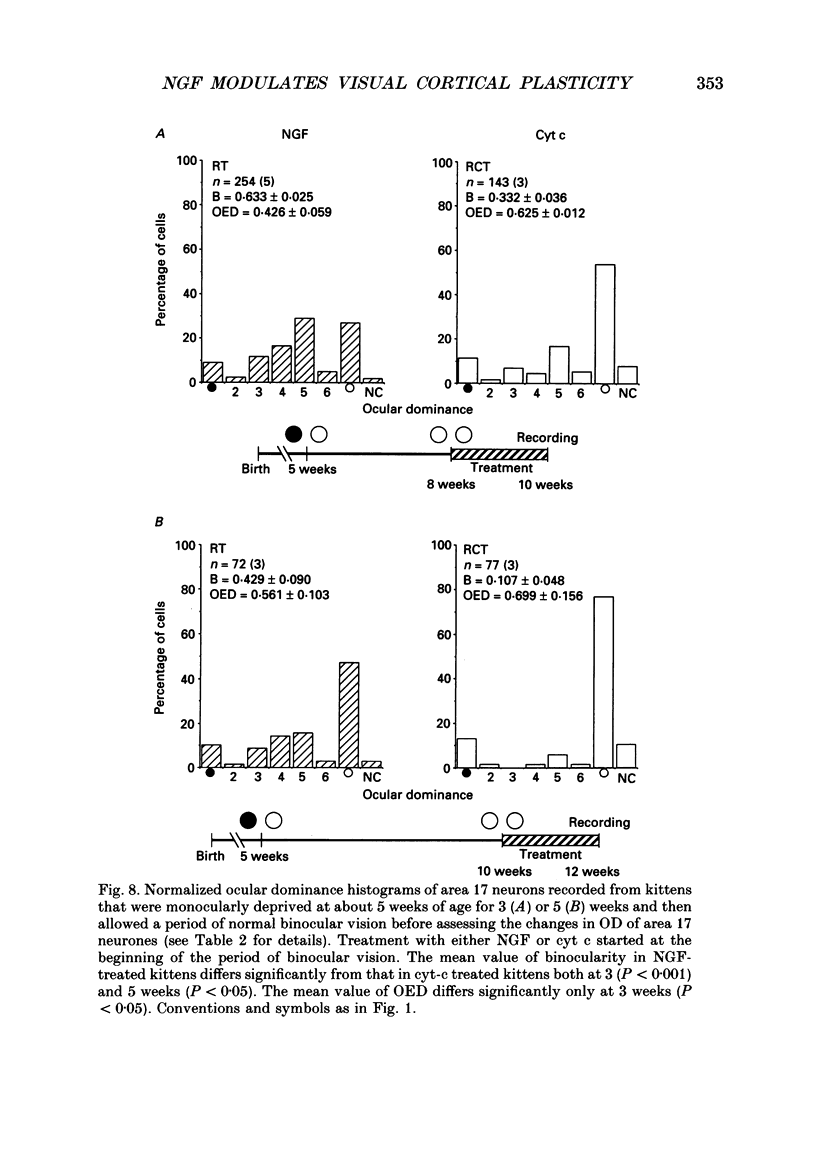
1. The effect of intraventricular administration of nerve growth factor (NGF) by means of a cannula-minipump system was studied in kittens monocularly deprived during the critical period. The ocular dominance of area 17 neurones of NGF-treated and control kittens was determined by conventional extracellular recordings. The soma size of cells in A and A1 laminae of the lateral geniculate nucleus (LGN) was also evaluated in Cresyl Violet preparations. 2. Binocularly responsive neurons were found to be significantly more numerous in NGF-treated than in control kittens. The shrinkage of cells from the deprived LGN laminae normally observed in control kittens was prevented by NGF administration. 3. Following an initial period of monocular deprivation (MD) kittens subsequently treated with NGF showed a substantial recovery of functional binocular connections. 4. These findings indicate that the administration of NGF during the period of deprivation reduces the amblyopic effects of MD, while its administration to kittens with both eyes open following the initial deprivation promotes recovery of the deprived eye. 5. Neurotrophic factors may contribute to the regulation of experience-dependent modifications of synaptic connectivity in the visual cortex.
Full text
PDF


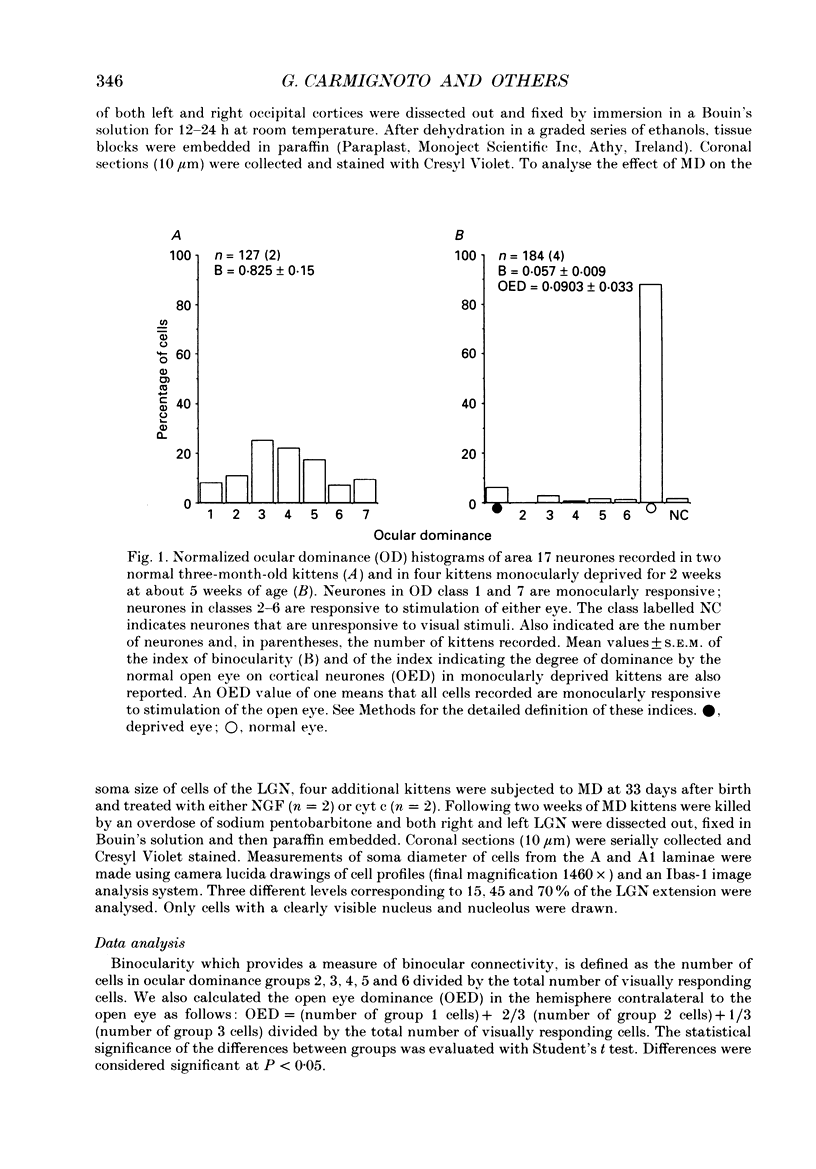
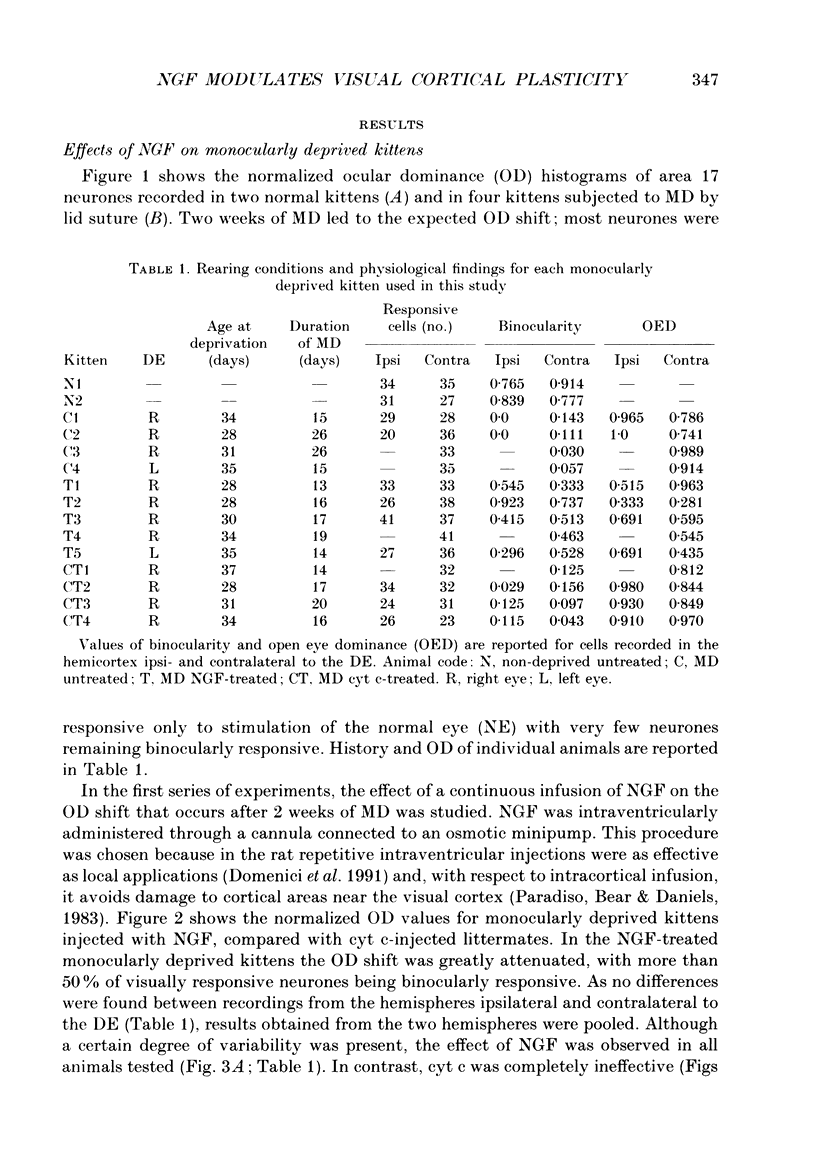
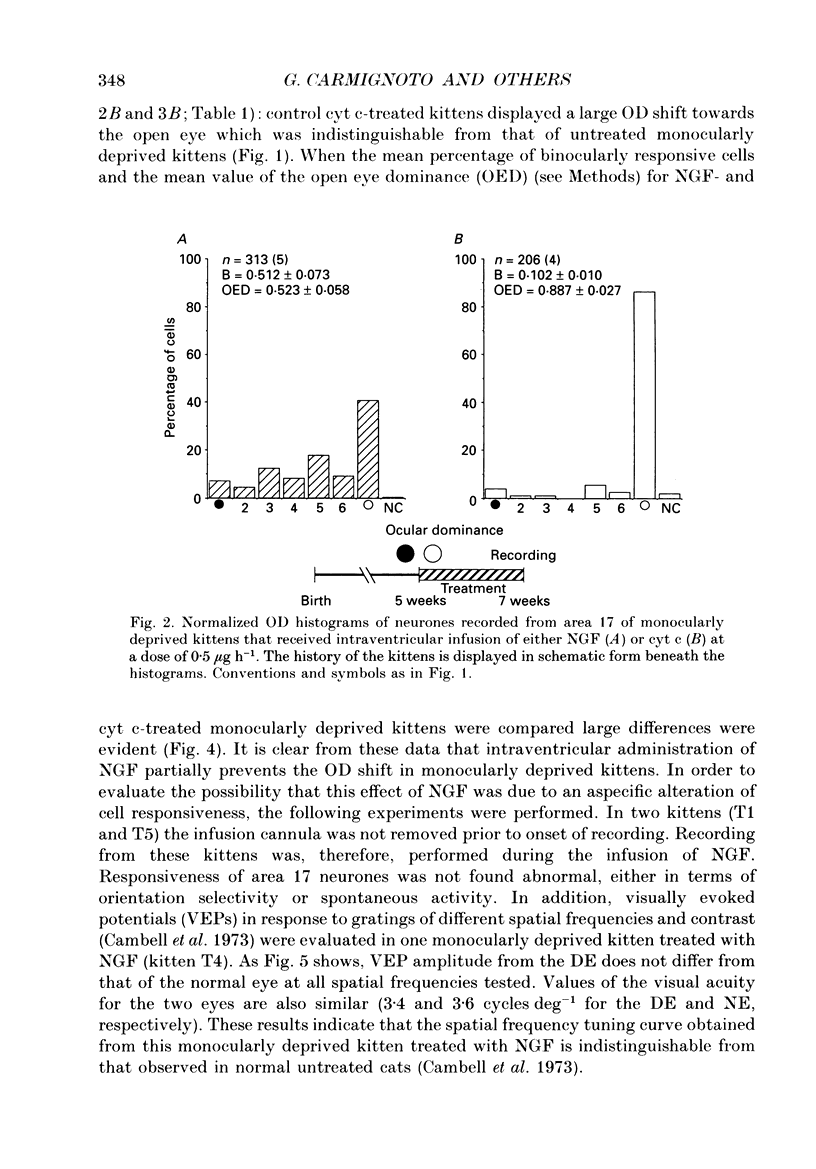





Images in this article
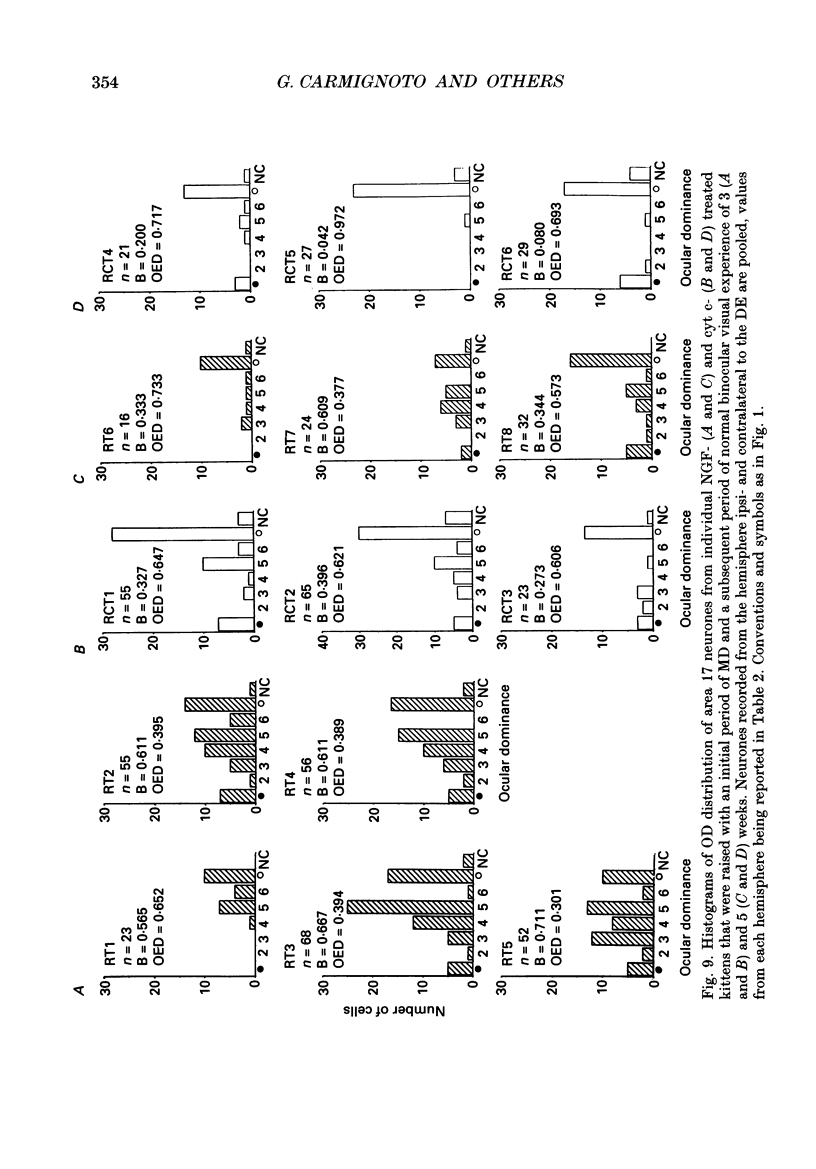
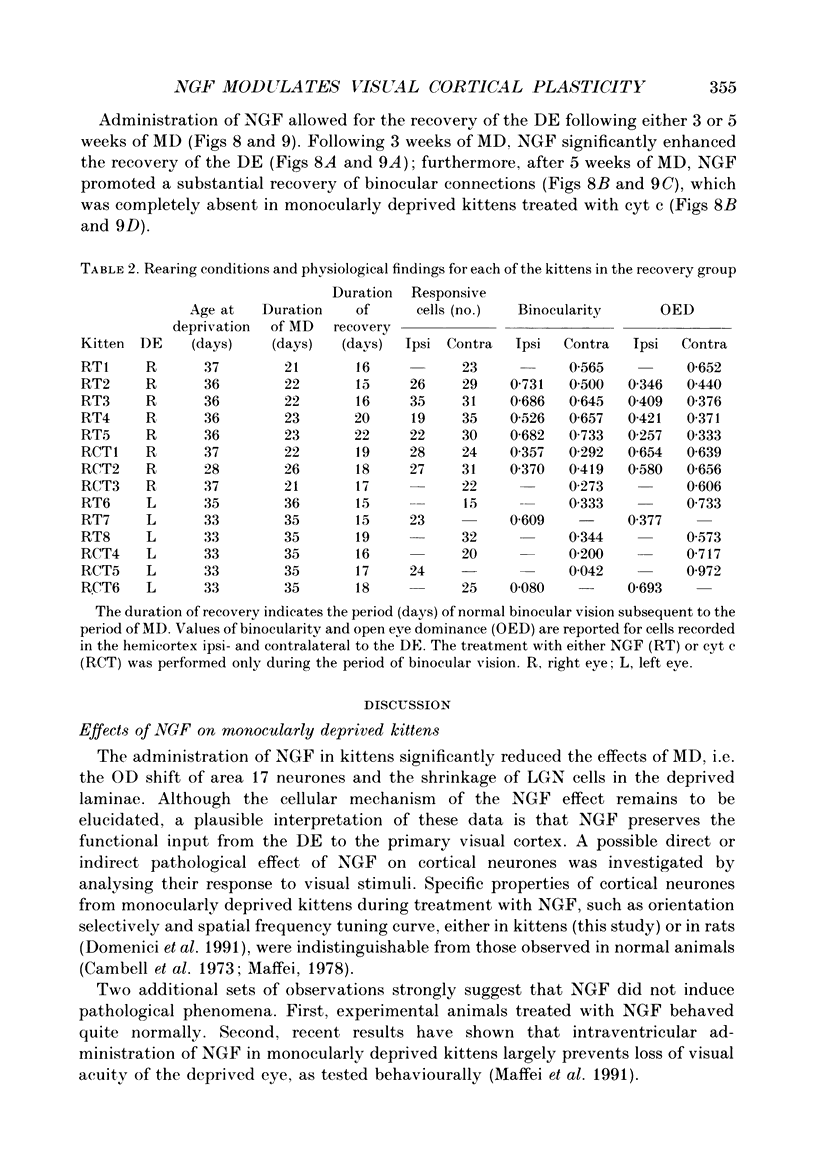
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986 Mar 13;320(6058):172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bisti S., Carmignoto G. Monocular deprivation in kittens differently affects crossed and uncrossed visual pathways. Vision Res. 1986;26(6):875–884. doi: 10.1016/0042-6989(86)90145-8. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Reversal of the physiological effects of monocular deprivation in kittens: further evidence for a sensitive period. J Physiol. 1974 Feb;237(1):195–216. doi: 10.1113/jphysiol.1974.sp010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L., Piccolino M. The contrast sensitivity of the cat. J Physiol. 1973 Mar;229(3):719–731. doi: 10.1113/jphysiol.1973.sp010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G., Comelli M. C., Candeo P., Cavicchioli L., Yan Q., Merighi A., Maffei L. Expression of NGF receptor and NGF receptor mRNA in the developing and adult rat retina. Exp Neurol. 1991 Mar;111(3):302–311. doi: 10.1016/0014-4886(91)90097-v. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992 Nov 6;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S., Dreyfus C. F., Black I. B. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J Neurosci. 1991 Feb;11(2):462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinopoulos A., Eadie L. A., Dori I., Parnavelas J. G. The development of basal forebrain projections to the rat visual cortex. Exp Brain Res. 1989;76(3):563–571. doi: 10.1007/BF00248913. [DOI] [PubMed] [Google Scholar]
- Domenici L., Berardi N., Carmignoto G., Vantini G., Maffei L. Nerve growth factor prevents the amblyopic effects of monocular deprivation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8811–8815. doi: 10.1073/pnas.88.19.8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Ibáez C. F., Ebendal T., Olson L., Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Gall C. M., Isackson P. J. Limbic seizures increase neuronal production of messenger RNA for nerve growth factor. Science. 1989 Aug 18;245(4919):758–761. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Binocular competition in the control of geniculate cell growth. J Comp Neurol. 1972 Jan;144(1):117–129. doi: 10.1002/cne.901440106. [DOI] [PubMed] [Google Scholar]
- Guillery R. W., Stelzner D. J. The differential effects of unilateral lid closure upon the monocular and binocular segments of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1970 Aug;139(4):413–421. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti F., Hartikka J., Knusel B. Function of neurotrophic factors in the adult and aging brain and their possible use in the treatment of neurodegenerative diseases. Neurobiol Aging. 1989 Sep-Oct;10(5):515–533. doi: 10.1016/0197-4580(89)90118-8. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970 Feb;206(2):419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lu B., Yokoyama M., Dreyfus C. F., Black I. B. Depolarizing stimuli regulate nerve growth factor gene expression in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin S. O., Shooter E. M. Molecular investigations on the high-affinity nerve growth factor receptor. Neuron. 1991 Jan;6(1):153–163. doi: 10.1016/0896-6273(91)90130-r. [DOI] [PubMed] [Google Scholar]
- Mitchell D. E., Cynader M., Movshon J. A. Recovery from the effects of monocular deprivation in kittens. J Comp Neurol. 1977 Nov 1;176(1):53–63. doi: 10.1002/cne.901760104. [DOI] [PubMed] [Google Scholar]
- Pioro E. P., Cuello A. C. Distribution of nerve growth factor receptor-like immunoreactivity in the adult rat central nervous system. Effect of colchicine and correlation with the cholinergic system--I. Forebrain. Neuroscience. 1990;34(1):57–87. doi: 10.1016/0306-4522(90)90304-m. [DOI] [PubMed] [Google Scholar]
- Rauschecker J. P. Mechanisms of visual plasticity: Hebb synapses, NMDA receptors, and beyond. Physiol Rev. 1991 Apr;71(2):587–615. doi: 10.1152/physrev.1991.71.2.587. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tébar A., Dechant G., Barde Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Shatz C. J. Impulse activity and the patterning of connections during CNS development. Neuron. 1990 Dec;5(6):745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Varon S. Three independent biological assays for nerve growth factor: no measurable activity in human sera. Exp Neurol. 1982 Jun;76(3):655–665. doi: 10.1016/0014-4886(82)90132-7. [DOI] [PubMed] [Google Scholar]
- Stent G. S. A physiological mechanism for Hebb's postulate of learning. Proc Natl Acad Sci U S A. 1973 Apr;70(4):997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. SINGLE-CELL RESPONSES IN STRIATE CORTEX OF KITTENS DEPRIVED OF VISION IN ONE EYE. J Neurophysiol. 1963 Nov;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Yan Q., Johnson E. M., Jr Immunohistochemical localization and biochemical characterization of nerve growth factor receptor in adult rat brain. J Comp Neurol. 1989 Dec 22;290(4):585–598. doi: 10.1002/cne.902900411. [DOI] [PubMed] [Google Scholar]
- Zafra F., Hengerer B., Leibrock J., Thoenen H., Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990 Nov;9(11):3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]