Abstract
To study the molecular structure and function of gene products in situ, we developed a molecular immunolabeling technology. Starting with cDNA from hybridomas producing monoclonal antibodies against biotin, catalase, and superoxide dismutase, we bioengineered recombinant single-chain variable fragment antibodies (scFv) and their derivatives containing metal-binding domains (scFv:MBD). As tested with surface plasmon resonance and enzyme-linked immunosorbent assay, affinity binding constants of the scFv (5.21 × 106 M−1) and scFv:MBD (4.17 × 106 M−1) were close to those of Fab proteolytic fragments (9.78 × 106 M−1) derived from the parental IgG antibodies. After saturation of MBD with nickel or cobalt, scFv:MBD was imaged with electron spectroscopic imaging at each element's specific energy loss, thus generating the element's map. Immunolabeling with scFv:MBD resulted in a significant improvement of the labeling fidelity over that obtained with Fab or IgG derivatives, as it produced a much heavier specific labeling and label-free background. As determined with radioimmunoassay, labeling effectiveness with scFv:MBD was nearly the same as with scFv, but much higher than with scFv conjugated to colloidal gold, Nanogold, or horseradish peroxidase. This technology opens possibilities for simultaneous imaging of multiple molecules labeled with scFv:MBD at the molecular resolution within the same sample with electron spectroscopic imaging. Moreover, the same scFv:MBD can also be imaged with fluorescence resonance energy transfer and lifetime imaging as well as positron emission tomography and magnetic resonance imaging. Therefore, this technology may serve as an integrative factor in life science endeavors.
With the rapid advances in functional genomics and proteomics, there is an urgent need for structural and functional information on how the myriad gene products of the cell combine and interact to form biomolecular assemblies, biomolecular “machines,” organelles, and cells. This rapidly growing area of research calls for molecular imaging technology that would allow us to pinpoint the location of many individual molecules within complex biomolecular assemblies. Ideally, the same markers should be detectable with various research instruments, allowing us direct interdisciplinary correlation of the data. Antibody-based markers should facilitate correlations of the data on gene expression available from biochemistry, molecular biology, and molecular imaging laboratories, as well as the location and functions of the gene products. In this sense, molecular imaging should also serve as an integrative factor in life science endeavors (1). Efficient pursuit of biomolecular imaging depends on resolution of the instruments and accuracy of the labels.
The repertoire of imaging instruments capable of resolving separate, multiple, individually labeled molecules in situ, within complex bioassemblies and cells is practically limited to the transmission electron microscope (TEM) and its “offspring,” the energy-filtering TEM (EFTEM). High-resolution TEMs can reach a spatial resolution of 0.89 Å (2–4). Although recent advances in other imaging techniques, including scanning near-field optics and atomic-force imaging, are very impressive, neither their resolution nor their sensitivity is sufficiently high for identification of the individual immunolabeled molecules in situ (5–7). Through provision of an atomic resolution, TEMs are extending capabilities for in situ imaging of immunolabels far beyond any other imaging modality available at the present time.
To determine spatial relationships between molecules within a complex multimolecular bioassembly, these molecules must be marked with unique labels that are significantly smaller than and easily distinguished from the molecules that are to be labeled. In general, molecular labels must fulfill two functions: targeting and reporting. To fulfill the labels' targeting functions, antibodies have been the first choice (8–11). A significant improvement of labeling efficiency was achieved when instead of the entire IgG (150 kDa) molecule, only its proteolytic fragment Fab (50 kDa) was used, but even that was not small enough to penetrate into dense biomolecular assemblies. Recombinant single-chain variable fragment antibody (scFv) is a much better candidate for this task. scFv consists of heavy and light variable chains constituting about 240 amino acids with a molecular mass of 26 kDa (12–16). Therefore, scFv is the smallest targeting domain currently available.
To achieve reporting functions, quantum dots (17, 18), metal-ligands (19, 20), colloidal gold beads (21), metal clusters (22, 23), metal-binding domains (MBDs) (24), carborane clusters (25), photoconverted fluorochromes (26), and enzyme molecules (8, 9) have been used. However, features of these reporters were not satisfactory for simultaneous localization of multiple molecules within the same sample at molecular resolution. In most cases, the reporters were big enough to create steric hindrance problems (17). Often, the core of a small reporter had to be covered with stabilizing and functional group shells (17, 18, 23), which significantly increased its final size. Electrostatic charges were generating repulsion forces (16). In multiple labeling, various antibodies could be distinguished from each other only after being conjugated to beads of different diameters, which caused changes in their labeling efficiency (8, 9, 13). Moreover, enhancement and enzymatic reactions often led to the products' drift or diffusion (27, 28). Taking into account all these facts, our attention has been attracted to electron spectroscopic imaging (ESI) using the EFTEM by its ability to map multiple elements within the same sample (29–32). Exploiting this unique feature, we bioengineered labels containing MBDs and incorporated atoms of selected elements into them so that the distribution of the labels could be pinpointed on the basis of the maps of the elements gathered through ESI (24, 33, 34).
In this publication, we report a molecular labeling technology that is based on bioengineered scFv antibodies designed with MBDs.
Materials and Methods
Antibodies and Reporters.
Colloidal gold beads (CG) (≈3.4 nm in diameter) were prepared by condensation of metallic gold from a supersaturated solution (10). The beads were adsorbed to antibodies above their isoelectric points. Monomaleimido-Nanogold (MMI-NG) (15 kDa) (Nanoprobes, Yaphank, NY; 1.4-nm diameter core consisting of 67 gold atoms within a 3.4-nm organic shell) was mixed with the buffer containing antibodies. The product was purified by gel filtration and ion-exchange chromatography (17). Horseradish peroxidase (HRP) (40 kDa) (Sigma) was activated through the sulfosuccinimidyl-4-(N-maleimidomethyl)-1-carboxylate (SMCC) hetero-bifunctional linker (Pierce) in sodium borate. After purification, it was coupled to antibodies. An MBD consisting of five glutamic acid and six histidine residues was synthesized (Perkin-Elmer, Shelton, CT), activated with SMCC, coupled to antibodies (26), and saturated with 3–24 nickel atoms. Polyclonal antibodies against peroxisomal membrane protein 3 (Pex3) (gift from P. Hazra and S. Subramani, Univ. of California, San Diego), catalase (CAT), superoxide dismutase (SOD) (Abcam, Cambridge, U.K.) were IgG class raised in rabbits. IgG molecules were digested into Fab′ fragments. Sulfhydryl groups of Fab′ were used to covalently couple biotin (Pierce). For generating mouse monoclonal antibodies, BALB/c mice were immunized through s.c. injections of biotin, SOD, or CAT (35). Fusion with NS-I murine myeloma cells was facilitated with polyethylene glycol. Based on the ELISA tests of supernatants, the hybridoma cell lines SSbio221, SScat198, and SSpod311 were selected. After the BALB/c mice had been primed with pristane, the hybridoma cells were injected intraperitoneally. From the ascites fluid, IgG fractions were purified on protein A/G columns (Pierce) and digested into Fab′. For bioengineering of scFv from the hybridomas, total mRNA was isolated and cDNA was generated through reverse transcription (36). The HV-linker-HL coding sequence was ligated into pSTE-215(Yol) in place of 215-Vh-Yol-linker-215-Vl-core-st (gift from S. Dubel, EMBL, Heidelberg) (37, 38). The plasmid construct contained the coding sequences for FLAG to facilitate the product's detection and Cys to facilitate coupling with a reporter molecule (39). In addition, the coding sequence for five glutamic acid and six histidine residues (MBD) was cloned into the amino or carboxyl terminus (40). These constructs were transformed into Escherichia coli XL1 Blue (Stratagene) (41). The antibodies were purified from the periplasmic fraction on chelating Sepharose columns (Amersham Pharmacia) saturated with nickel or cobalt (41). Specificity of the antibodies was checked with immunoblotting (42) from SDS/PAGE (43).
ELISA.
The antibodies were adsorbed to immunoplates at 37°C for 1 h. They were immunolabeled with HRP-conjugated anti-FLAG antibody in Hepes-buffered saline (HBS; 0.1 M NaCl/0.1 M Na Hepes, pH 7.3/5 mM NaN3) for 30 min at room temperature (44–46). 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate (Pierce) was used and the absorbance was read at 450 nm.
Surface Plasmon Resonance (SPR).
Biotin was immobilized on an SA sensor chip (Amersham Pharmacia). Binding kinetics were measured at various concentrations of scFv between 0.1 and 1 mM in HBS with contact times of 8 min at a flow rate of 5 ml/min (46–48). The chip was regenerated with 1 mM HCl.
Cells: Fixation, Freezing, and Embedding.
Peroxisomes and mitochondria in yeast and mammalian cells were used as test specimens. The yeast cells were grown in LB medium supplemented with 0.5% methanol to induce peroxisome proliferation (a gift from P. Hazra). The neuronal and glial primary cultures were grown in Dulbecco's medium supplemented with 10% bovine serum (gift from L. Ogden and D. Berg, Univ. of California, San Diego, and G. Konat, West Virginia Univ., Morgantown). For preembedding labeling, the cells were fixed with 4% formaldehyde/0.1% glutaraldehyde buffered with 1 M sorbitol/0.1 M Na Hepes, pH 7.3/5 mM NaN3 (SHA). The cells were then permeabilized with 0.1% digitonin in SHA, blocked with glycine, and labeled with antibodies in SHA containing 4% skimmed milk. The labeled cells were spun down into 0.4% gelatin and cut into ≈0.5-mm3 cubes in ice-cold HBS. These were dehydrated with 66% ethylene glycol at 4°C, followed by 80% ethanol at −15°C, and then infiltrated by gradually increasing the concentration of embedding media: Epon and LR white (Ted Pella, Redding, CA) (49, 50). For immunolabeling on thawed sucrose-infused cryosections, the cells were fixed with formaldehyde/glutaraldehyde, rinsed and pelleted into the gelatin cushion, and infused with buffered 330 mM sucrose (51). The samples were shaped into thin cones, mounted onto pins, and frozen in a rapid-freezer (24). For immunocytochemistry on resin sections, the cells were prepared as above, but without labeling or permeabilization. Alternatively, rapidly frozen cells were freeze-substituted with acetone at −80°C, infiltrated, and embedded as described above (49, 50). Ultramicrotomy of the cells in the resin blocks was performed at room temperature with an Ultracut E (Reichert), which for cryo-ultramicrotomy of frozen cells was equipped with an FD4E chamber (49–51).
Preembedding Immunolabeling.
Before, between, and after labeling steps, the cells were rinsed thoroughly through five cycles of spinning (2,200 rpm in an Eppendorf centrifuge, 4°C) and resuspension into HBS with BSA (0.5 mg/ml). The cells were labeled with antibodies (1 μg/ml) at room temperature for 30 min. To facilitate imaging after immunolabeling, NG, CG, and MBD conjugates were enhanced through autometallography (20). To quench endogenous peroxidase, the samples were incubated with 0.001 M NaN3 and 0.001 M H2O2 before the antibody–enzyme step. For detection, the labeled cells were immersed in a solution of 0.1% diaminobenzidine (DAB; Sigma) containing 0.001 M NiCl2 (31).
Radioimmunoassay (RIA).
Effectiveness of labeling with antibodies carrying various reporter molecules (CG, NG, HRP, and MBD) was compared with RIA. Radioiodination with 125I (Amersham Pharmacia) of anti-FLAG Fab′ from sheep was performed through chloramine-T oxidation followed by removal of the unconjugated 125I by gel filtration (specific activity ≈ 1 μCi/μg; 1 μCi = 37 kBq) (52). Radioiodinated antibodies were applied at 20 ng with 50,000–70,000 cpm for 30 min at room temperature. As a control, biotinylated antibodies were absorbed to immunoplates for 1 h at 37°C, rinsed with HBS, and labeled with 125I-Fab′ as above. The bound radioactivity was measured with a γ-counter (Packard).
Immunolabeling on Sections.
Serial resin and cryosections were mounted on gold grids (50, 51) and labeled as above. Labeled cryosections were reembedded with LR white after labeling (52).
Imaging.
Images were acquired on JEOL JEM1200EX and Leo 912 or 922 TEMs and processed on a stand-alone graphic station (SIS, Boulder, CO; Adobe, San Jose, CA; HyproDesign, Tempe, AZ).
Results
Preembedding Immunolabeling.
Preembedding labeling with scFv-NG.
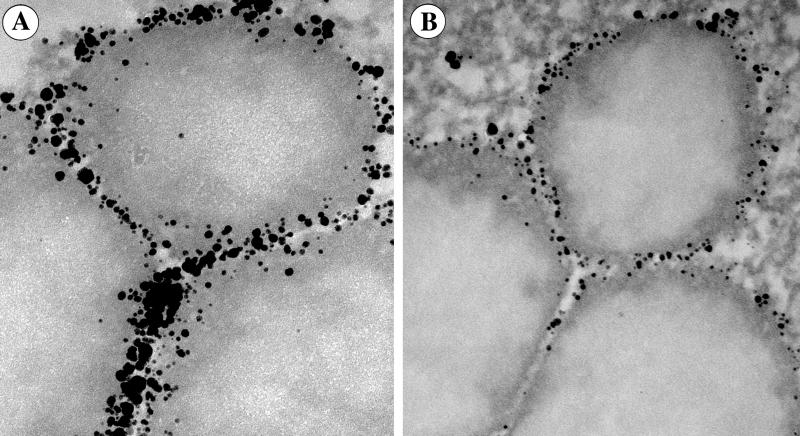
The advantage of using scFv-based labels is clear from comparing images of the cells labeled with scFv and Fab (Fig. 1). scFv and Fab were conjugated with NG through covalent bonds (scFv-NG and Fab-NG), thus the beads reflected the true distribution of the labels. scFv-NG and Fab-NG were used as the secondary antibodies against biotin, which was coupled to Fab fragments of antibodies against Pex3 used as the primary antibody. Apparently, the use of scFv versus Fab′ made a huge difference in the ability of the scFv-based labels to access the antigens. scFv-labeled peroxisomal membrane (Fig. 1A) was covered with a dense band of NG. This labeling was much heavier than that present in the Fab-labeled sample (Fig. 1B). Quantitative data are presented in Figs. 2 and 3. Labeling with scFv-NG resulted in 997 beads per μm of the membrane cross section, and 211 with Fab, but labeling with 10-nm IgG CG resulted in ≈5 beads per μm, which corresponds to the bead counts on peroxisomes labeled with classical techniques as documented in previous publications (33–37). Moreover, it is worth pointing out that only the scFv-based labels were able to penetrate into the narrow spaces between peroxisomes and label the antigens there (Fig. 1). Anti-Pex3 scFv directly conjugated to NG gave the smallest radius of uncertainty, thus the highest resolution. However, the two-step approach resulted in a higher sensitivity as shown in Fig. 4. These antigens were not accessible even to Fab-NG conjugates (Fig. 1B), a circumstance that could lead to false-negative results. Moreover, the background was clean in the scFv-labeled samples; thus there was no false-positive labeling.
Figure 1.
Peroxisomes in yeast cells were first labeled with the biotinylated Fab fragment antibody against Pex3. This was followed by the second immunolabeling with scFv (A) or Fab (B) against biotin conjugated to NG through MMI linker. Labeling with scFv-NG results in a much higher labeling density than with Fab-NG. Notice that the antigens in the tight interperoxisomal spaces are heavily labeled with the scFv-, but remain unlabeled with Fab-based labels. (Horizontal field width = 685 nm.)
Figure 2.
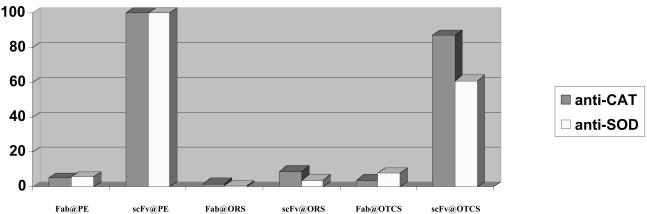
Effectiveness of different immunolabeling strategies for CAT and SOD was evaluated on the basis of calculation of NG bead density per unit of the cell volume (CAT: 100% = 89,657 beads per μm3; SOD: 100% = 10,246 beads per μm3). After labeling of the cells with the biotinylated primary Fab against CAT and SOD, conjugates of NG with Fab and scFv were used for immunolabeling in pre-embedding (@PE), on-resin-section (@ORS), and on-thawed-sucrose-infused-cryosection (@OTCS) approaches.
Figure 3.
Sensitivity of immunolabeling in neuronal cells was assessed either in one step with anti-CAT and anti-SOD scFv covalently conjugated to NG (scFv-NG) or in two steps in which the first anti-CAT and anti-SOD scFv biotinylated antibodies (scFv-B) were followed by the second anti-biotin scFv conjugated with NG (scFv-NG). Immunolabeling efficiency was evaluated by counting the beads and calculating labeling density, NG beads per unit of volume, and is plotted as a percentage. The two-step method has high sensitivity. The one-step method offers high resolution.
Figure 4.
Fidelity of immunolabeling with the antibodies carrying various reporter molecules was evaluated on samples of 100,000 neuronal cells, which were labeled in three steps: (i) with biotinylated anti-CAT antibody; (ii) the secondary anti-biotin scFv containing FLAG peptide only (scFv), or additionally containing MBD at the amino terminus (scFv:MBD), or conjugated with NG (scFv-NG), CG (scFv-CG), or HRP (scFv-HRP); (iii) with 125I-radioiodinated anti-FLAG Fab antibodies. Measurements of radioactivity detected (cpm) in a γ-counter were calculated as percentage of the cpm registered with scFv:MBD as 100%. Cryoimmobilization protocol (Cryo.) results in higher labeling effectiveness than chemical fixation (Chem. Fix.). scFv:MBD results in much higher labeling than the other reporters.
For quantitative evaluation of the results in preembedding labeling, the thickness of the sections is an important factor contributing to the final count. With a uniform distribution of beads, a difference in the section's thickness would result in a proportional difference in the bead count. To exclude such errors, the setting of the ultramicrotome was always at 50 nm; this section thickness was verified by means of fold thickness measurement and with use of the film thickness monitor. To ensure that every bead was counted, it had to stand out clearly against the background and to generate a good signal-to-noise ratio. Two factors contributed to this ratio: embedment and stain. The choice of postlabeling embedment influenced contrast in the microscope and resistance to the electron beam. With LR white (LRW), almost no staining was necessary to reveal the ultrastructure and to visualize the beads sharply. In Epon-embedded cells, the unstained ultrastructure was nearly invisible, but the samples were the most stable under the beam. Clearly, LRW and Epon had distinctively different electron-scattering cross sections. Another factor that contributed greatly to efficient imaging was the choice of staining. Osmium tetroxide, known to erode shells of silver enhancements, was avoided altogether. Sodium vanadate, potassium ferricyanide, and uranyl acetate were used. This modification of classical staining and embedding protocols affected the appearance of peroxisomes and mitochondria. The faint staining was just sufficient to outline the labeled ultrastructure and expose the labels.
Preembedding labeling with scFv-HRP.
Biotinylated anti-Pex3 followed by anti-biotin scFv-HRP resulted in a very distinct band along peroxisomal membrane. In the case of enzyme-based reporters, labeling efficiency should translate into local concentration of the reaction products, which are generated by the antibody-bound enzymes. In practice, these products often drifted and/or diffused. Therefore, labeling intensity was hard to quantify, and the radius of uncertainty was increased. Attempts to improve ultrastructural resolution through reduction of the precipitate's volume resulted in reduced contrast, making faint diaminobenzidine precipitates barely distinguishable from the structures. Therefore, quantitative evaluation of the labeling effectiveness could be made through RIA (Fig. 4).
Preembedding labeling with scFv:MBD.
For ESI, the samples had to meet two requirements: sections had to be uniformly thin to ensure a single scattering event (30, 50, and 80 nm thick for work at 80, 120, and 200 kV, respectively), and reporting atoms had to be present in a sufficient number to meet the minimum detectable number criterion (MDN) (3). This was accomplished with scFv:MBD (Fig. 5).
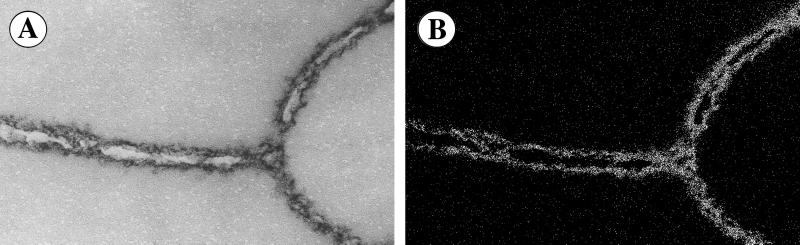
Figure 5.
Peroxisomes in yeast cells were labeled with the biotinylated Fab fragment antibody against Pex3. This was followed by secondary immunolabeling with chimeric scFv:MBD saturated with nickel (scFv:MBDNi). Peroxisomal ultrastructure is visualized at the zero-loss (A). The distribution of the immunolabels is visualized at the nickel's spectroscopic map (B). The distribution of the labels can be referred to the peroxisomal ultrastructure. (Horizontal field width = 702 nm.)
An ultrastructural overview with ESI at the zero energy-loss is illustrated in Fig. 5A. The distribution of antibodies containing nickel atoms imaged with ESI at the nickel's specific edge is presented in Fig. 5B. It is worth mentioning here that the location of the metal definitely pointed to the location of the antibody, because the MBD was a part of the fusion protein. As such, it could not drift away from the antibody. Fab covalently linked to MBD had identical reliability. The two images can be superimposed to match the label with the ultrastructure. scFv offered the smallest possible size of the label's targeting portion. It achieved labeling effectiveness close to that of scFv alone (Fig. 4).
Comparison of Affinity Constants and Labeling Fidelity.
To evaluate contribution of antibody affinity to labeling fidelity, binding affinity constants were calculated from ELISA and SPR data. Binding affinity constants for Fab, scFv, scFv:MBD (MBD at amino terminus), and scFv:MBD (MBD at carboxyl terminus) were, respectively, 9.78 × 106 M−1, 5.21 × 106 M−1, 4.17 × 106 M−1, and not measurable.
For comparison of labeling fidelity of antibodies conjugated to different reporter molecules, these antibodies were detected in situ. After the last step of immunolabeling for electron microscopy was completed, all of the reporter-carrying antibodies that were marked with FLAG peptide were labeled with 125I-anti-FLAG antibodies. In a control test involving labeling of the reporter-carrying antibodies adsorbed to immunoplates, the presence of covalently linked reporter molecules did not interfere with efficiency of labeling with radioiodinated antibodies. The relative radioactivity counts are listed in Fig. 4.
Immunolabeling on Sections.
On-thawed-sucrose-infused-cryo-section immunolabeling (OTCS).
The cells were chemically fixed and frozen, followed by cryosectioning and labeling on thawed sections. The scFv-based labeling effectiveness was significantly higher than that obtained with Fab and IgG conjugates (Fig. 2) (53–55). The background was free of unspecific labeling. It can be assumed that the bioengineered scFv was small enough to penetrate into the peroxisomal bioarchitecture contained within the depth of the sections through a molecular-sieve-like network of chemically cross-linked molecules. This was in contrast to other labels (Fab-NG, Fab-CG, or Fab-HRP), which were retained on the section surface on a manner similar to that reported earlier (51, 52, 56).
On-resin-section immunolabeling (ORS).
In addition to the density of the native structure and networking induced by cross-linking fixative, embedment created an additional obstacle for antibodies to reach antigens within sections. Increasing hydrophilic features, porosity of resins, their etching, extraction, swelling, or partial polymerization might improve accessibility of antigens (49, 50, 52). However, heavily cross-linked cytoplasm matrix was still the primary barrier retaining the IgG-based labels on the section's surface (51, 52, 56). That matrix kept even the smallest 1-nm ImmunoGold on the section's surface without any penetration into its depth (52, 56). To obtain efficient labeling, neither extraction of embedment, nor rinsing off sucrose, nor treatment with detergents was very efficient in achieving penetration of the labels. Reducing the size of the labels was necessary and sufficient to achieve this goal.
Discussion
The most striking result of the work presented here is the significant increase in labeling efficiency achieved with scFv-based labels over that obtained with IgG- or Fab-based labels. This increase was observed after employing four major reporter molecules. Moreover, it was achieved with use of two major immunocytochemical approaches: pre- and postembedding immunolabeling. Molecular labeling patterns were demonstrated at the ultrastructural level with TEM and ESI using EFTEM.
Maximum sensitivity, specificity, resolution, and signal strength are sought in immunocytochemical methods to reveal a lifelike molecular organization of a biostructure with high fidelity. In essence, it can be accomplished only if all and only the targeted molecules are labeled and detected. Improved immunolabeling fidelity described in this publication has its origins in molecular interactions that occurred between the antibodies and the labeled structures. They are analyzed below.
Affinity constants of scFv and its parental Fab were nearly identical. It is worth stressing here that the linker's sequence and the metal-binding insert's location in scFv were essential for retaining the paratope affinity, as discussed earlier (44). Therefore, it can be assumed that the molecular size and organic nature of the labels described here facilitated their penetration, leading to increased labeling efficiency with scFv compared with Fab (Fig. 1). In ELISA, immunoblotting, or SPR, where the antibodies had unrestricted access to antigens, neither the size of the antibody nor the properties of the reporter mattered. However, these tests were informative concerning affinity and specificity of binding. Usefulness for immunocytochemistry could be tested only on cells for labeling cell surface receptors (e.g., CD68, transferrin, etc.) and far more challenging immunocytochemistry of intracellular compartments (e.g., Pex3, titin, etc.).
For two reasons the labels should be small and neutral. First, labeling small densely packed epitopes can be accomplished only if the labels can be packed proportionally (e.g., actin polymer), that is, if they can get close to each other. It was demonstrated—e.g., with 10-nm CG—that electrostatic repulsion forces and label size significantly reduced labeling density, as compared with 1 nm (23). Second, if the molecules located deep within a dense cellular matrix were targeted through preembedding or embedment-free section labeling, then steric hindrance and repulsion forces prevented large labels from entering the structure and significantly reduced the label count (56, 57). However, avidity of the monovalent Fab and scFv was lower than that of polyvalent IgG and IgM. Nevertheless, small monovalent labels of lower avidity not only reflected quantitatively the real occurrence of the labeled molecules but also visualized the lifelike distribution of these molecules with a higher fidelity. In essence, this technology increases the sensitivity of detection of molecules (i.e., some molecules that would remain undetected and gave false-negative result with the classical protocols have been revealed with the present method). Moreover, this method increases the specificity of immunolabeling, because it excludes the presence of reporters in the structures with a known absence of antigens (i.e., false indication that the molecules are present in sites where they are not: false-positive). Finally, this method gives the highest resolution when the scFv antibodies are directly conjugated to reporter molecules.
For efficient detection of the labels, a strong signal was essential. If the reporters are to be detected through the same imaging modality as the labeled structure, then they have to be big enough, or accumulated, or enhanced to a sufficient electron density to boost the signal of the labels above the signal from the stained structures (e.g., 10-nm gold bead ≈ cluster of 10 beads of 1 nm ≈ silver/gold-enhanced 1-nm bead). All these approaches took place at the expense of resolution or fidelity. Therefore, the approaches presented in this project, in which the reporters were detected at their energy-loss edges and the labeled structure at the zero loss, allowed us to avoid that compromise. Our imaging approach allowed us to increase not only sensitivity (two-step scFv labeling approach) but also resolution of immunolabeling (primary antibody carrying the reporter molecule). Bioengineering of chimeric proteins with MBDs, which were identical in size and charge, but different in metal cargo, opened new possibilities for multiple labeling. These chimeric immunolabels could be detected at the very specific energy losses.
Finally, we developed the technology for imaging MBDs with molecular resolution in situ within cells. Other articles have shown that organometallic clusters are suitable for magnetic resonance imaging (MRI), positron emission tomography (PET), fluorescence resonance energy transfer (FRET), and lifetime imaging (58–60). Therefore, the scFv-MBD has become a universal marker spanning a whole spectrum of imaging modes, from the structure of a biomolecule to its distribution within the entire human body. It opens avenues for the correlation of research data at the molecular level. The scFv-MBD labels may help with the molecular interpretation of the phenomena involved in binding and turnover of antibodies used in PET or MRI. Additionally, new markers can be tested in cell cultures at the molecular level with EFTEM or FRET, until the exact mechanisms of the markers' turnover are known. Thereafter, they can be safely transferred to clinical practice involving MRI. Therefore, this labeling technology will potentially have a great impact not only on progress in functional genomics and proteomics but also on the advances in immunology-based diagnosis and therapy.
Acknowledgments
We thank Dr. Joyce Sexton (Univ. of Wisconsin) for editing of the manuscript and Dr. Ray Egerton (Univ. of Alberta) for calculations of the spectra. We acknowledge with thanks the generous support that we have received from the National Science Foundation as Grants 9420056, 9522771, 9902020, and 0094016 to M.M.
Abbreviations
- TEM
transmission electron microscope or microscopy
- EFTEM
energy-filtering TEM
- scFv
recombinant single-chain variable fragment antibody
- MMI
monomaleimido
- CG
colloidal gold
- NG
Nanogold
- HRP
horseradish peroxidase
- Pex3
peroxisomal membrane protein 3
- CAT
catalase
- SOD
superoxide dismutase
- MBD
metal-binding domain
- ESI
electron spectroscopic imaging
- SMCC
sulfosuccinimidyl-4-(N-maleimidomethyl)-1-carboxylate
- SPR
surface plasmon resonance
References
- 1.Malecki M. Trends Cell Biol. 2000;10:494–499. doi: 10.1016/s0962-8924(00)01805-5. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe M A. Microsc Microanal. 2000;6:1192–1193. [Google Scholar]
- 3.Egerton R F. J Electron Microsc. 1999;48:711–716. [Google Scholar]
- 4.Egerton R F, Crozier P A. Micron. 1997;28:117–124. [Google Scholar]
- 5.Ros R, Schwesinger F, Anselmetti D, Kubon M, Schafer R, Plückthun A, Tiefenauer L. Proc Natl Acad Sci USA. 1998;95:7402–7405. doi: 10.1073/pnas.95.13.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirsch A K, Subramaniam V, Jenei A, Jovin T M. J Microsc. 1999;194:448–454. doi: 10.1046/j.1365-2818.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- 7.Roberts C J, Davies M C, Tendler S J, Williams P M, Davies J, Dawkes A C, Yearwood G D, Edwards J C. Ultramicroscopy. 1996;62:149–155. doi: 10.1016/0304-3991(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakane P K, Pierce G B. J Cell Biol. 1967;33:307–318. doi: 10.1083/jcb.33.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Striker G E, Donati E J, Petrali J P, Sternberger L A. Exp Mol Pathol Suppl. 1966;3:52–58. [PubMed] [Google Scholar]
- 10.McLean J D, Singer S J. Proc Natl Acad Sci USA. 1970;65:122–128. doi: 10.1073/pnas.65.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobot J A, Newman G R. Scanning Microsc. 1996;10:121–145. [PubMed] [Google Scholar]
- 12.Skerra A, Plückthun A. Science. 1988;240:1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- 13.Better M, Chang C P, Robinson R R, Horwitz A H. Science. 1988;240:1041–1043. doi: 10.1126/science.3285471. [DOI] [PubMed] [Google Scholar]
- 14.Dattelbaum J D, Abugo O O, Lakowicz J R. Bioconjug Chem. 2000;11:533–536. doi: 10.1021/bc990174+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huston J S, Levinson D, Mudgett-Hunter M, Tai M S, Novotny J, Margolies M N, Ridge R J, Bruccoleri R E, Haber E, Crea R. Proc Natl Acad Sci USA. 1988;85:5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubel S, Breitling F, Klewinghaus I, Little M. Cell Biophys. 1992;21:69–79. doi: 10.1007/BF02789479. [DOI] [PubMed] [Google Scholar]
- 17.Ribrioux S, Kleymann G, Haase W, Heitmann K, Ostermeier C, Michel H. J Histochem Cytochem. 1996;44:207–213. doi: 10.1177/44.3.8648079. [DOI] [PubMed] [Google Scholar]
- 18.Lacoste T D, Michalet X, Pinaud F, Chemla D S, Alivisatos A P, Weiss S. Proc Natl Acad Sci USA. 2000;97:9461–9466. doi: 10.1073/pnas.170286097. . (First Published August 8, 2000; 10.1073/pnas.170286097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han M, Gao X, Su J Z, Nie S. Nat Biotechnol. 2001;19:631–635. doi: 10.1038/90228. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz J R, Piszczek G, Kang J S. Anal Biochem. 2001;288:62–75. doi: 10.1006/abio.2000.4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faulk W P, Taylor G M. Immunochemistry. 1971;8:1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- 22.Hainfeld J F. In: Science of Specimen Preparation for Microscopy and Microanalysis. Malecki M, Roomans G, editors. Vol. 10. Chicago: SMI Press; 1996. pp. 309–322. [Google Scholar]
- 23.Robinson J M, Takizawa T, Vandré D D. J Histochem Cytochem. 2000;48:487–492. doi: 10.1177/002215540004800406. [DOI] [PubMed] [Google Scholar]
- 24.Malecki M. In: Science of Specimen Preparation for Microscopy and Microanalysis. Malecki M, Roomans G, editors. Vol. 10. Chicago: SMI Press; 1996. pp. 1–16. [Google Scholar]
- 25.Kessels M M, Qualmann B, Sierralta W D. In: Science of Specimen Preparation for Microscopy and Microanalysis. Malecki M, Roomans G, editors. Vol. 10. Chicago: SMI Press; 1996. pp. 327–344. [Google Scholar]
- 26.Deerinck T J, Martone M E, Lev-Ram V, Green D P, Tsien R Y, Spector D L, Huang S, Ellisman M H. J Cell Biol. 1994;126:901–910. doi: 10.1083/jcb.126.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danscher G. Histochemistry. 1981;71:1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- 28.Bobrow M N, Harris T D, Shaughnessy K J, Litt G J. J Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- 29.Fukushige T, Hendzel M J, Bazett-Jones D P, McGhee J D. Proc Natl Acad Sci USA. 1999;96:11883–11888. doi: 10.1073/pnas.96.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark J M, Bazett-Jones D P, Herfort M, Roth M B. Proc Natl Acad Sci USA. 1998;95:2163–2168. doi: 10.1073/pnas.95.5.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chadee D N, Hendzel M J, Tylipski C P, Allis C D, Bazett-Jones D P, Wright J A, Davie J R. J Biol Chem. 1999;274:24914–24920. doi: 10.1074/jbc.274.35.24914. [DOI] [PubMed] [Google Scholar]
- 32.Bazett-Jones D P, Mendez E, Czarnota G J, Ottensmeyer F P, Allfrey V G. Nucleic Acids Res. 1996;24:321–329. doi: 10.1093/nar/24.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malecki M, Tiongco L, Hsu A, Takeuchi N. Microsc Microanal. 2000;6:338–339. [Google Scholar]
- 34.Malecki M, Dahlke J, Haig M. In: Electron Microscopy. Calderon Benavides H A, Yacaman M J, editors. Vol. 1. Bristol, U.K.: Institute of Physics; 1998. pp. 709–710. [Google Scholar]
- 35.Muller H P, van Tilburg N H, Derks J, Klein-Breteler E, Bertina R M. Blood. 1981;58:1000–1006. [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Dubel S, Breitling F, Kontermann R, Schmidt T, Skerra A, Little M. J Immunol Methods. 1995;178:201–209. doi: 10.1016/0022-1759(94)00257-w. [DOI] [PubMed] [Google Scholar]
- 38.Ge L, Knappik A, Pack P, Freund C, Plückthun A. In: Antibody Engineering. Borrebaeck C A K, editor. Vol. 8. Oxford: Oxford Univ. Press; 1995. pp. 229–266. [Google Scholar]
- 39.Kipriyanov S M, Dubel S, Breitling F, Kontermann R E, Heymann S, Little M. Cell Biophys. 1995;26:187–204. doi: 10.1007/BF02791580. [DOI] [PubMed] [Google Scholar]
- 40.Goel A, Colcher D, Koo J S, Booth B J, Pavlinkova G, Batra S K. Biochim Biophys Acta. 2000;1523:13–20. doi: 10.1016/s0304-4165(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 41.Skerra A, Pfitzinger I, Plückthun A. Bio/Technology. 1991;9:273–278. doi: 10.1038/nbt0391-273. [DOI] [PubMed] [Google Scholar]
- 42.Siegel R W, Allen B, Pavlik P, Marks J D, Bradbury A. J Mol Biol. 2000;302:285–293. doi: 10.1006/jmbi.2000.4070. [DOI] [PubMed] [Google Scholar]
- 43.Laemmli U K. Nature (London) 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro D A, Adkinson M A, Kaattari S L. In: Immunological Methods Manual. Lefkovits I, editor. Vol. 4. San Diego: Academic; 1997. pp. 2354–2364. [Google Scholar]
- 45.Mao S, Gao C, Lo C H L, Wirsching P, Wong C H, Janda K D. Proc Natl Acad Sci USA. 1999;96:6953–6958. doi: 10.1073/pnas.96.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanes J, Jermutus L, Weber-Bornhauser S, Bosshard H R, Plückthun A. Proc Natl Acad Sci USA. 1998;95:14130–14135. doi: 10.1073/pnas.95.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao C, Mao S, Lo C H L, Wirsching P, Lerner R A, Janda K D. Proc Natl Acad Sci USA. 1999;96:6025–6030. doi: 10.1073/pnas.96.11.6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwesinger F, Ros R, Strunz T, Dario Anselmetti D, Guntherodt H J, Honegger A, Jermutus L, Tiefenauer L, Plückthun A. Proc Natl Acad Sci USA. 2000;97:9972–9977. doi: 10.1073/pnas.97.18.9972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman G R, Hobot J A. J Histochem Cytochem. 1987;35:1192–1193. doi: 10.1177/35.9.3302021. [DOI] [PubMed] [Google Scholar]
- 50.Malecki M, Small J V. Protoplasma. 1987;139:160–167. [Google Scholar]
- 51.Tokuyasu K. J Microsc. 1986;143:139–149. doi: 10.1111/j.1365-2818.1986.tb02772.x. [DOI] [PubMed] [Google Scholar]
- 52.Wilbur D S. Bioconjug Chem. 1992;3:433–470. doi: 10.1021/bc00018a001. [DOI] [PubMed] [Google Scholar]
- 53.Elgersma Y, Kwast L, van den Berg M, Snyder W B, Distel B, Subramani S, Tabak H F. EMBO J. 1997;16:7326–7341. doi: 10.1093/emboj/16.24.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaar K. Eur J Cell Biol. 1992;59:233–254. [PubMed] [Google Scholar]
- 55.Baerends R J S, Faber K N, Kiel J A K W, van der Klei I J, Harder W, Veenhuis M. FEMS Microbiol Rev. 2000;24:291–301. doi: 10.1111/j.1574-6976.2000.tb00543.x. [DOI] [PubMed] [Google Scholar]
- 56.Baschong W, Stierhof Y D. Microsc Res. 1998;42:66–79. doi: 10.1002/(SICI)1097-0029(19980701)42:1<66::AID-JEMT8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 57.Ris H, Malecki M. J Struct Biol. 1993;111:148–157. doi: 10.1006/jsbi.1993.1045. [DOI] [PubMed] [Google Scholar]
- 58.Lakowicz J R, Gryczynski I, Gryczynski Z, Nowaczyk K, Murphy C J. Anal Biochem. 2000;280:128–136. doi: 10.1006/abio.2000.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jansen H M, Paans A M, van der Vliet A M, Veenma-van der Duin L, Bolwijn-Meijer C J, Pruim J, Willemsen A T, Franssen E J, Minderhoud J M, Korf J. Clin Neurol Neurosurg. 1997;99:6–10. doi: 10.1016/s0303-8467(96)00558-6. [DOI] [PubMed] [Google Scholar]
- 60.Van Beers B E, Sempoux C, Materne R, Delos M, Smith A M. J Magn Reson Imaging. 2001;13:594–599. doi: 10.1002/jmri.1083. [DOI] [PubMed] [Google Scholar]