Abstract
The reorientation of the microtubule organizing center during cell migration into a wound in the monolayer was directly observed in living wound-edge cells expressing γ-tubulin tagged with green fluorescent protein. Our results demonstrate that in CHO cells, the centrosome reorients to a position in front of the nucleus, toward the wound edge, whereas in PtK cells, the centrosome lags behind the nucleus during migration into the wound. In CHO cells, the average rate of centrosome motion was faster than that of the nucleus; the converse was true in PtK cells. In both cell lines, centrosome motion was stochastic, with periods of rapid motion interspersed with periods of slower motion. Centrosome reorientation in CHO cells required dynamic microtubules and cytoplasmic dynein/dynactin activity and could be prevented by altering cell-to-cell or cell-to-substrate adhesion. Microtubule marking experiments using photoactivation of caged tubulin demonstrate that microtubules are transported in the direction of cell motility in both cell lines but that in PtK cells, microtubules move individually, whereas their movement is more coherent in CHO cells. Our data demonstrate that centrosome reorientation is not required for directed migration and that diverse cells use distinct mechanisms for remodeling the microtubule array during directed migration.
INTRODUCTION
During directed migration into a wound, cells develop a polarized morphology visualized, for example, by the assembly of dynamic protrusions in the direction of cell migration (Elbaum et al., 1999; Nobes and Hall, 1999). Previous studies have noted that the microtubule organizing center (MTOC), or centrosome, reorients to a location in front of the nucleus, toward the direction of cell migration (for review, see Schliwa and Honer, 1993). This reorientation has been postulated to contribute to the establishment of cell polarity, to efficient migration, and to the delivery of membranous material to the site of lamellar extension. However, centrosome reorientation is not observed in all migrating cells: the centrosome lags behind the nucleus during migration of hepatocyte growth factor–treated PtK cells, and in some cells, centrosome reorientation can be modulated by growth conditions (Danowski et al., 2001; Schutze et al., 1991). Recently, we have observed that in both wound-edge and individually migrating cells, microtubules are transported forward, in the direction of cell migration, independently of the motion of the centrosome (Yvon and Wadsworth, 2000). These observations led us to test the hypothesis that centrosome reorientation is not a requirement for directed migration and to determine whether diverse cells use distinct mechanisms for remodeling the microtubule cytoskeleton during directed migration.
To examine microtubule remodeling in wound-edge cells, we used γ-tubulin tagged with green fluorescent protein (GFP) to monitor centrosome position and photoactivation of tubulin fluorescence to mark the microtubule lattice in two cell lines, CHO and PtK (Mitchison, 1989; Khodjakov et al., 1997; Khodjakov and Rieder, 1999; Yvon and Wadsworth, 2000). Our data demonstrate that centrosome repositioning in wound-edge cells occurs in CHO cells but not in PtK cells. In both cell lines, marks on microtubules are moved forward in the direction of migration; in CHO cells, the mark moves in a coherent manner, whereas in PtK cells, microtubules move individually. Microtubule turnover, measured from dissipation of fluorescence after photoactivation, is rapid in CHO and slow in PtK cells. Interestingly, when CHO cells are transfected with E- or N-cadherin or grown on fibronectin-coated coverslips, centrosome reorientation is not detected. Suppression of microtubule dynamics with taxol or nocodazole also inhibits centrosome reorientation. Our data demonstrate that microtubule remodeling during migration can proceed by distinct pathways and that centrosome reorientation is not a universal feature of cell polarization and directed migration.
MATERIALS AND METHODS
Materials
All materials for cell culture were obtained from Life Technologies-BRL (Gaithersburg, MD), with the exception of fetal calf serum, which was obtained from Atlanta Biologicals (Norcross, GA). Unless otherwise noted, all other chemicals were obtained from Sigma Chemical (St. Louis, MO).
Cell Culture
PtK1, PtK2, and LLCPK cells were cultured with 5% CO2 at 37°C in MEM supplemented with 1.0 mM sodium pyruvate, 10% fetal calf serum, and antibiotics. CHO cells (CHO-K1; Kao and Puck, 1968; Rodionov et al., 1999) were cultured under the same conditions in Ham's F-10. For observation, cells were plated on etched glass coverslips (Bellco Glass, Vineland, NJ) and allowed to grow to confluence before wounding in one of two ways. Most monolayers were manually wounded with forceps and subsequently used for immunocytochemistry or for microinjection. For experiments in which cells were microinjected before wounding, cells adjacent to those that had been injected were removed with a micromanipulator and microneedle. For some experiments, CHO cells were plated on coverslips coated with 20–40 μg/ml fibronectin.
Transfection and Establishment of Permanent Cell Lines
PtK1 cells permanently expressing a construct consisting of the full-length human γ-tubulin fused in frame with the enhanced GFP were used for these experiments (Khodjakov and Rieder, 1999). Additional cells expressing GFP–γ-tubulin were prepared by transfecting the appropriate cells with the γ-tubulin–enhanced GFP construct by use of lipofectamine (Life Technologies BRL, Gaithersburg, MD) according to the manufacturer's instructions. Cells expressing the GFP–γ-tubulin construct were selected with G418-containing medium. CHO cells were also transiently transfected with a vector encoding N-cadherin (gift of Dr. A. Bershadsky, Weizmann Institute, Rehovot, Israel) and fixed at 48 h after transfection.
Because some experiments were performed on PtK1 and some on PtK2 cells, we refer to these collectively throughout this article as PtK cells.
Indirect Immunofluorescence Staining
Cells were fixed for 10 min in −20°C methanol, rehydrated in PBS containing 0.1% Tween-20 and 0.02% sodium azide (PBS-Tw-Az), and stained. Primary antibodies included monoclonal (Sigma clone GTU 88; 1:100 dilution) and polyclonal (Sigma, 1:5000 dilution) antibodies to γ-tubulin, a monoclonal antibody to α-tubulin (Sigma clone DM1a; 1:100 dilution), and a polyclonal antibody to detyrosinated (glu) microtubules (generous gifts of Drs. Chloe Bulinski and Gregg Gundersen; 1:100 dilution). Primary staining was followed by incubation in either Cy3-labeled goat antimouse (Jackson Immunoresearch, West Grove, PA; 1:200 dilution), fluorescein-conjugated goat antimouse (Organon Teknika, Durham, NC; 1:31 dilution), or fluorescein-conjugated goat anti-rabbit (Organon Teknika; 1:31 dilution) secondary antibodies. For inhibition of cytoplasmic dynein, confluent cells were microinjected with anti-dynein antibody (Sigma clone 70.1; needle concentration of 40–60 mg/ml) and allowed to recover for 1 h, and then a wound was made with a microneedle such that the injected cells were at the wound edge. Coverslips were fixed in formaldehyde 2–3 h after wounding and were stained with polyclonal γ-tubulin antibody, Cy3-conjugated anti-rabbit secondary antibody, and finally with FITC-conjugated antimouse secondary antibody so that injected cells could be identified. Cells were mounted in Vectashield (Vector Laboratories, Burlingame, CA) and sealed with nail polish. Cells were observed on a Nikon Eclipse TE 300 inverted microscope with a 60× or 100× objective lens.
Image Acquisition
Living cells expressing GFP–γ-tubulin were observed with a Nikon Eclipse TE 300 inverted microscope equipped with a 100× 1.3 numerical aperture objective lens. Images were acquired with a Princeton Instruments micromax interline transfer cooled CCD camera (Roper Instruments, NJ) and Metamorph software (Universal Imaging, Brandywine, PA). An electronic shutter (Ludl Electrical Products, Hawthorne, NY) also driven by Metamorph controlled exposure to the epi-illumination. A standard filter cube (B-2E/C) was used for collection of GFP fluorescence. Time-lapse sequences of GFP and phase images were initiated 10–45 min after wounding and were collected with an exposure time of 0.3–0.7 s at an interval of 3–8 min between exposures.
Preparation of labeled tubulins, photoactivation, and image collection were performed exactly as described previously (Yvon et al., 2001).
Data Analysis
For calculating the extent and direction of movement of the photoactivated microtubules, the measure distance function of Metamorph imaging software (Universal Imaging) was used with a dynamic data exchange connection to an Excel spreadsheet (Microsoft, Redmond, WA). The behavior of the centrosome was quantified by use of the track points function of Metamorph linked to an Excel spreadsheet. The motion of the nucleus was also tracked by use of the track points function; in some cells, a point on the nuclear envelope was used to follow nuclear motion, whereas in other cases, the nucleolus was used. To measure the position of the cell edge, the edge was traced with the regions tool in Metamorph, and the average distance moved was determined with the measure distance function of Metamorph. Graphic analysis of the data was performed in Excel.
Determination of Centrosome Position in Fixed Cells
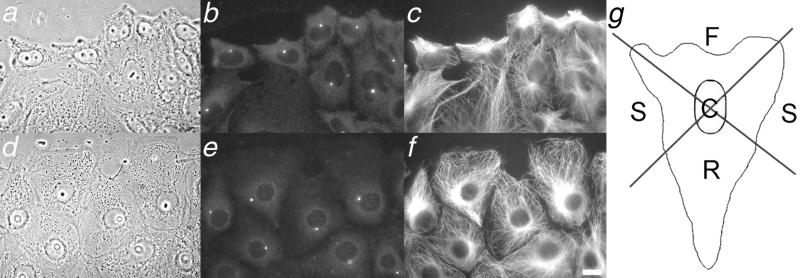
To determine centrosome position, cells were visually divided into four quadrants (Figure 3g). The lateral boundaries of the leading edge were defined as the point at which the leading edge met the neighboring cell; lines from the boundary of the leading edge back to the nucleus were used to define the side and rear regions of the cell. Centrosomes falling in either side quadrant were combined for the side region. The side region represents ∼ 25% of the total cell area (see Figure 3). For cells in which the centrosome was located directly over the nucleus, the position was scored as “center.”
Figure 3.
Centrosome behavior in wound-edge CHO and PtK cells. Phase images (a and d) are shown, along with corresponding immunolocalization of γ-tubulin (b and e) and microtubules (c and f). Bar, 5 mm. Note the greater proportion of centrosomes oriented toward the wound edge in CHO cells than in PtK cells. (g) Diagram of cellular regions used to score centrosome position. Front, sides, rear and center regions are labeled F, S, R, and C, respectively. The wounds are toward the top of the figure; images were acquired ∼ 2.5 h after wounding.
RESULTS
Centrosome Reorientation in Wound-Edge CHO cells
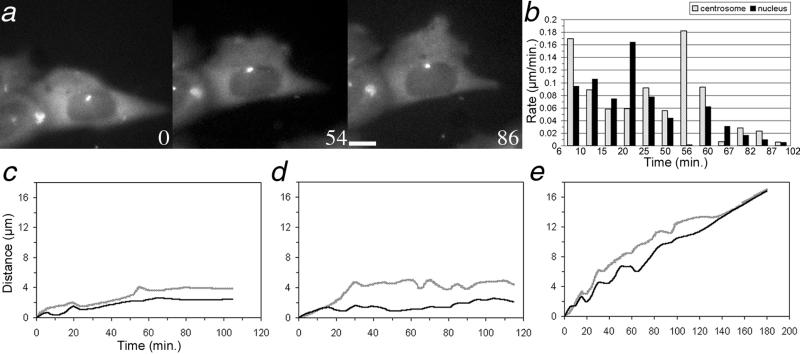
Previous experiments have demonstrated that the centrosome reorients to a position in front of the nucleus, in the direction of cell migration, in some wound-edge cells (Schliwa, 1999; Etienne-Manneville and Hall, 2001; Palazzo et al., 2001). To characterize this motility directly in living cells, centrosome behavior was followed in cells expressing GFP–γ-tubulin (Khodjakov and Rieder, 1999). In the example shown in Figure 1a, the centrosome progressed in the direction of cell motility more rapidly than the nucleus, such that it relocated from a position over the nucleus to one directly in front of it. This is shown graphically in Figure 1c; note that once the centrosome arrived at a position ahead of the nucleus, the two usually progressed together, moving approximately the same distance over the remainder of the sequence. Additional examples of centrosome behavior in wound-edge CHO cells (Figure 1, d and e) also demonstrate centrosome movement to the front of the cell; in most instances, the reorientation of the centrosome was complete within 60 min of wounding.
Figure 1.
(a) Time-lapse sequence of a living wound-edge CHO cell expressing GFP-γ–tubulin; time, in minutes, is given in the lower corner of each panel. Bar, 5 μm. The direction of cell motility is toward the top of the page. Note the movement of the centrosome to the front of the nucleus between panels 0 and 54 and its subsequent persistence in that position. Cells behind the wound-edge cell were not expressing GFP–γ-tubulin and thus are not visible in the fluorescence image. (b) Histogram of rates of movement of the centrosome and nucleus for the cell shown in a; the end of the time interval used to measure the rate is shown on the x axis. (c–e) Plots of the distance the centrosome and nucleus moved from their initial positions over time. (b) Graph corresponds to the cell shown in a. In each example, the centrosome moves forward a greater distance than the nucleus such that it reorients and remains in front of the nucleus.
Centrosome Lagging in Wound-Edge PtK Epithelial Cells
Centrosome behavior was also observed in live PtK epithelial cells expressing GFP–γ-tubulin. In these cells, the centrosome lagged behind the nucleus (Figure 2a). Graphic analysis of this cell (Figure 2c) showed that the nucleus initiated a period of rapid motion before the centrosome. The lagging of the centrosome was most striking for cells in which the initial location of the centrosome was behind or to the side of the nucleus (Figure 2, a, c, and e). When the centrosome was initially in front of the nucleus, the behavior of the two tended to be coordinated (Figure 2d), although this was not always the case.
Figure 2.
(a) Time-lapse sequence of a living wound-edge PtK cell expressing GFP–γ-tubulin; time, in minutes, is given in the lower corner of each panel. Bar, 5 μm. The direction of cell motility is toward the top of the page. Note the lagging of the centrosome behind the nucleus over time (small white dot). Other cells in this field of view were not expressing GFP–γ-tubulin and thus are not visible in the fluorescence image. (b) Histogram of rates of movement of the centrosome and nucleus for the cell shown in a; the end of the time interval used to determine the rates is shown on the x axis. (c–e) Plots of the distance the centrosome and nucleus moved from their initial positions over time. (b) Graph corresponds to the cell shown in a. In a and b, the nucleus moves a greater distance than the centrosome, leaving it behind. In c, the initial position of the centrosome is in front of the nucleus, and it remains there throughout the course of observation.
To determine whether the lack of centrosome reorientation was a common feature of epithelial cells, centrosome reorientation was also monitored in LLCPK-1 cells expressing GFP–γ-tubulin. Centrosome reorientation was not detected in these cells, consistent with our observations in PtK cells (our unpublished results).
Quantification of Centrosome Position in Fixed Cells
Because only a limited number of living cells were examined by time-lapse microscopy, we quantified centrosome position in cells fixed at various times after wounding and stained with antibodies to γ-tubulin (Figure 3 and Table 1). The data demonstrate that at 4 hours after wounding, the centrosome was located in front of the nucleus in 73.2% of CHO cells, consistent with previous observations of wound-edge endothelial, BSC, and NRK cells, 3T3 fibroblasts, and astrocytes (Gotlieb et al., 1981; Kupfer et al., 1982; Gundersen and Bulinski, 1988; Euteneuer and Schliwa, 1992; Palazzo et al., 2001) and consistent with our observations of living cells. In PtK cells at the wound edge, the position of the centrosome shifted from an essentially random distribution at 30 min after wounding (Table 1) to a biased one 4 hours after wounding, with centrosomes positioned behind the nucleus in 52% of cells. To determine whether centrosome repositioning proceeded more slowly in PtK cells than in CHO cells, centrosome distribution in PtK cells was also scored at 18 h after wounding; wounds were sufficiently wide that cell migration had not ceased in these experiments. In this experiment, centrosome position was random with respect to the direction of migration, as determined by one-way analysis of variance (Table 1). Thus, analysis of many fixed cells supports our observations of a limited number of live cells that centrosome reorientation is not a universal feature of wound-edge cells. That is, centrosome reorientation occurs in CHO cells but not PtK cells.
Table 1.
Centrosome position in CHO and PtK2 wound-edge cells
| Cell line | Time | Position of the centrosome
|
|||
|---|---|---|---|---|---|
| Front | Side | Rear | Center | ||
| CHO | 30 min (n = 260) | 29.0 ± 8.0 | 27.7 ± 1.5 | 25.0 ± 2.7 | 18.2 ± 8.0 |
| 1 hr (n = 226) | 54.5 ± 5.2a | 13.4 ± 3.0 | 3.5 ± 1.3 | 28.6 ± 9.6 | |
| 4 hr (n = 296) | 73.2 ± 0.28a | 9.8 ± 1.6 | 2.4 ± 1.4 | 15.5 ± 0.14 | |
| 18 hr (n = 377) | 73.6 ± 3.7a | 10.6 ± 3.7 | 3.3 ± 1.7 | 12.5 ± 2.2 | |
| PtK2 | 30 min (n = 264) | 31.8 ± 1.4 | 31.4 ± 3.0 | 28.4 ± 2.8 | 8.4 ± 3.7 |
| 1 hr (n = 232) | 39.7 ± 7.3 | 29.9 ± 6.6 | 19.0 ± 3.2 | 11.4 ± 6.5 | |
| 4 hr (n = 284) | 18.7 ± 4.4 | 22.5 ± 3.0 | 52.1 ± 9.1a | 6.7 ± 3.6 | |
| 18 hr (n = 439) | 31.2 ± 2.1 | 32.4 ± 4.3 | 31.2 ± 6.1 | 5.2 ± 2.5 | |
Centrosome position was scored according to Figure 3g; data are given as percentage ± SD of the total cells scored at the corresponding time after wounding.
All statistics analyzed by one-way analysis of variance.
Centrosome position exhibits a biased distribution.
Our immunofluorescence observations (Figure 3) also showed a dramatic difference in the distribution of microtubules in CHO and PtK cells. In CHO cells, both at the wound edge and internally in the monolayer, most of the microtubules emanate from the centrosome region (Figure 3c). Furthermore, in wound-edge CHO cells, more microtubules extend from the centrosome toward the wound edge than away from the wound. Note also that CHO cells lack a well-defined lamellipodium and that microtubules extend to the extreme cell periphery (Figure 3c). In contrast, microtubules in PtK cells are present as a dense cytoplasmic array that lacks centrosomal organization. In wound-edge PtK cells, a parallel array of microtubules, aligned with the direction of migration, extends into the prominent lamellae; however, microtubules are excluded from the actin-rich lamellipodium at the extreme cell periphery (Forscher and Smith, 1988; Waterman-Storer and Salmon, 1997; Wadsworth, 1999).
Discontinuous Motile Behavior of the Centrosome
To understand the basis for centrosome lagging or leading behavior (Figures 1 and 2), the rates of movement of the centrosome, nucleus, and leading edge were determined for each cell analyzed. The average rates of centrosome and nuclear motion were not statistically different between PtK and CHO cells. However, in CHO cells, the average rate of centrosome motion was faster than that of the nucleus in all 8 cells examined. In PtK cells, the average rate of the centrosome was slower than that of the nucleus in 9 of 11 cells examined. In the other two PtK cells, the centrosome was located in front of the nucleus at the start of the experiment, and the rates of motion for the centrosome and nucleus were not different (see Figure 2d).
When examined at multiple, shorter intervals over the observation period, the motion of the centrosome and nucleus were observed to be stochastic and uncoordinated. The histogram in Figure 1b shows the average rates of movement of the centrosome and nucleus for the CHO cell shown in Figure 1a; the last time point of the interval for which the rates were determined is indicated on the x axis. In this example, during the first 6 minutes of observation, the centrosome moved much faster than the nucleus (Figure 1b); during the next 4-minute interval, the nucleus moved slightly faster than the centrosome. By the 1-hour point, the centrosome had moved faster than the nucleus in enough instances that it ended up in a leading position.
Conversely, the histogram in Figure 2b, corresponding to the cell in Figure 2a, illustrates several time periods in which the nucleus of this PtK cell moves more rapidly than the centrosome, resulting in the latter being left behind. In both cell types, the motile behavior of both the centrosome and nucleus was stochastic, with frequent switches between periods of rapid and slower motion over the course of observation. In many cases, the rates of motion were out of phase such that a lagging and catching up behavior of the centrosome and nucleus was observed.
Microtubule Transport in Wound-Edge Cells
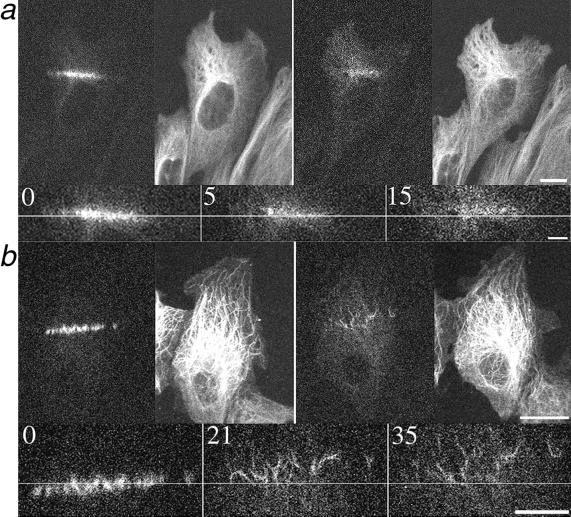
Previous work has demonstrated that microtubules in motile cells are transported forward, in the direction of cell motility, in an actomyosin-dependent manner (Yvon and Wadsworth, 2000). To determine whether microtubule transport contributes to microtubule rearrangement in wound-edge cells, we used photoactivation of caged fluorescein-labeled tubulin to mark the microtubule lattice. As shown in Figure 4, microtubules were transported forward, in the direction of cell migration, in both CHO and PtK cells at the wound edge, consistent with our previous observations of microtubule transport. The average rate at which marked microtubules moved in CHO and PtK cells was 0.13 ± 0.048 μm/min (n = 7) and 0.12 ± 0.065 μm/min (n = 10) for CHO and PtK cells, respectively.
Figure 4.
Microtubule transport in live wound-edge CHO (a) and PtK (b) cells. Cells were comicroinjected with rhodamine-labeled tubulin to visualize all the microtubules and with caged fluorescein tubulin for local photoactivation. The pairs of larger panels show all microtubules on the right (rhodamine fluorescence) and photoactivated microtubules on the left (fluorescein fluorescence) immediately and 15 (a) or 35 (b) minutes after photoactivation. Bars, 10 μm. The smaller panels are magnified views of photoactivated microtubules; time after photoactivation is given, in minutes, in the upper corner of each panel. The lines are provided as a reference; arrowheads in b mark individual microtubules. Bars, 5 μm. The direction of movement is upward for both cells. Note the extensive movement of individual microtubules in PtK cells and the coherent motion of microtubules in CHO cells.
The photoactivation experiments revealed two distinctions between microtubule behavior in CHO and PtK cells. First, microtubule turnover was faster in CHO cells, as evident by the more rapid dissipation of photoactivated fluorescence than in PtK cells (Figure 4), a predictable outcome in light of previous measurements of microtubule dynamic instability in fibroblasts and epithelial cells (Shelden and Wadsworth, 1993). The half-time for microtubule turnover was ∼ 19 min in PtK cells at the wound edge (our unpublished results), and although the rapid dissipation of fluorescence in CHO cells precluded accurate measurements, we estimate the half-time to be ∼ 5 min in these cells (Saxton et al., 1984; Sammak and Borisy, 1988; Schulze and Kirschner, 1988).
A second distinction between microtubule behavior in the two cell types was that photoactivated marks on microtubules were transported in a coherent manner in CHO cells, whereas in PtK cells, microtubules or small groups of microtubules were transported individually (Figure 4). This is consistent with the observation that microtubules in CHO cells are associated with the centrosome, and thus, when the centrosome moves, they move as a unit. Conversely, microtubules in wound-edge PtK cells move individually (our unpublished results), consistent with the noncentrosomal microtubule array in these cells (Keating et al., 1997).
Centrosome Reorientation in Fibroblasts Requires Cytoplasmic Dynein/Dynactin
Our observations that centrosome reorientation occurs in CHO cells and that microtubules are transported in these cells in a coherent manner suggest that the microtubule array may be moved as a unit in these cells. Previous experiments have shown that cytoplasmic dynein is required for the proper positioning of the mitotic spindle, Golgi apparatus, nucleus, and some components of the centrosome (Eshel et al., 1993; Li et al., 1993; Burkhardt et al., 1997; Busson et al., 1998; Gonczy et al., 1999; Quintyne et al., 1999); recent experiments further demonstrate a role for dynein/dynactin in MTOC reorientation in 3T3 fibroblasts and migrating astrocytes (Etienne-Manneville and Hall, 2001; Palazzo et al., 2001). We tested the contribution of cytoplasmic dynein/dynactin to centrosome reorientation in wound-edge CHO cells using microinjection of an antibody to a cytoplasmic dynein intermediate chain, clone 70.1, that blocks cytoplasmic dynein function by inhibiting its association with the intact dynactin complex (Compton, 1998; Quintyne et al., 1999). The position of the centrosome in 70.1 injected cells was scored according to Figure 3g (see MATERIALS AND METHODS) in cells fixed ∼ 3 hours after wounding. The distribution was determined, by one-way analysis of variance, to be random, with 31% in the front, 29% on the side, 22% in the back, and 18% in the center, demonstrating that cytoplasmic dynein/dynactin does indeed play a role in MTOC reorientation in CHO cells at the wound edge (Table 2). Cells injected with 70.1 antibodies were characterized by less-well-organized microtubule arrays (Yvon et al., 2001) and, although lamellae did form at the leading edge, locomotion was somewhat reduced compared with control cells.
Table 2.
Centrosome position in treated CHO wound-edge cells
| Cell treatment | Percentage of
centrosomes in front
|
||
|---|---|---|---|
| 4 hours | 8 hours | 18 hours | |
| CHO | 73.2 ± 0.28 | 38.8 ± 5.8 | 73.6 ± 3.7 |
| (n = 296) | (n = 417) | (n = 377) | |
| + 70.1 antibody | 30.5 ± 5.1 | — | — |
| (n = 226) | |||
| + Fibronectin 20 μg/ml | 29.3 ± 4.6 | 40.7 ± 7.4 | 66.2 ± 6.1a |
| (n = 196) | (n = 314) | (n = 304) | |
| + N-cadherin transfection | 26.9 ± 6.9 | 38.0 ± 7.2 | 35.0 ± 3.8 |
| (n = 327) | (n = 159) | (n = 120) | |
| + Enhanced GFP transfection | 62.2 ± 4.6a | — | — |
| (n = 200) | |||
| + Nocodazole 100 nM | 29.7 ± 5.1 | 51.4 ± 10.2 | 46.6 ± 8.1 |
| (n = 420) | (n = 317) | (n = 305) | |
| + Taxol 100 nM [with 1 hr pretreatment] | 41.9 ± 2.9 | 44.6 ± 5.2 | 43.2 ± 9.0 |
| (n = 443) | (n = 307) | (n = 305) | |
| + Taxol 10 μM | 24.2 ± 3.0 | — | — |
| (n = 385) | |||
Centrosome position was scored according to Figure 3g; data are given as percentage ± SD of the total cells scored at the corresponding number of hours after wounding.
All statistics analyzed by one-way analysis of variance.
Centrosome position in the front was not significantly different from control cells.
Cell Adhesion Alters Centrosome Behavior in Wound-Edge CHO Cells
Recent experiments show that cell adhesion modulates centriole motile behavior in telophase cells (Piel et al., 2001). To test the contribution of cell adhesion to centrosome behavior in wound-edge cells, CHO cells, which do not express detectable levels of cadherin (Figure 5a), were transfected with E- or N-cadherin and wounded, and the behavior of the centrosome was quantified. As shown in Figure 5, b and c, wound-edge CHO cells expressing cadherin show distinct cadherin staining at cell borders, and centrosome position is random (Table 2). The contribution of cell adhesion was also evaluated by plating CHO cells on fibronectin-coated coverslips (see MATERIALS AND METHODS). Under these conditions as well, centrosome position was random (Table 2), demonstrating that centrosome behavior can be modulated by cell substrate and cell-to-cell interactions in wound-edge cells (Chausovsky et al., 2001; Piel et al., 2001). To determine the mechanism by which adhesion altered centrosome behavior, the organization and dynamic turnover of microtubules in CHO cells expressing cadherin were measured. Microtubules in cells expressing high levels of cadherin were more resistant to nocodazole-induced disassembly than microtubules in control cells, indicating an increase in microtubule stability (our unpublished results); however, we did not detect a difference in microtubule organization in the transfected cells.
Figure 5.
Cadherin expression in CHO cells alters centrosome behavior. Immunolocalization of cadherin (a and b) and γ-tubulin (c). (a) Control CHO cells do not express detectable levels of cadherin. CHO cells transiently transfected with cadherin and expressing cadherin at sites of cell-to-cell contact (b); the position of the centrosome is detected after staining for γ-tubulin. Compare the position of the centrosome in these cells with those shown in Figure 3. The wound is at the top of the figure. Bar, 5 mm.
Dynamic Microtubules Are Required for Centrosome Reorientation in Wound-Edge CHO Cells
Our results from photoactivation experiments (Figure 4) and previous measurements of individual microtubule dynamics in CHO and PtK cells (Shelden and Wadsworth, 1993) suggest that differences in microtubule dynamic turnover might contribute to the observed differences in centrosome behavior in these cells. To examine this issue, wound-edge cells were treated with taxol and nocodazole at concentrations previously shown to suppress microtubule dynamic turnover with little effect on polymer level (Vasquez et al., 1996; Yvon et al., 1998). In cells treated with 100 nM nocodazole or taxol, centrosome position was random (Table 2) (Yvon et al., 1998).
One explanation for the lack of centrosome reorientation after these various treatments is that cell motion is slowed, thus reducing the rate of centrosome repositioning. Measurements of the rate of cell motion showed that taxol induced a significant reduction in cell motion but treatment with nocodazole or growth on fibronectin did not. This indicates that the lack of centrosome reorientation was not simply a result of a reduction in the rate of cell migration (Etienne-Manneville and Hall, 2001). Interestingly, however, centrosome position in cells grown on fibronectin was not random at 18 h, suggesting that growth on fibronectin may, in fact, slow, rather than block, centrosome reorientation (Table 2).
DISCUSSION
Centrosome Reorientation Is Not a Universal Feature of Wound-Edge Cells
Our experiments demonstrate that different cell types use distinct pathways for remodeling the microtubule array during directed cell migration. In CHO cells, the centrosome reoriented toward the direction of migration, whereas in PtK epithelial cells, the centrosome lagged. Recent experiments have shown that activation of Cdc42 is required for centrosome reorientation and the initiation of protrusion formation in astrocytes (Etienne-Manneville and Hall, 2001). Our results show that centrosome reorientation and the development of polarity are separable events and indicate that Cdc42 must initiate the formation of protrusions independently of centrosome reorientation in epithelial cells. Our data are consistent with previous observations that reorientation of the centrosome is not universally required for the establishment of cell polarity and directed cell migration (Schliwa and Honer, 1993). Finally, our direct observations show that centrosome motion in directionally migrating cells is stochastic, with periods of rapid motion and relative stasis (Danowski et al., 2001). A similar behavior of the centrosome has also been observed in stationary cells, and inhibition studies show that both actin and microtubules contribute to centrosome motility (Piel et al., 2000).
Centrosome Repositioning Requires Cytoplasmic Dynein and Dynamic Microtubules
The results of our experiments demonstrate that in cases in which centrosome repositioning is observed, this motion requires cytoplasmic dynein, consistent with recent observations in 3T3 cells and astrocytes (Etienne-Manneville and Hall, 2001; Palazzo et al., 2001). Previous work has shown that spindle positioning in Caenorhabditis elegans (Gonczy et al., 1999), mammalian epithelial cells (Busson et al., 1998), and yeast (Eshel et al., 1993; Li et al., 1993) also requires cytoplasmic dynein. In these cases, dynamic interactions of the plus ends of astral microtubules with cortical cytoplasmic dynein are postulated to drive spindle positioning. The observation that the extension of centrosomal microtubules to the cell cortex is required for centrosome repositioning in wound-edge BSC-1 cells (Euteneuer and Schliwa, 1992) and the requirement for cytoplasmic dynein/dynactin suggests that a mechanism similar to that used in spindle positioning may contribute to centrosome reorientation in diverse cells.
Our experiments also demonstrate that dynamic microtubules are necessary for centrosome reorientation in CHO cells (Etienne-Manneville and Hall, 2001), an observation that is inconsistent with previous observations that microtubules oriented in the direction of migration are stabilized (Gundersen and Bulinski, 1988). These previous studies, however, are complicated by the fact that stable microtubules, identified by staining for detyrosinated tubulin, compose only a subset of the total microtubule array. Furthermore, not all cells contain detyrosinated microtubules (Euteneuer and Schliwa, 1992), which could result from the lack of the necessary enzymes, rather than the lack of stable microtubules. Importantly, it has recently been shown that centrosome reorientation can be induced in 3T3 cells in the absence of microtubule stabilization (Palazzo et al., 2001) and conversely, that stable microtubules are present in PtK cells that lack centrosome reorientation. These observations are consistent with the view that centrosome reorientation is critically dependent on a dynamic, not a stable microtubule array and that microtubule stabilization is an independent process.
Cell Adhesion Modulates Centrosome Behavior and Microtubule Dynamics in Wound-Edge Cells
Our experiments show that changing cell adhesion to the substratum or to neighboring cells can modulate the behavior of the centrosome in CHO cells. This observation is strikingly similar to the recent observation that the motion of the centriole to the midbody in telophase cells is not observed when cells are plated on adhesive surfaces (Piel et al., 2001). How might changing cell adhesion alter centriole or centrosome behavior? It has been well established that adhesion complexes are linked to the actin cytoskeleton (Sastry and Burridge, 2000) and that changes in actomyosin contractility can alter adhesive contacts (Chrzanowska-Wodnicka and Burridge, 1996; Katz et al., 2000). Recent experiments further show that microtubules can target focal adhesions and that expression of cadherin can modulate microtubule dynamic behavior (Chausovsky et al., 2001; Kaverina et al., 1999). One possibility is that adhesions directly influence microtubule and centrosome behavior; alternatively, microtubule behavior could be altered indirectly by changes in contractility. This latter possibility is supported by the observation that the activity of actomyosin modulates the organization, turnover, and motion of microtubules in mammalian cells (Yvon et al., 2001) and is consistent with previous observations demonstrating that actin inhibitors affect centrosome positioning in leukocytes (Euteneuer and Schliwa, 1985).
Distinct Pathways for Microtubule Remodeling in Wound-Edge Cells
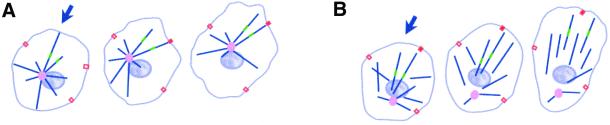
The data presented here and in other recent reports (Etienne-Manneville and Hall, 2001) support a model in which centrosome reorientation is achieved by interactions of dynamic centrosomal microtubules with activated cortical motors (Etienne-Manneville and Hall, 2001; Palazzo et al., 2001; see Figure 6). This is consistent with previous reports showing that cells in which centrosome reorientation occurs have centrosomally focused microtubule arrays (Gotlieb et al., 1981; Kupfer et al., 1982; Gundersen and Bulinski, 1988; Euteneuer and Schliwa, 1992; Etienne-Manneville and Hall, 2001; Palazzo et al., 2001) Given such a model, then, what features of epithelial cells might account for the lack of centrosome reorientation? In epithelial cells, the microtubule array is predominantly noncentrosomal, microtubule dynamics are reduced compared with fibroblastic cells, and few microtubules extend into the extreme cell periphery (Figures 3 and 6) (Bre et al., 1990; Shelden and Wadsworth, 1993; Keating and Borisy, 1999; Waterman-Storer et al., 2000). Thus, the lack of centrosome reorientation in PtK cells is likely to result from specific aspects of microtubule dynamic behavior, geometric constraints (Holy and Leibler, 1994), and the location and activation of motor proteins (Nedelec et al., 1997). Interestingly, treatments that suppressed centrosome reorientation in CHO cells also modified these features of microtubule behavior and organization.
Figure 6.
Model for centrosome reorientation in wound edge cells. (a) In CHO cells, most microtubules (blue lines) are focused at the centrosome (pink circle) and extend to the cell periphery. Activated motors in the cortex (red squares) contribute to centrosome repositioning; the microtubules and centrosome reorient as a unit. Photoactivated marks on the microtubules are shown in green. Blue arrow indicates location of wound. (b) In PtK cells, noncentrosomal microtubules persist due to the presence of stabilizing minus-end caps (not illustrated). Slower turnover results in fewer interactions between centrosomal microtubules and cortical motors. Microtubule are transported individually to the front of the cell.
Although the centrosome in PtK cells lags behind the nucleus during migration into the wound, it does ultimately achieve a position near the cell centroid, a phenomenon that has also been observed in other cell types (for references, see Euteneuer and Schliwa, 1992; Danowski et al., 2001). How is this centering motion accomplished? Various experiments have demonstrated that microtubules can exert pushing forces (Holy et al., 1997; Shaw et al., 1997; Tran et al., 2001) as well as respond to pulling forces (Eshel et al., 1993; Li et al., 1993; Busson et al., 1998; Gonczy et al., 1999). Microtubule pushing against the cell cortex or a balance between pushing and pulling activities would tend to position the centrosome at the geometric center of the cell (Holy et al., 1997).
Summary
Our data show that cells use distinct pathways to remodel the microtubule cytoskeleton during migration into a wound in the monolayer. One pathway involves the motion of the centrosome and associated microtubules as a unit and requires dynamic microtubules and dynein/dynactin activity. In the alternative pathway, noncentrosomal microtubules move as individuals, and the centrosome lags. In both pathways, centrosome motion is directed toward the wound but is stochastic and shows periods of rapid and slower motion relative to the nucleus. Cell-type–specific features of cytoskeletal organization are likely to be responsible for the pattern of microtubule remodeling that is observed in different cell types.
ACKNOWLEDGMENTS
We thank members of the Wadsworth laboratory for help and support throughout the duration of this project. Special thanks to Kimberly Salaycik for performing measurements of cell migration. This work was supported by grants from the National Institutes of Health (to P.W. and A.K.).
Abbreviations used:
- GFP
green fluorescent protein
- MTOC
microtubule organizing center
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–11–0539. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–11–0539.
REFERENCES
- Bre M-H, Pepperkok R, Hill AM, Levilliers N, Ansorge W, Stelzer EHK, Karsenti E. Regulation of microtubule dynamics and nucleation during polarization in MDCK II cells. J Cell Biol. 1990;111:3013–3021. doi: 10.1083/jcb.111.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busson S, Dujardin D, Moreau A, Dompierre J, DeMay JR. Dynein and dynactin are localized to astral microtubules and at cortical sites in mitotic epithelial cells. Curr Biol. 1998;8:541–544. doi: 10.1016/s0960-9822(98)70208-8. [DOI] [PubMed] [Google Scholar]
- Chausovsky A, Bershadsky AD, Borisy GG. Cadherin-mediated regulation of microtubule dynamics. Nat Cell Biol. 2001;2:797–804. doi: 10.1038/35041037. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DA. Focusing on spindle poles. J Cell Sci. 1998;111:1477–1481. doi: 10.1242/jcs.111.11.1477. [DOI] [PubMed] [Google Scholar]
- Danowski BA, Khodjakov A, Wadsworth P. Centrosome behavior in motile HGF-treated PtK2 cells expressing GFP-gamma tubulin. Cell Motil Cytoskeleton. 2001;50:59–68. doi: 10.1002/cm.1041. [DOI] [PubMed] [Google Scholar]
- Elbaum M, Chausovsky A, Levy ET, Shtutman M, Bershadsky AD. Microtubule involvement in regulating cell contractility and adhesion-dependent signaling: a possible mechanism for polarization of cell motility. Biochem Soc Symp. 1999;65:147–172. [PubMed] [Google Scholar]
- Eshel D, Urrestarazu LA, Vissers S, Jauniaux J-C, van Vliet-Reedijk JC, Planta RJ, Gibbons IR. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci USA. 1993;90:11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKC. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Euteneuer U, Schliwa M. Evidence for an involvement of actin in the positioning and motility of centrosomes. J Cell Biol. 1985;101:96–103. doi: 10.1083/jcb.101.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U, Schliwa M. Mechanism of centrosome positioning during the wound response in BSC-1 cells. J Cell Biol. 1992;116:1157–1166. doi: 10.1083/jcb.116.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forscher P, Smith S. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–150. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb AI, May LM, Subrahmanyan L, Kalnins VI. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981;91:589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci USA. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Dogterom M, Yurke B, Leibler S. Assembly and positioning of microtubule asters in microfabricated chambers. Proc Natl Acad Sci USA. 1997;94:6228–6231. doi: 10.1073/pnas.94.12.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Leibler S. Dynamic instability of microtubules as an efficient way to search in space. Proc Natl Acad Sci USA. 1994;91:5682–5685. doi: 10.1073/pnas.91.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F-T, Puck TT. Genetics of somatic mammalian cells, VII: induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci USA. 1968;60:1275–1280. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B-Z, Zamir E, Bershadsky AD, Kam Z, Yamada KM, Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of cell-matrix adhesions. Mol Biol Cell. 2000;11:1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146:1033–1044. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating TJ, Borisy GG. Centrosomal and non-centrosomal microtubules. Biol Cell. 1999;91:321–329. [PubMed] [Google Scholar]
- Keating TJ, Peloquin JG, Rodionov VI, Momcilovic D, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Rieder CL. A synergy of technologies: combining laser microsurgery with green fluorescent protein tagging. Cell Motil Cytoskeleton. 1997;38:311–317. doi: 10.1002/(SICI)1097-0169(1997)38:4<311::AID-CM1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J Cell Biol. 1999;146:585–596. doi: 10.1083/jcb.146.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Louvard DD, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci USA. 1982;79:2603–2607. doi: 10.1073/pnas.79.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-Y, Yeh E, Hays T, Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci USA. 1993;90:10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelec FJ, Surrey T, Maggs AC, Leibler S. Self-organization of microtubules and motors. Nature. 1997;389:305–308. doi: 10.1038/38532. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo AF, Joseph HL, Chen Y-J, Dujardin DL, Alberts AS, Pfister KK, Vallee RB, Gundersen GG. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr Biol. 2001;11:1536–1541. doi: 10.1016/s0960-9822(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel M, Nordberg J, Euteneuer U, Bornens M. Centrosome-dependent exit of cytokinesis in animal cells. Science. 2001;291:1550–1553. doi: 10.1126/science.1057330. [DOI] [PubMed] [Google Scholar]
- Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at fibroblast centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov V, Nadezhdina E, Borisy G. Centrosomal control of microtubule dynamics. Proc Natl Acad Sci USA. 1999;96:115–120. doi: 10.1073/pnas.96.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak PJ, Borisy GG. Direct observation of microtubule dynamics in living cells. Nature. 1988;332:724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- Saxton WM, Stemple DL, Leslie RJ, Salmon ED, Zavortink M, McIntosh JR. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984;99:2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Centrosomes, microtubules and cell migration. Biochem Soc Symp. 1999;65:223–231. [PubMed] [Google Scholar]
- Schliwa M, Honer B. Microtubules, centrosomes and intermediate filaments in directed cell movement. Trends Cell Biol. 1993;3:377–380. doi: 10.1016/0962-8924(93)90086-g. [DOI] [PubMed] [Google Scholar]
- Schulze E, Kirschner M. New features of microtubule behavior observed in vivo. Nature. 1988;334:356–359. doi: 10.1038/334356a0. [DOI] [PubMed] [Google Scholar]
- Schutze K, Maniotis A, Schliwa M. The position of the microtubule-organizing center in directionally migrating fibroblasts depends on the nature of the substratum. Proc Natl Acad Sci USA. 1991;88:8367–8371. doi: 10.1073/pnas.88.19.8367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL, Yeh E, Maddox P, Salmon ED, Bloom K. Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J Cell Biol. 1997;139:985–994. doi: 10.1083/jcb.139.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelden E, Wadsworth P. Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993;120:935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Marsh L, Doyle V, Inoue S, Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol. 2001;153:397–411. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez RJ, Howell B, Yvon AC, Wadsworth P, Cassimeris L. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol Biol Cell. 1996;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P. Regional regulation of microtubule dynamics in polarized, motile cells. Cell Motil Cytoskeleton. 1999;42:48–59. doi: 10.1002/(SICI)1097-0169(1999)42:1<48::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C, Duey DY, Weber KL, Keech J, Cheney RE, Salmon ED, Bement WM. Microtubules remodel actomyosin networks in Xenopus egg extracts via two mechanisms of F-actin transport. J Cell Biol. 2000;150:361–376. doi: 10.1083/jcb.150.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability, induces microtubule breakage and generates non-centrosomal microtubules. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon AC, Gross DJ, Wadsworth P. Antagonistic forces generated by myosin II and cytoplasmic dynein regulate microtubule turnover, movement, and organization in interphase cells. Proc Natl Acad Sci USA. 2001;15:8656–8661. doi: 10.1073/pnas.141224198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon AC, Wadsworth P. Region specific microtubule transport in motile cells. J Cell Biol. 2000;151:1003–1012. doi: 10.1083/jcb.151.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon AC, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1998;10:947–959. doi: 10.1091/mbc.10.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]