Abstract
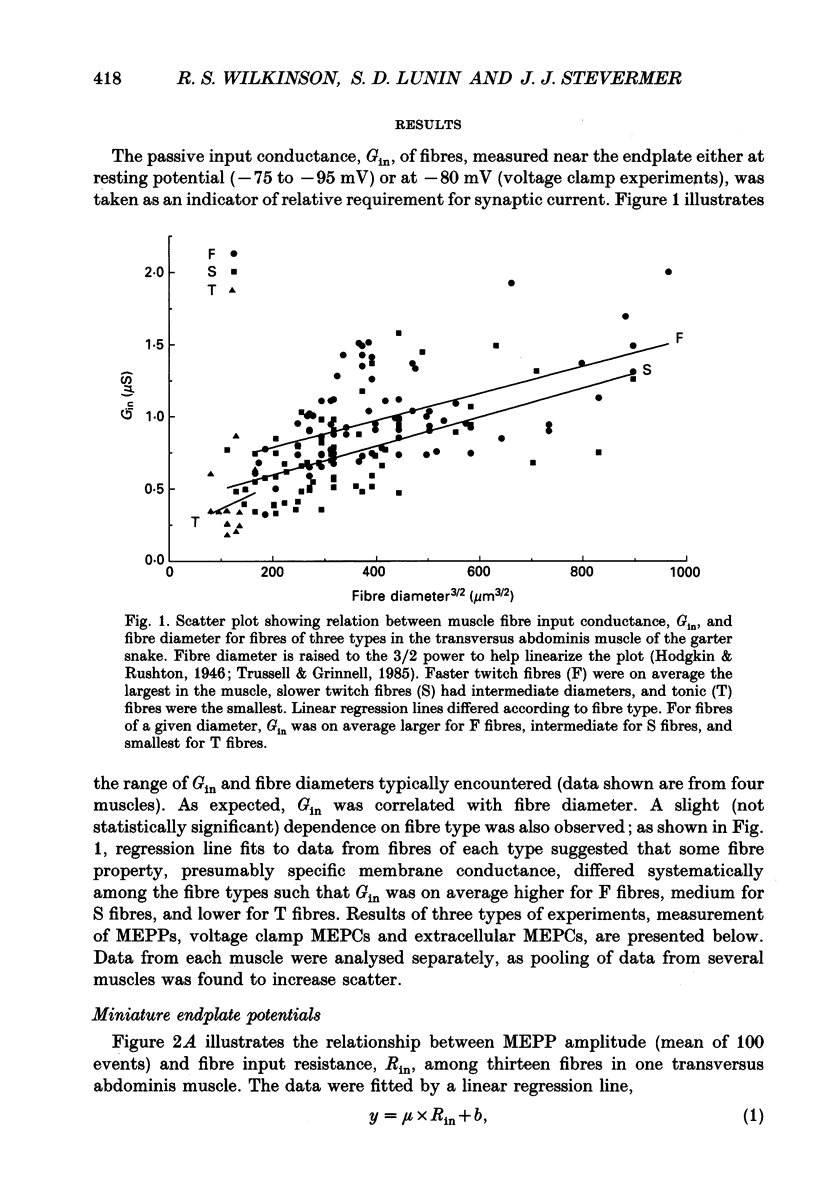
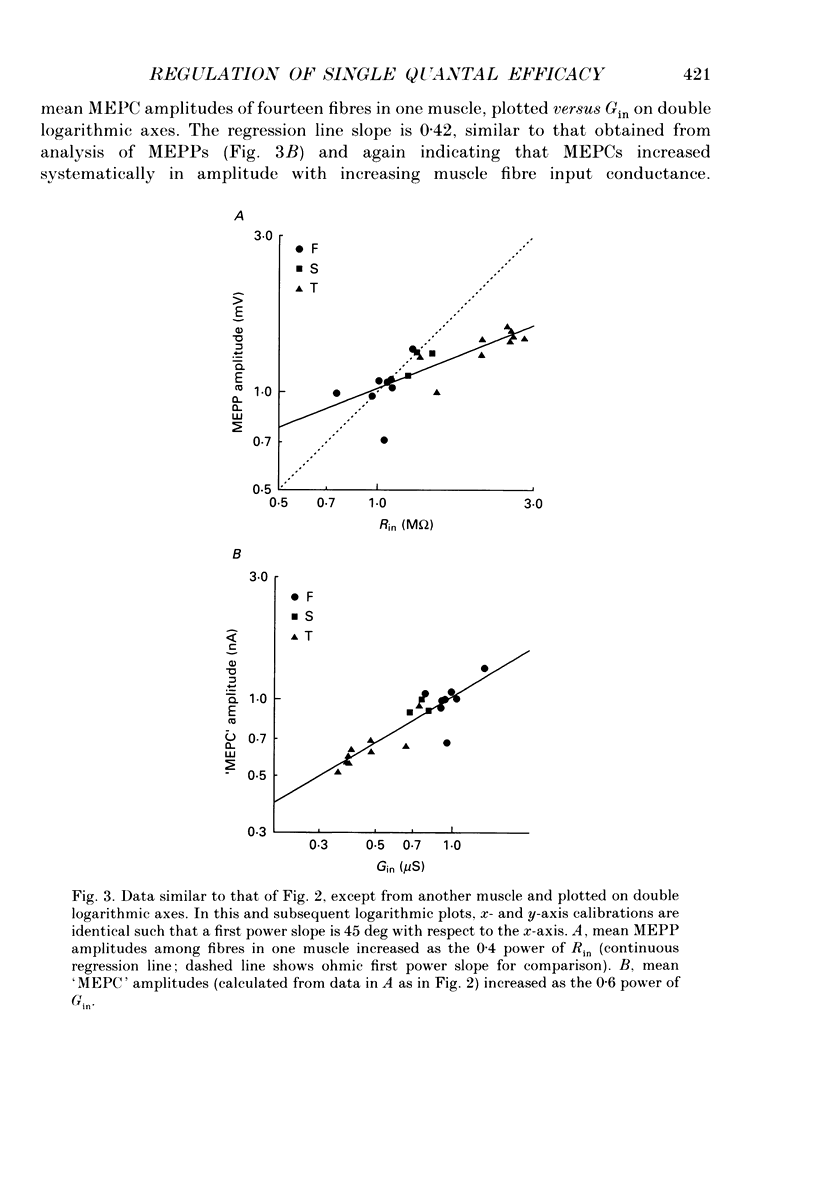
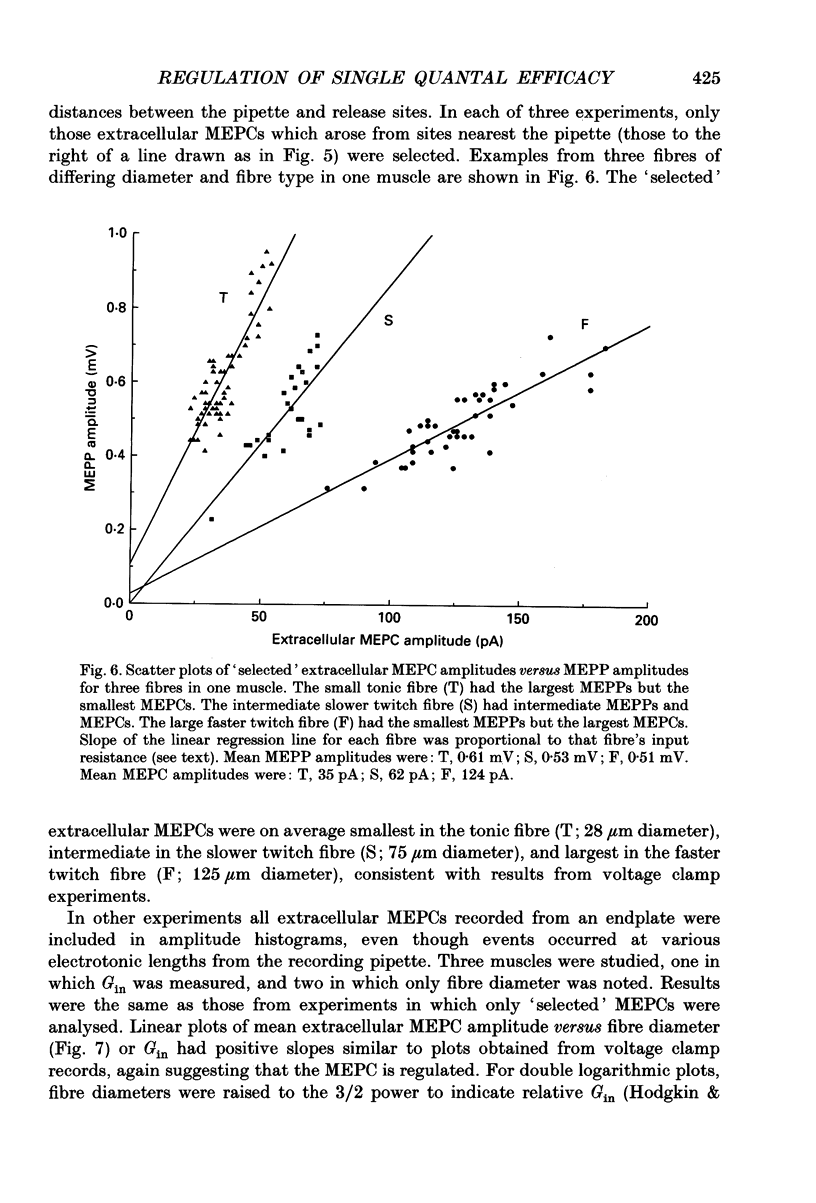
1. Postsynaptic responses to spontaneous quantal transmitter release have been compared among neuromuscular junctions in a thin snake muscle. For each junction the type, diameter, and input conductance, G(in) of the postsynaptic muscle fibre were determined. Particularly among fibres of a given type, G(in) was directly correlated with fibre diameter. 2. Miniature endplate potentials (MEPPs) were recorded intracellularly near endplates visualized with Nomarski optics. Mean MEPP amplitude decreased with increasing G(in) among fibres in one muscle. However, the dependence of mean amplitude upon G(in) was not ohmic, as would be expected if the underlying single quantal currents (miniature endplate currents, MEPCs) were of similar amplitude at all junctions. Instead, the relation between MEPPs and G(in) suggested that mean MEPC amplitudes, calculated as mean MEPP amplitude x G(in), increased with increasing G(in). 3. MEPCs were recorded directly using the two-microelectrode voltage clamp technique. Mean MEPC amplitudes depended systematically on G(in), again such that MEPCs were on average larger in fibres with higher G(in). 4. MEPCs were recorded extracellularly from small regions of endplates (underlying a few nerve terminal boutons). Amplitudes of MEPCs depended on G(in) or fibre diameter in the same manner as amplitudes of MEPCs recorded by intracellular voltage clamp. 5. When the anticholinesterase agent neostigmine was added to the bath, amplitude and duration of MEPPs, MEPCs, and extracellular MEPCs increased. However, the systematic dependence of mean MEPC amplitude on G(in) or fibre diameter remained. 6. Evoked subthreshold endplate potentials (EPPs) were recorded under conditions of low extracellular Ca2+. Endplate currents (EPP amplitudes x G(in)) were systematically larger in fibres with larger G(in), indicating regulation of evoked synaptic current in the muscle. The regulation was found to be due to a combination of increased quantal content and larger single quantal currents in larger (higher G(in)) fibres. 7. Synaptic size, assessed either by area of cholinesterase staining or number of terminal boutons, increased with increasing fibre diameter. Assuming that quantal content is proportional to synaptic size, this relation was sufficient to account for the observed increase in quantal content with increasing G(in) among fibres in the muscle, but was not alone sufficient to account for the observed regulation of evoked current. 8. It is concluded that the efficacy of individual transmitter quanta released at the snake neuromuscular junction is regulated such that large muscle fibres receive larger single quantal currents. Regulation of single quantal current contributes substantially to overall regulation of synaptic strength in the muscle.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balice-Gordon R. J., Breedlove S. M., Bernstein S., Lichtman J. W. Neuromuscular junctions shrink and expand as muscle fiber size is manipulated: in vivo observations in the androgen-sensitive bulbocavernosus muscle of mice. J Neurosci. 1990 Aug;10(8):2660–2671. doi: 10.1523/JNEUROSCI.10-08-02660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Caldwell J. H., Campbell D. T. Na channels in skeletal muscle concentrated near the neuromuscular junction. Nature. 1985 Feb 14;313(6003):588–590. doi: 10.1038/313588a0. [DOI] [PubMed] [Google Scholar]
- Brown T. H., Kairiss E. W., Keenan C. L. Hebbian synapses: biophysical mechanisms and algorithms. Annu Rev Neurosci. 1990;13:475–511. doi: 10.1146/annurev.ne.13.030190.002355. [DOI] [PubMed] [Google Scholar]
- Connor E. A., Fiekers J. F., Neel D. S., Parsons R. L., Schnitzler R. M. Comparison of cholinergic activation and desensitization at snake twitch and slow muscle fibre end-plates. J Physiol. 1984 Jun;351:657–674. doi: 10.1113/jphysiol.1984.sp015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Parsons R. L. Characteristics of the acetylcholine-operated channel at twitch and slow fibre neuromuscular junctions of the garter snake. J Physiol. 1981 Jan;310:145–158. doi: 10.1113/jphysiol.1981.sp013541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erxleben C., Kriebel M. E. Characteristics of spontaneous miniature and subminiature end-plate currents at the mouse neuromuscular junction. J Physiol. 1988 Jun;400:645–658. doi: 10.1113/jphysiol.1988.sp017141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erxleben C., Kriebel M. E. Subunit composition of the spontaneous miniature end-plate currents at the mouse neuromuscular junction. J Physiol. 1988 Jun;400:659–676. doi: 10.1113/jphysiol.1988.sp017142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- Fields R. D., Ellisman M. H. Functionally significant plasticity of synaptic morphology: studies on the ribbon synapse of the ampullae of Lorenzini. Neuroscience. 1988 May;25(2):705–720. doi: 10.1016/0306-4522(88)90271-0. [DOI] [PubMed] [Google Scholar]
- Grinnell A. D., Trussell L. O. Synaptic strength as a function of motor unit size in the normal frog sartorius. J Physiol. 1983 May;338:221–241. doi: 10.1113/jphysiol.1983.sp014670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. B., Ribchester R. R. The relationship between end-plate size and transmitter release in normal and dystrophic muscles of the mouse. J Physiol. 1979 Nov;296:245–265. doi: 10.1113/jphysiol.1979.sp013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A. A., Grinnell A. D. Transmitter release from frog motor nerve terminals depends on motor unit size. Nature. 1980 Oct 16;287(5783):649–651. doi: 10.1038/287649a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S. Properties of motor units in the transversus abdominis muscle of the garter snake. J Physiol. 1987 Dec;393:355–374. doi: 10.1113/jphysiol.1987.sp016827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Wilkinson R. S., Rich M. M. Multiple innervation of tonic endplates revealed by activity-dependent uptake of fluorescent probes. 1985 Mar 28-Apr 3Nature. 314(6009):357–359. doi: 10.1038/314357a0. [DOI] [PubMed] [Google Scholar]
- Markus E. J., Petit T. L. Synaptic structural plasticity: role of synaptic shape. Synapse. 1989;3(1):1–11. doi: 10.1002/syn.890030102. [DOI] [PubMed] [Google Scholar]
- Martin A. R. The effect of membrane capacitance on non-linear summation of synaptic potentials. J Theor Biol. 1976 Jun;59(1):179–187. doi: 10.1016/s0022-5193(76)80031-8. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth P. M., Rosser B. W., Wilkinson R. S. Metabolic and contractile uniformity of isolated motor unit fibres of snake muscle. J Physiol. 1991 Mar;434:41–55. doi: 10.1113/jphysiol.1991.sp018458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibers of uniform input resistance. J Neurosci. 1982 Feb;2(2):216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Regulation of synaptic position, size, and strength in anuran skeletal muscle. J Neurosci. 1983 Jan;3(1):161–176. doi: 10.1523/JNEUROSCI.03-01-00161.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge R. M. Different types of extrafusal muscle fibres in snake costocutaneous muscles. J Physiol. 1971 Sep;217(2):393–418. doi: 10.1113/jphysiol.1971.sp009578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Loring R. H. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog Neurobiol. 1985;25(4):297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Schubert D. The possible role of adhesion in synaptic modification. Trends Neurosci. 1991 Apr;14(4):127–130. doi: 10.1016/0166-2236(91)90078-9. [DOI] [PubMed] [Google Scholar]
- Stefani E., Steinbach A. B. Resting potential and electrical properties of frog slow muscle fibres. Effect of different external solutions. J Physiol. 1969 Aug;203(2):383–401. doi: 10.1113/jphysiol.1969.sp008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. A comment on Martin's relation. Biophys J. 1976 Aug;16(8):891–895. doi: 10.1016/S0006-3495(76)85739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell L. O., Grinnell A. D. The regulation of synaptic strength within motor units of the frog cutaneous pectoris muscle. J Neurosci. 1985 Jan;5(1):243–254. doi: 10.1523/JNEUROSCI.05-01-00243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vautrin J., Mambrini J. Synaptic current between neuromuscular junction folds. J Theor Biol. 1989 Oct 23;140(4):479–498. doi: 10.1016/s0022-5193(89)80110-9. [DOI] [PubMed] [Google Scholar]
- Walrond J. P., Reese T. S. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985 May;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A. Estimates of statistical release parameters from crayfish and frog neuromuscular junctions. J Physiol. 1975 Jan;244(1):207–221. doi: 10.1113/jphysiol.1975.sp010792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. S., Lichtman J. W. Regular alternation of fiber types in the transversus abdominis muscle of the garter snake. J Neurosci. 1985 Nov;5(11):2979–2988. doi: 10.1523/JNEUROSCI.05-11-02979.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


