Abstract
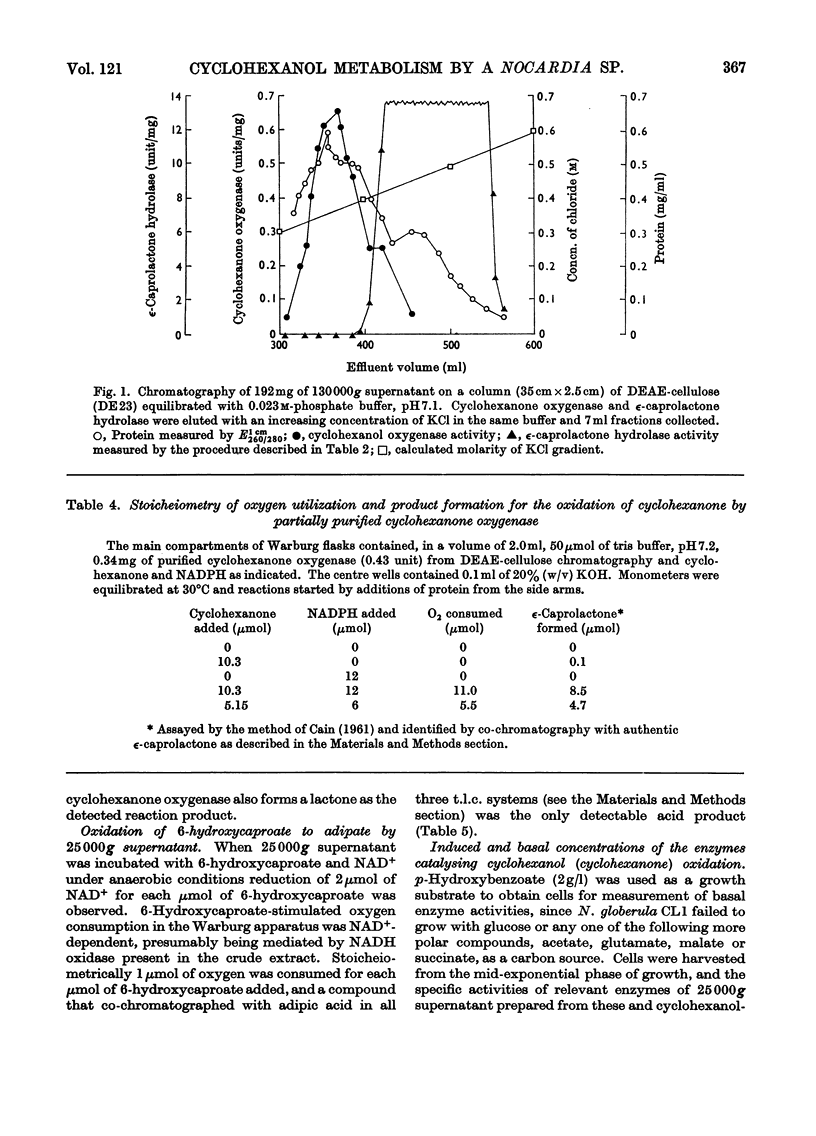
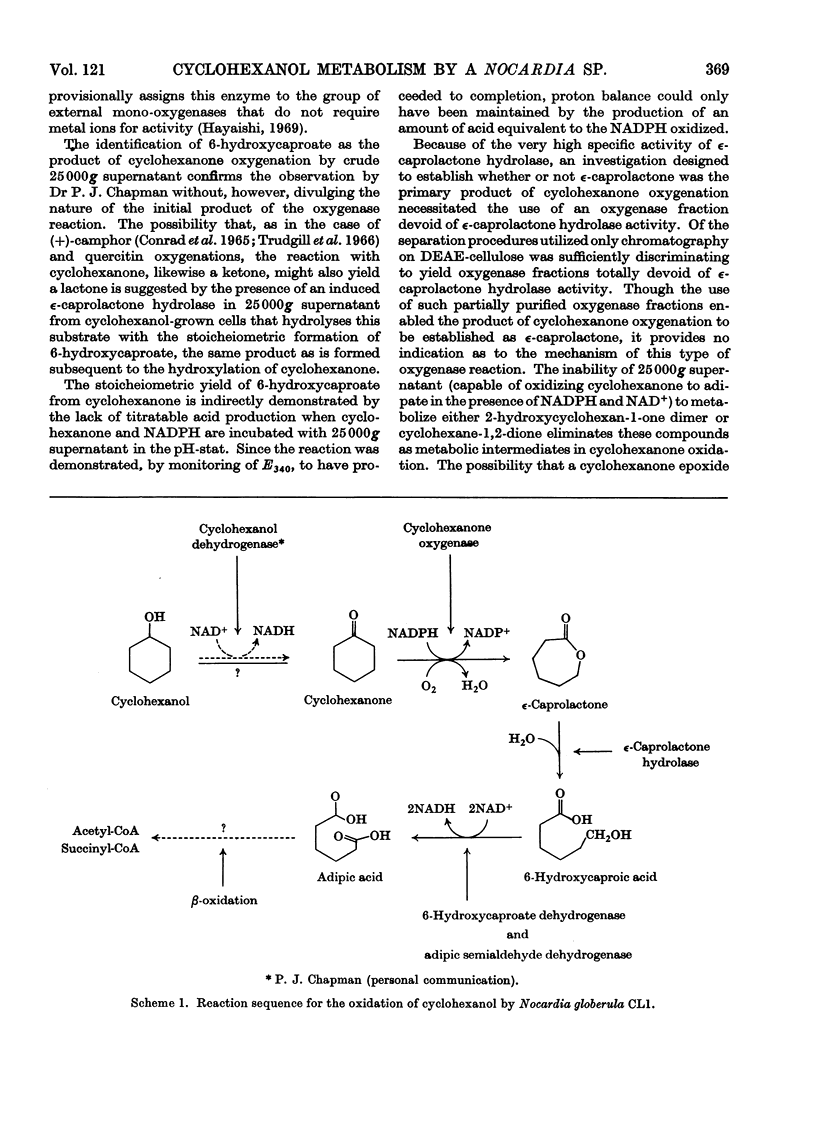
1. Nocardia globerula CL1, isolated by enrichment on cyclohexanol and grown with it as carbon source, oxidized it with a Qo2 of 39μl/h per mg dry wt. and the overall consumption of 2.2μmol of oxygen/mol of substrate. Cyclohexanone, 2-hydroxycyclohexan-1-one dimer and cyclohexane-1,2-dione were oxidized with Qo2 values similar to that for cyclohexanol whereas ∈-caprolactone and 6-hydroxycaproate were oxidized very slowly and adipate not all. 2. Disrupted cell suspensions could not be shown to catalyse the conversion of cyclohexanol into cyclohexanone. 3. A cyclohexanol-induced cyclohexanone oxygenase (specific activity 0.55μmol of NADPH oxidized/min per mg of protein) catalysed the consumption of 1mol of NADPH and 1mol of O2 in the presence of 1mol of cyclohexanone. NADPH oxidation did not occur under anaerobic conditions. The only detected reaction product with 25000g supernatant was 6-hydroxycaproate. 4. Extracts of cyclohexanol-grown cells contained a lactone hydrolase (specific activity 15.6μmol hydrolysed/min per mg of protein), which converted ∈-caprolactone into 6-hydroxycaproate. 5. Incubation of 6-hydroxycaproate with 25000g supernatant in the presence of NAD+ resulted in NAD+ reduction under anaerobic conditions, oxygen consumption under aerobic conditions and the conversion of 6-hydroxycaproate into adipate. 6. Cyclohexanone oxygenase fractions devoid of ∈-caprolactone hydrolase catalysed the stoicheiometric formation of ∈-caprolactone from cyclohexanone in the presence of excess of NADPH. 7. The reaction sequence for the oxidation of cyclohexanone by N. globerula CL1 is: cyclohexanol → cyclohexanone → ∈-caprolactone → 6-hydroxycaproate → adipate. 8. It is suggested that the adipate may be further dissimilated by β-oxidation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALIKHAN M. Y., HALL A. N., ROBINSON D. S. PRODUCTS OF THE OXIDATION OF SELECTED ALKANES BY A GRAM-NEGATIVE BACTERIUM. Antonie Van Leeuwenhoek. 1964;30:417–427. doi: 10.1007/BF02046755. [DOI] [PubMed] [Google Scholar]
- CAIN R. B. The metabolism of protocatechuic acid by a vibrio. Biochem J. 1961 May;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUS D., WALKER N. THE DECOMPOSITION OF TOLUENE BY SOIL BACTERIA. J Gen Microbiol. 1964 Jul;36:107–122. doi: 10.1099/00221287-36-1-107. [DOI] [PubMed] [Google Scholar]
- CONRAD H. E., DUBUS R., NAMTVEDT M. J., GUNSALUS I. C. MIXED FUNCTION OXIDATION. II. SEPARATION AND PROPERTIES OF THE ENZYMES CATALYZING CAMPHOR LACTONIZATION. J Biol Chem. 1965 Jan;240:495–503. [PubMed] [Google Scholar]
- Cory E. J., Russey W. E., Ortiz de Montellano P. R. 2,3-oxidosqualene, an intermediate in the biological synthesis of sterols from squalene. J Am Chem Soc. 1966 Oct 20;88(20):4750–4751. doi: 10.1021/ja00972a056. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- ELLIOTT T. H., TAO R. C., WILLIAMS R. T. STEREOCHEMICAL ASPECTS OF THE METABOLISM OF THE ISOMERIC METHYLCYCLOHEXANOLS AND METHYLCYCLOHEXANONES. Biochem J. 1965 Apr;95:59–69. doi: 10.1042/bj0950059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT T. H., TAO R. C., WILLIAMS R. T. THE METABOLISM OF METHYLCYCLOHEXANE. Biochem J. 1965 Apr;95:70–76. doi: 10.1042/bj0950070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D. T. Microbial degradation of aromatic compounds. Science. 1967 Sep 13;161(3846):1093–1097. [PubMed] [Google Scholar]
- KATAGIRI M., HAYAISHI O. Enzymatic degradation of beta-ketoadipic acid. J Biol Chem. 1957 May;226(1):439–448. [PubMed] [Google Scholar]
- KESTER A. S., FOSTER J. W. DITERMINAL OXIDATION OF LONG-CHAIN ALKANES BY BACTERIA. J Bacteriol. 1963 Apr;85:859–869. doi: 10.1128/jb.85.4.859-869.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEACH A. A., O'SHEA P. C. THE DETERMINATION OF PROTEIN MOLECULAR WEIGHTS OF UP TO 225,000 BY GEL-FILTRATION ON A SINGLE COLUMN OF SEPHADEX G-200 AT 25 DEGREES AND 40 DEGREES. J Chromatogr. 1965 Feb;17:245–251. doi: 10.1016/s0021-9673(00)99864-9. [DOI] [PubMed] [Google Scholar]
- MASON H. S. Mechanisms of oxygen metabolism. Science. 1957 Jun 14;125(3259):1185–1188. doi: 10.1126/science.125.3259.1185. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Suhara Y., Kobayashi T. Separation of aliphatic dibasic acids by thin-layer chromatography. J Chromatogr. 1969 Jan 14;39(1):88–90. doi: 10.1016/s0021-9673(01)97983-x. [DOI] [PubMed] [Google Scholar]
- OOYAMA J., FOSTER J. W. BACTERIAL OXIDATION OF CYCLOPARAFFINIC HYDROCARBONS. Antonie Van Leeuwenhoek. 1965;31:45–65. doi: 10.1007/BF02045875. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Van Tamelen E. E., Willett J. D., Clayton R. B., Lord K. E. Enzymic conversion of squalene 2,3-oxide to lanosterol and cholesterol. J Am Chem Soc. 1966 Oct 20;88(20):4752–4754. doi: 10.1021/ja00972a058. [DOI] [PubMed] [Google Scholar]
- YUGARI Y. Metabolism of cyclohexane-diol-(1,2)-trans by a soil bacterium. Biken J. 1961 Sep;4:197–207. [PubMed] [Google Scholar]


