Abstract
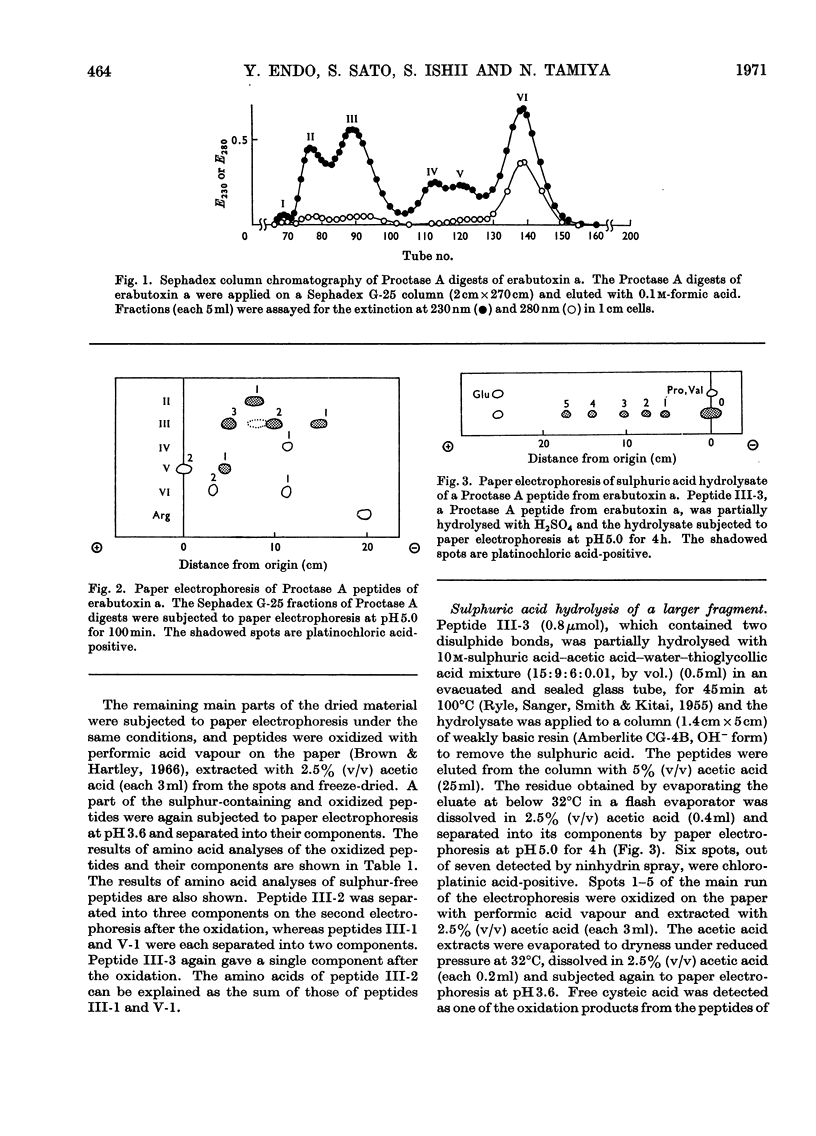
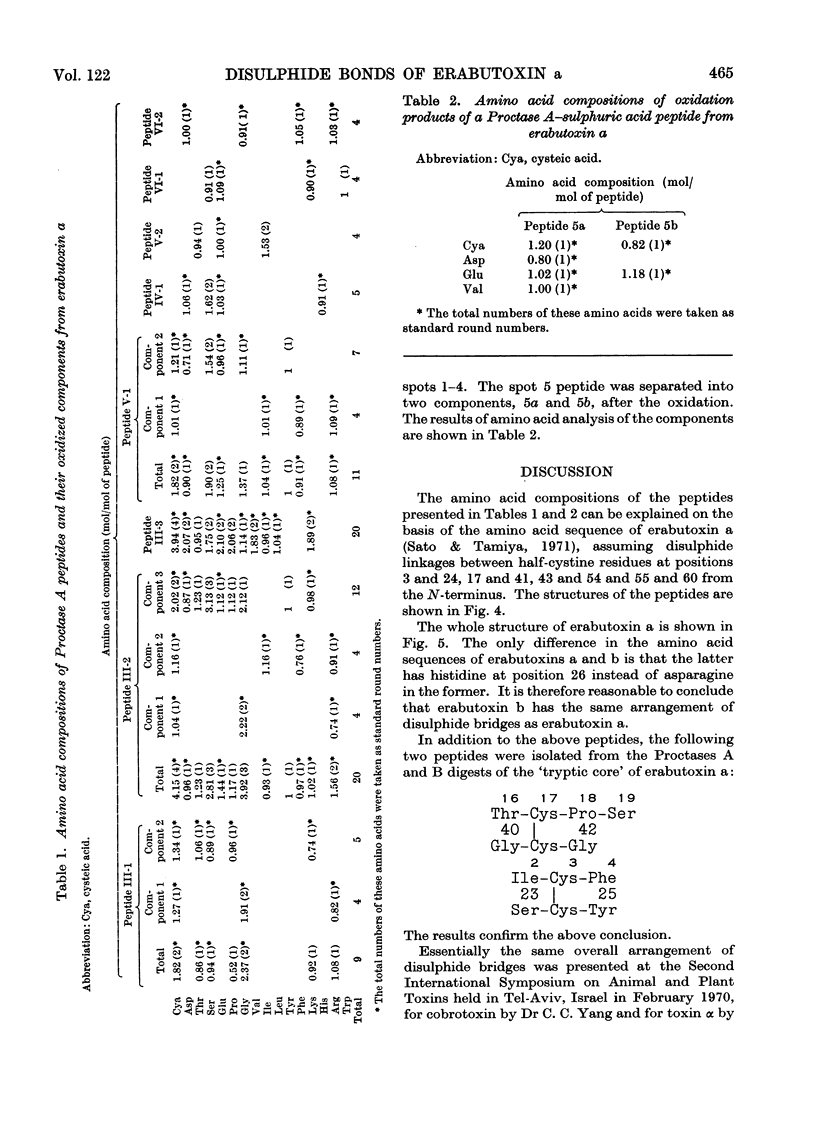
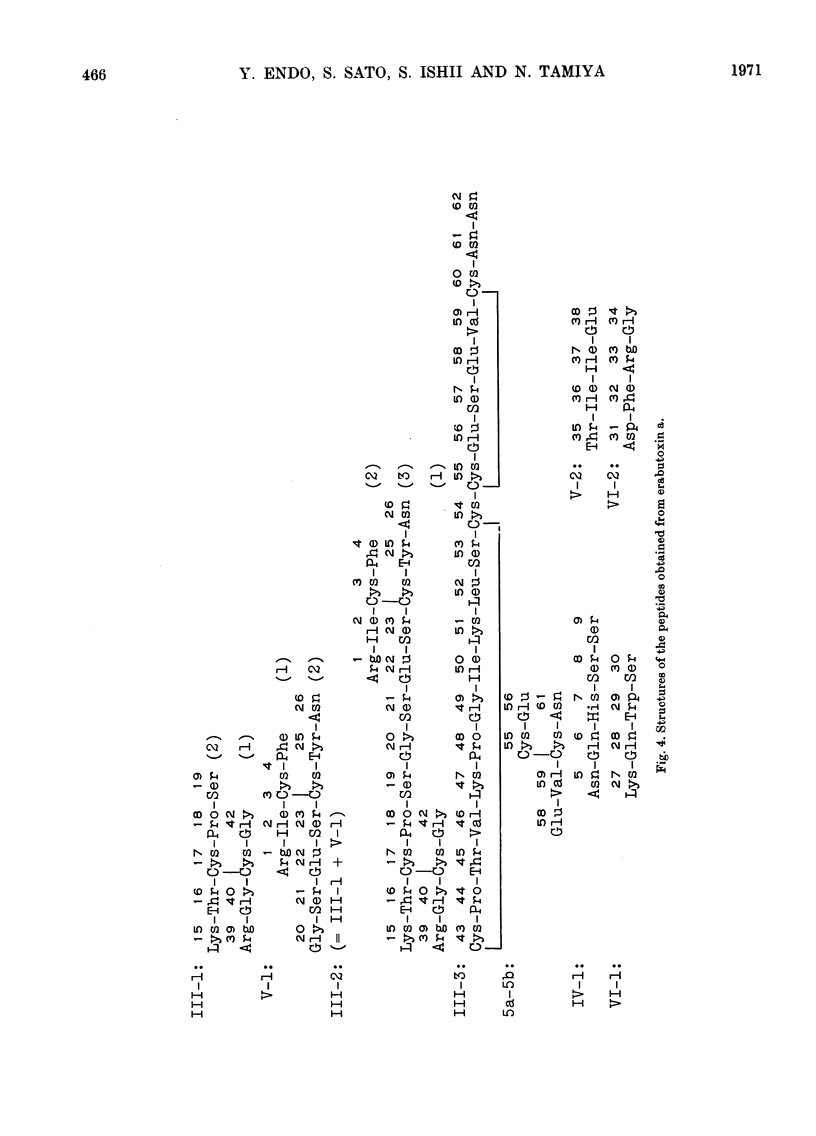
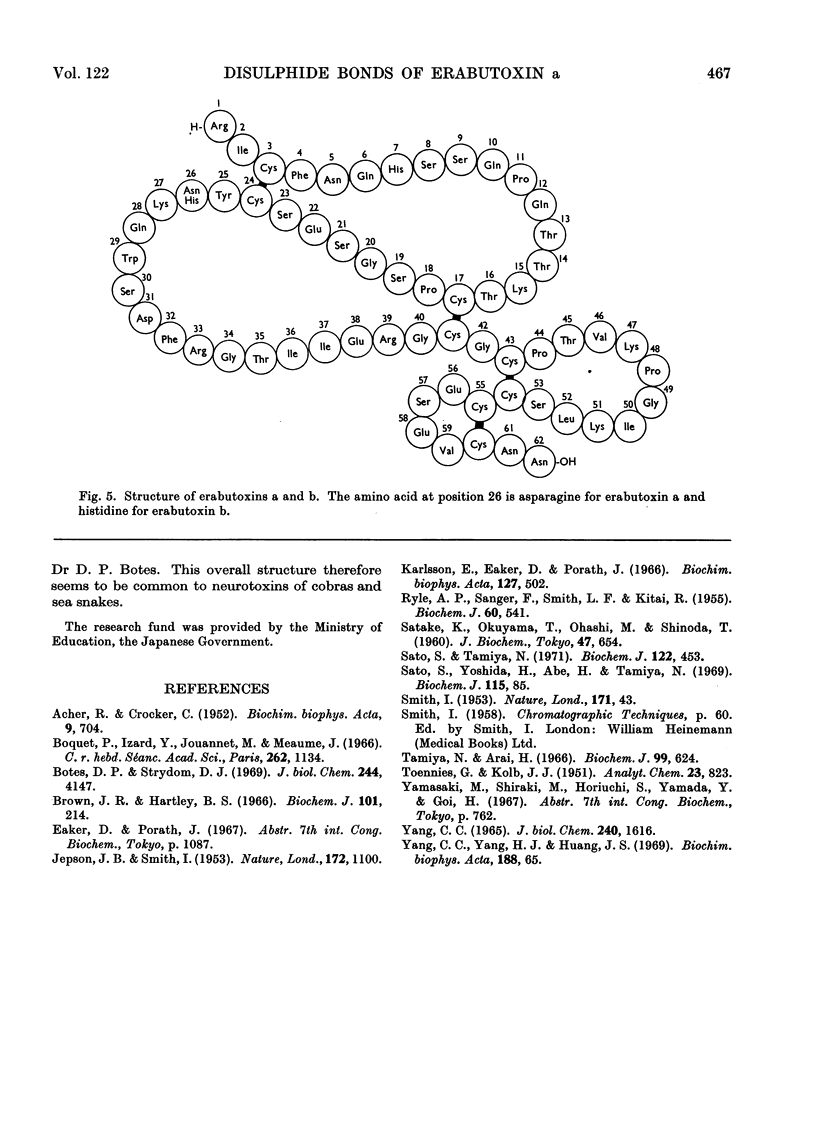
Erabutoxin a was partially hydrolysed with enzymes and sulphuric acid and the resulting peptides were separated from each other by column chromatography and paper electrophoresis. From the results of amino acid analyses of the sulphur-containing peptides and their oxidized components, all four disulphide bridges in the toxin molecule were located. The disulphide bonds were found between half-cystine residues at positions 3 and 24, 17 and 41, 43 and 54, and 55 and 60 from the N-terminus.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CROCKER C. Réactions colorées spécifiques de l'arginine et de la tyrosine réalisées après chromatographie sur papier. Biochim Biophys Acta. 1952 Dec;9(6):704–705. doi: 10.1016/0006-3002(52)90236-9. [DOI] [PubMed] [Google Scholar]
- Botes D. P., Strydom D. J. A neurotoxin, toxin alpha, from Egyptian cobra (Naja haje haje) venom. I. Purification, properties, and complete amino acid sequence. J Biol Chem. 1969 Aug 10;244(15):4147–4157. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- RYLE A. P., SANGER F., SMITH L. F., KITAI R. The disulphide bonds of insulin. Biochem J. 1955 Aug;60(4):541–556. doi: 10.1042/bj0600541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH I. Colour reactions on paper chromatograms by a dipping technique. Nature. 1953 Jan 3;171(4340):43–44. doi: 10.1038/171043a0. [DOI] [PubMed] [Google Scholar]
- Sato S., Tamiya N. The amino acid sequences of erabutoxins, neurotoxic proteins of sea-snake (Laticauda semifasciata) venom. Biochem J. 1971 May;122(4):453–461. doi: 10.1042/bj1220453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Yoshida H., Abe H., Tamiya N. Properties and biosynthesis of a neurotoxic protein of the venoms of s snakes Laticauda laticaudata and Laticauda colubrina. Biochem J. 1969 Oct;115(1):85–90. doi: 10.1042/bj1150085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya N., Arai H. Studies on sea-snake venoms. Crystallization of erabutoxins a and b from Laticauda semifasciata venom. Biochem J. 1966 Jun;99(3):624–630. doi: 10.1042/bj0990624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG C. C. CRYSTALLIZATION AND PROPERTIES OF COBROTOXIN FROM FORMOSAN COBRA VENOM. J Biol Chem. 1965 Apr;240:1616–1618. [PubMed] [Google Scholar]


