Abstract
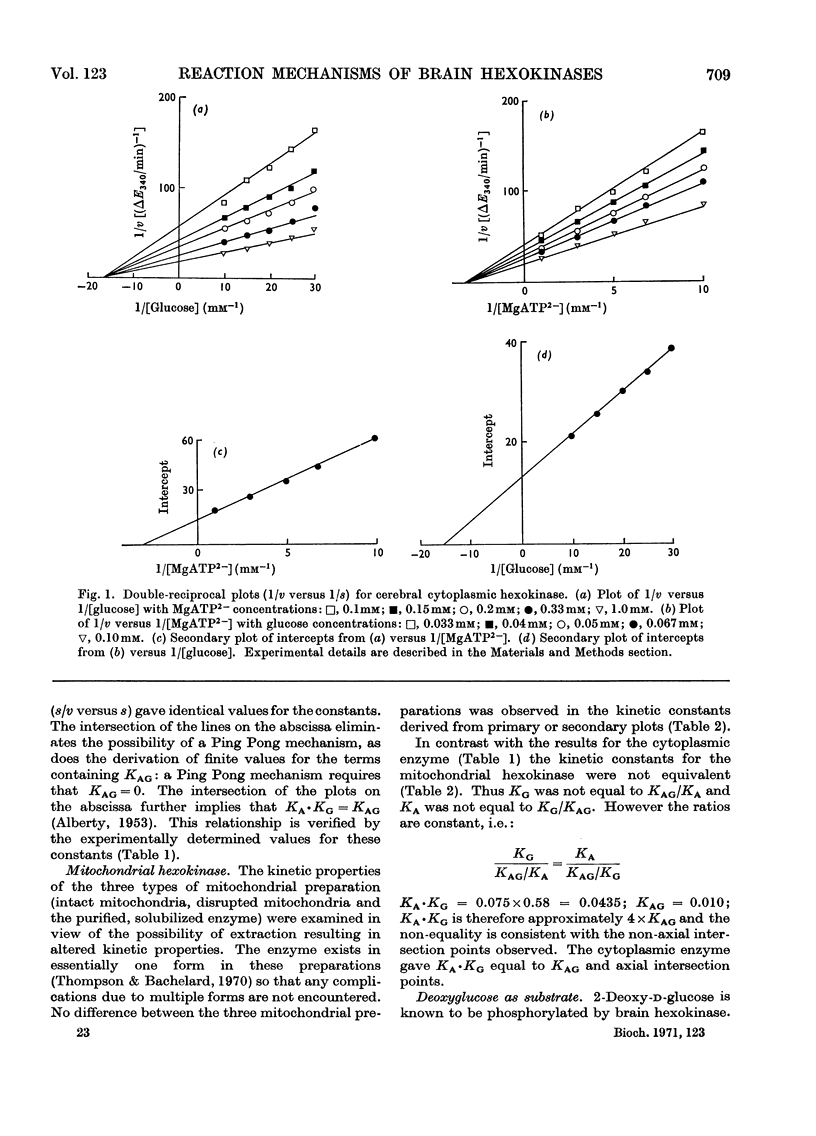
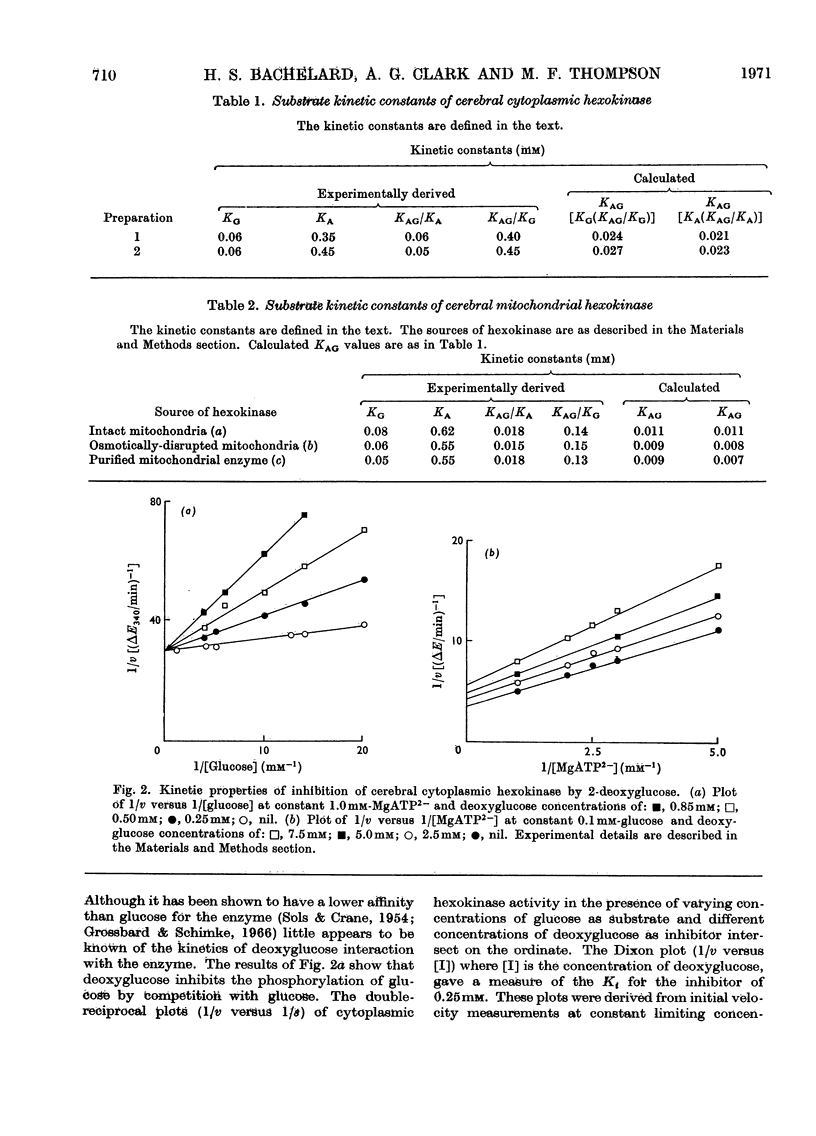
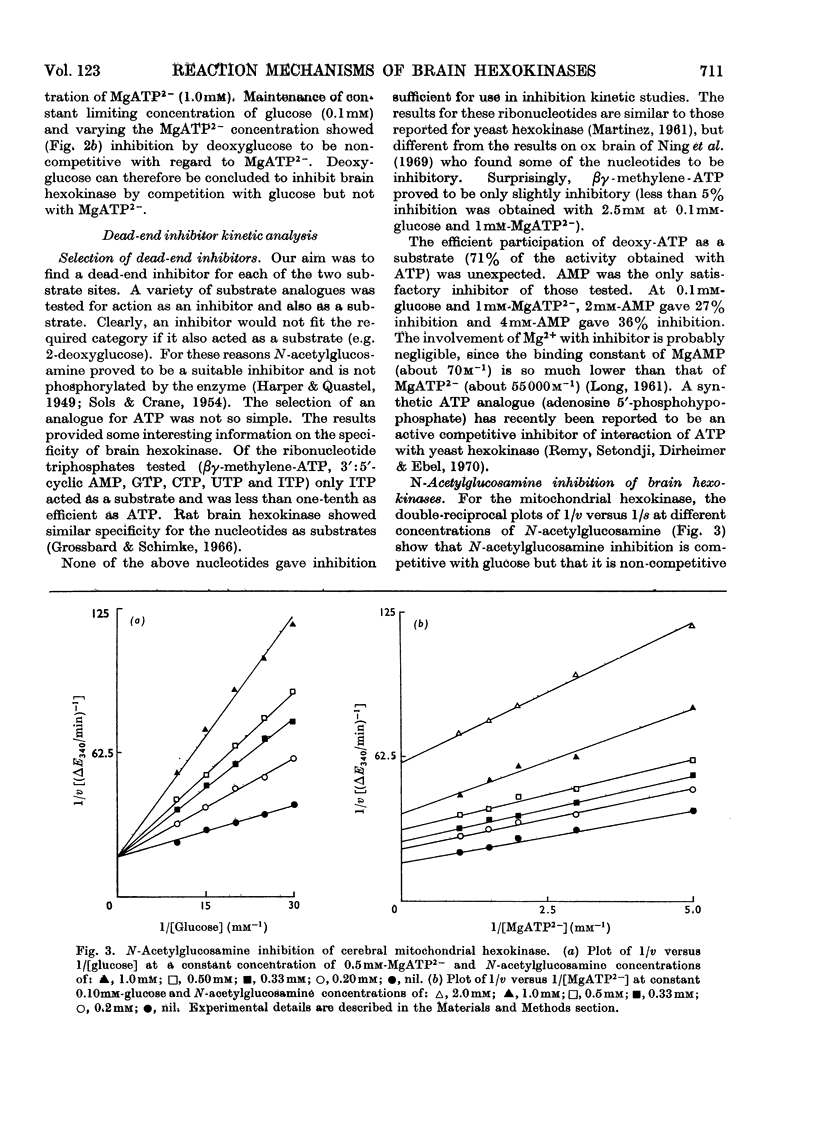
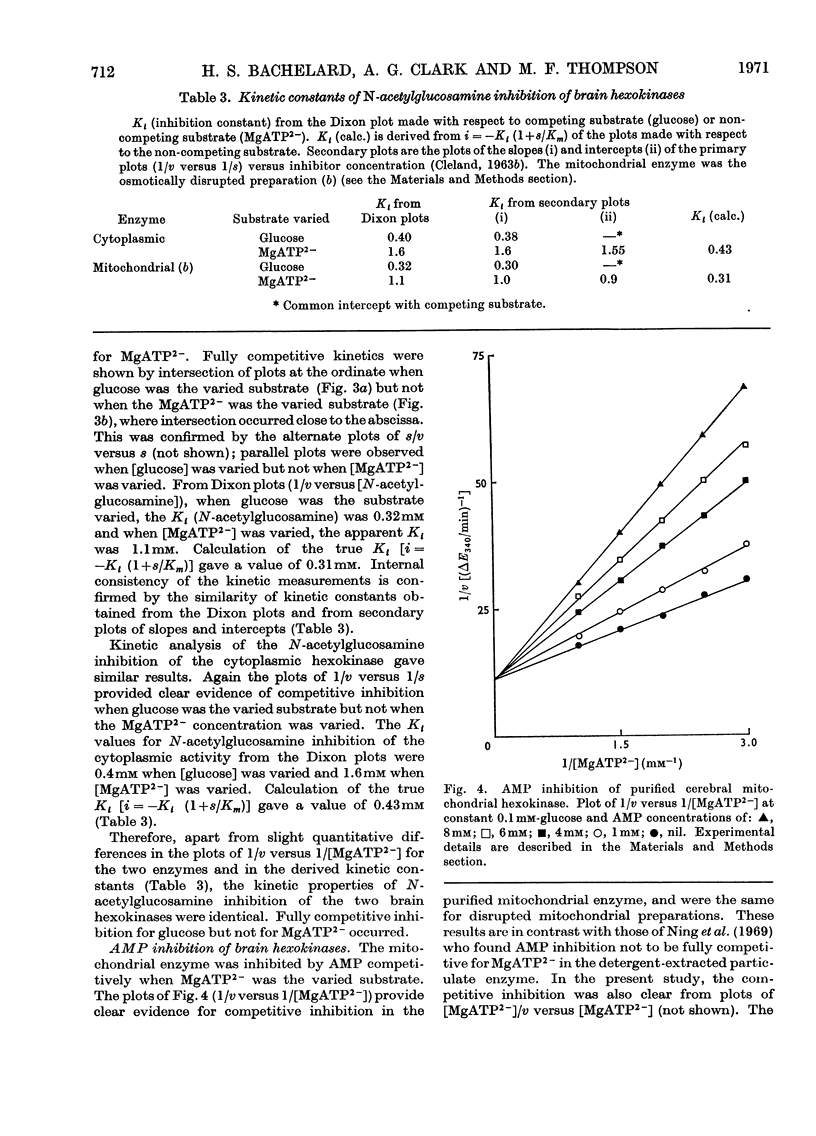
1. The substrate kinetic properties of cerebral hexokinases (mitochondrial and cytoplasmic) were studied at limiting concentrations of both glucose and MgATP2−. Primary plots of the enzymic activity gave no evidence of a Ping Pong mechanism in three types of mitochondrial preparation tested (intact and osmotically disrupted mitochondria, and the purified mitochondrial enzyme), nor in the purified cytoplasmic preparation. 2. Secondary plots of intercepts from the primary plots (1/v versus 1/s) versus reciprocal of second substrate of the mitochondrial activity gave kinetic constants which differed from those obtained directly from the plots of 1/v versus 1/s or of s/v versus s, although the ratios of the derived constants were consistent. The kinetic constants obtained with the cytoplasmic enzyme from primary and secondary plots were consistent. 3. Deoxyglucose, as alternative substrate, inhibited cytoplasmic hexokinase by competition with glucose, but did not compete when MgATP2− was the substrate varied. The Ki for deoxyglucose when glucose concentrations were varied was 0.25mm. 4. A range of ATP analogues was tested as potential substrates and inhibitors of hexokinase activity. GTP, ITP, CTP, UTP and βγ-methylene-ATP did not act as substrates, nor did they cause significant inhibition. Deoxy-ATP proved to be almost as effective a substrate as ATP. AMP inhibited but did not act as substrate. 5. N-Acetyl-glucosamine inhibited all preparations competitively when glucose was varied and non-competitively when MgATP2− was varied. AMP inhibition was competitive when MgATP2− was the substrate varied and non-competitive when glucose was varied. 6. The results are interpreted as providing evidence for a random reaction mechanism in all preparations of brain hexokinase, cytoplasmic and mitochondrial. The kinetic properties and reaction mechanism do not change on extraction and purification of the particulate enzyme. 7. The results are discussed in terms of the participation of hexokinase in regulation of cerebral glycolysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachelard H. S., Goldfarb P. S. Adenine nucleotides and magnesium ions in relation to control of mammalian cerebral-cortex hexokinase. Biochem J. 1969 May;112(5):579–586. doi: 10.1042/bj1120579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelard H. S. Specificity and kinetic properties of monosaccharide uptake into guinea pig cerebral cortex in vitro. J Neurochem. 1971 Feb;18(2):213–222. doi: 10.1111/j.1471-4159.1971.tb00559.x. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Clark A. G. Reversible inhibition in bimolecular rapid equilibrium random order enzyme systems. The effect of substrate-substrate and inhibitor-substrate interactions. Biochem J. 1970 May;117(5):997–1003. doi: 10.1042/bj1170997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copley M., Fromm H. J. Kinetic studies of the brain hexokinase reaction. A reinvestigation with the solubilized bovine enzyme. Biochemistry. 1967 Nov;6(11):3503–3509. doi: 10.1021/bi00863a023. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- FROMM H. J., ZEWE V. Kinetic studies of the brain hexokinase reaction. J Biol Chem. 1962 May;237:1661–1667. [PubMed] [Google Scholar]
- Fromm H. J., Ning J. Kinetic studies of solubilized brain hexokinase with D-fructose as a substrate. Biochem Biophys Res Commun. 1968 Aug 21;32(4):672–677. doi: 10.1016/0006-291x(68)90291-x. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- HARPUR R. P., QUASTEL J. H. Phosphorylation of d-glucosamine by brain extracts. Nature. 1949 Oct 22;164(4173):693–693. doi: 10.1038/164693a0. [DOI] [PubMed] [Google Scholar]
- Ning J., Purich D. L., Fromm H. J. Studies on the kinetic mechanism and allosteric nature of bovine brain hexokinase. J Biol Chem. 1969 Jul 25;244(14):3840–3846. [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Thompson M. F., Bachelard H. S. Cerebral-cortex hexokinase. Comparison of properties of solubilized mitochondrial and cytoplasmic activities. Biochem J. 1970 Jun;118(1):25–34. doi: 10.1042/bj1180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem. 1968 Jul 10;243(13):3640–3647. [PubMed] [Google Scholar]


