Abstract
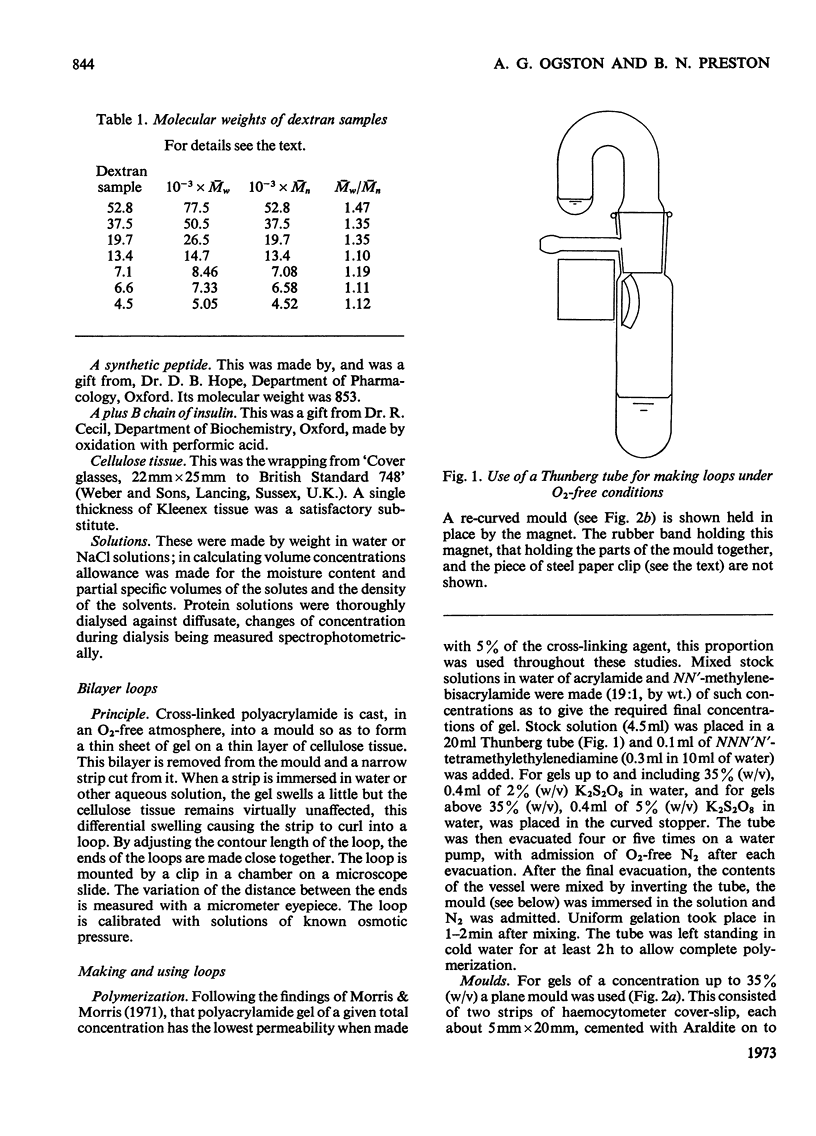
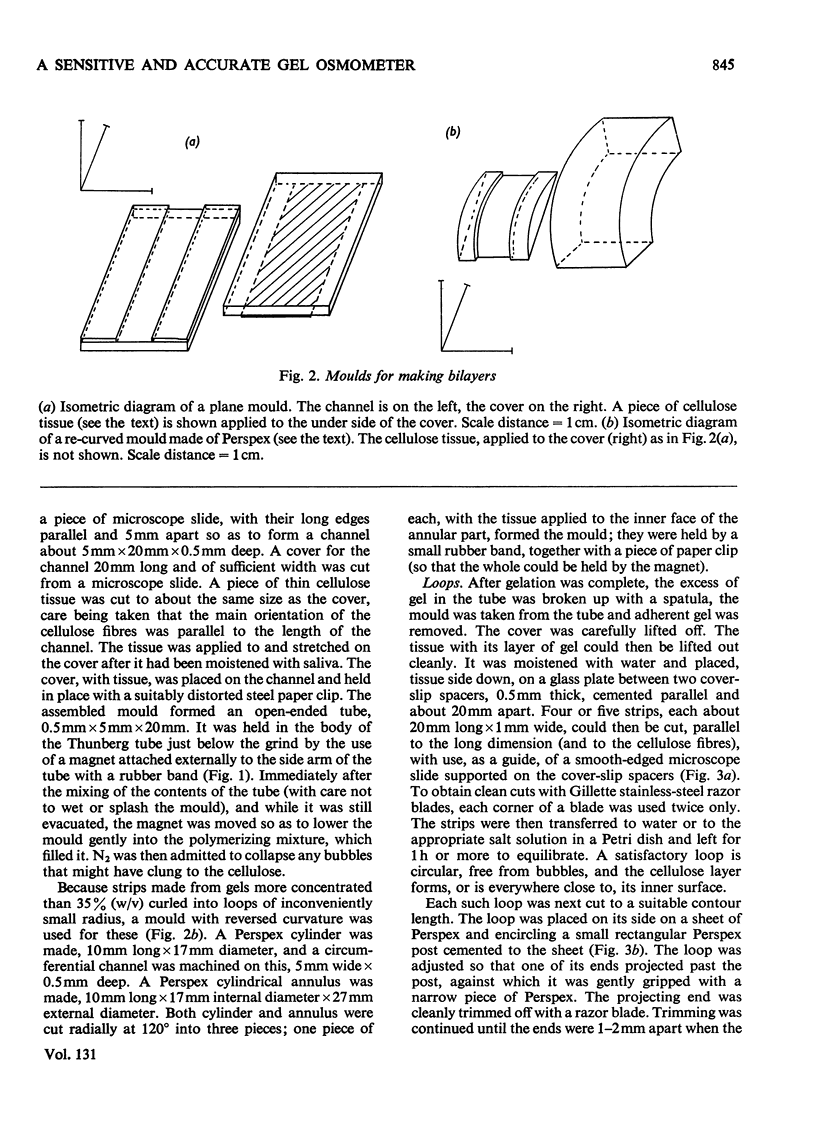
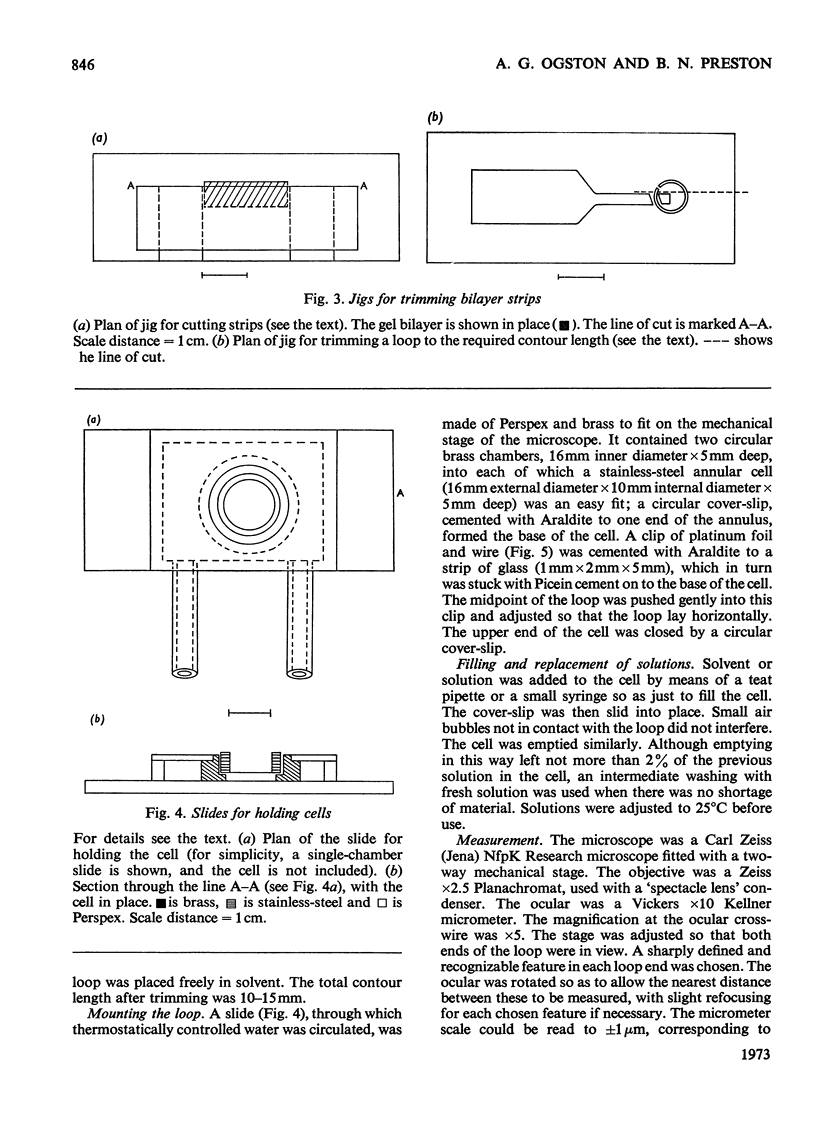
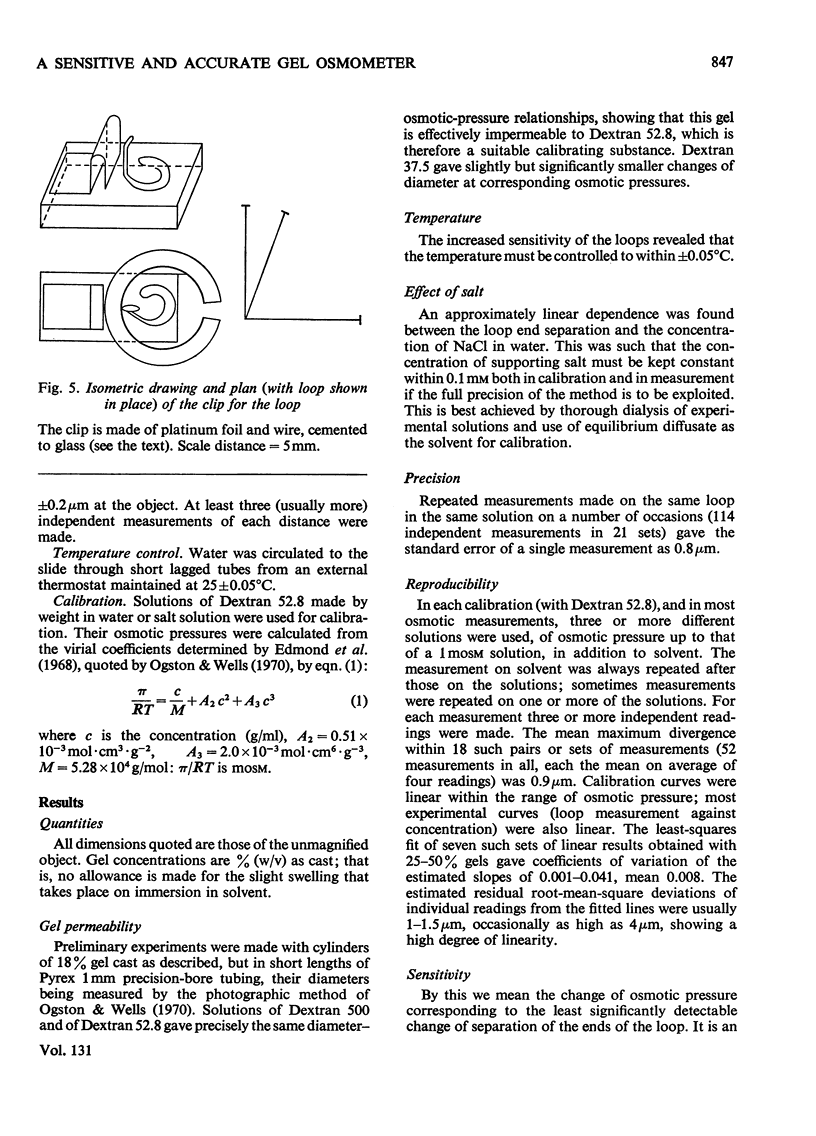
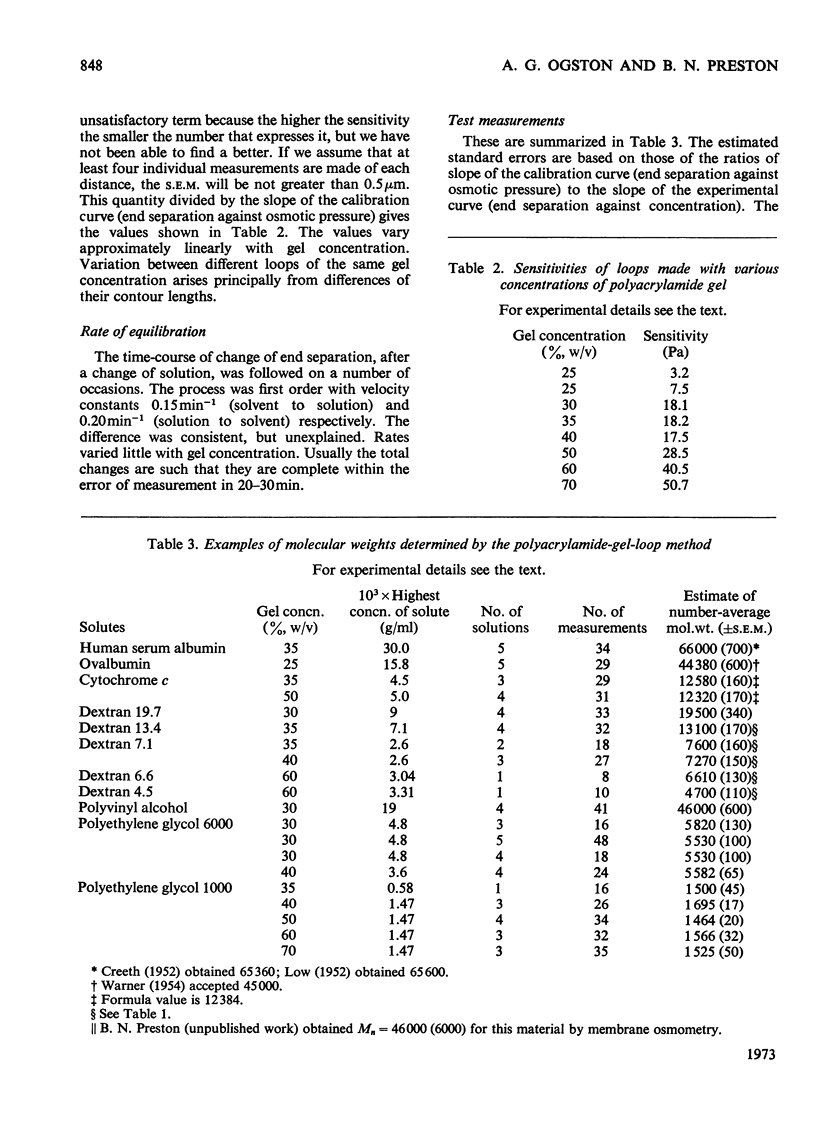
1. A bilayer strip, cut from a thin layer of cross-linked polyacrylamide gel cast on to cellulose tissue, forms an open circular loop whose ends are close together. Shrinkage of the gel, in response to the osmotic pressure of a non-penetrating solution, causes a proportional separation of the ends of the loop. This is measured with a microscope and micrometer eyepiece. 2. The resulting effective sensitivity is about 30 times that of the Sephadex-bead osmometer (Ogston & Wells, 1970), i.e. of the order of 5Pa, comparable with that of a membrane osmometer. Use of gel up to 70% (w/v) allows the measurement of molecular weights, as low as 1500 in favourable cases, with an accuracy of 1–2%. The useful range of osmotic pressure is up to 5kPa. A single measurement requires 0.5ml of solution. Equilibration is completed in 20–30min. 3. The method is illustrated by measurements on human serum albumin, ovalbumin, cytochrome c, samples of dextrans, polyvinyl alcohol, and polyethylene glycols 6000 and 1000.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CREETH J. M. The use of the Gouy diffusiometer with dilute protein solutions; an assessment of the accuracy of the method. Biochem J. 1952 Apr;51(1):10–17. doi: 10.1042/bj0510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond E., Farquhar S., Dunstone J. R., Ogston A. G. The osmotic behaviour of Sephadex and its effects on chromatography. Biochem J. 1968 Aug;108(5):755–763. doi: 10.1042/bj1080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond E., Ogston A. G. An approach to the study of phase separation in ternary aqueous systems. Biochem J. 1968 Oct;109(4):569–576. doi: 10.1042/bj1090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent T. C., Obrink B. On the restriction of the rotational diffusion of proteins in polymer networks. Eur J Biochem. 1972 Jun 23;28(1):94–101. doi: 10.1111/j.1432-1033.1972.tb01888.x. [DOI] [PubMed] [Google Scholar]
- Morris C. J., Morris P. Molecular-sieve chromatography and electrophoresis in polyacrylamide gels. Biochem J. 1971 Sep;124(3):517–528. doi: 10.1042/bj1240517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol L. W., Ogston A. G., Preston B. N. The equilibrium sedimentation of hyaluronic acid and of two synthetic polymers. Biochem J. 1967 Feb;102(2):407–416. doi: 10.1042/bj1020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. G., Silpananta P. The thermodynamics of interaction between Sephadex and penetrating solutes. Biochem J. 1970 Jan;116(2):171–175. doi: 10.1042/bj1160171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. G., Wells J. D. Osmometry with single sephadex beads. Biochem J. 1970 Aug;119(1):67–73. doi: 10.1042/bj1190067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]


