Abstract
1. The metabolism of methylamine in excised shoot tips of tea was studied with micromolar amounts of [14C]methylamine. Of the [14C]methylamine supplied 57% was utilized by tea shoots during the 10h experimental period. 2. The main products of [14C]methylamine metabolism in tea shoots were serine, γ-glutamylmethylamide, theobromine, caffeine and CO2. There was also incorporation of the label into glutamate, aspartate, RNA purine nucleotides and S-adenosylmethionine. 3. The formation of methylamine from γ-glutamylmethylamide was confirmed by feeding tea shoots with γ-glutamyl[14C]methylamide. The products of γ-glutamyl[14C]methylamide metabolism in tea plants were serine, theobromine, caffeine, glutamate and aspartate. 4. The results indicate that the oxidation of methylamine to formaldehyde is the first step of methylamine utilization. Labelled formaldehyde released by the metabolism of methylamine leads to the incorporation of the label into metabolites on the C1 pathways of this compound. It is also suggested that formaldehyde is further oxidized via formate to CO2. 5. The role of γ-glutamylmethylamide in methylamine metabolism in tea plants is discussed. 6. Results support the view that theobromine is the immediate precursor of caffeine.
Full text
PDF



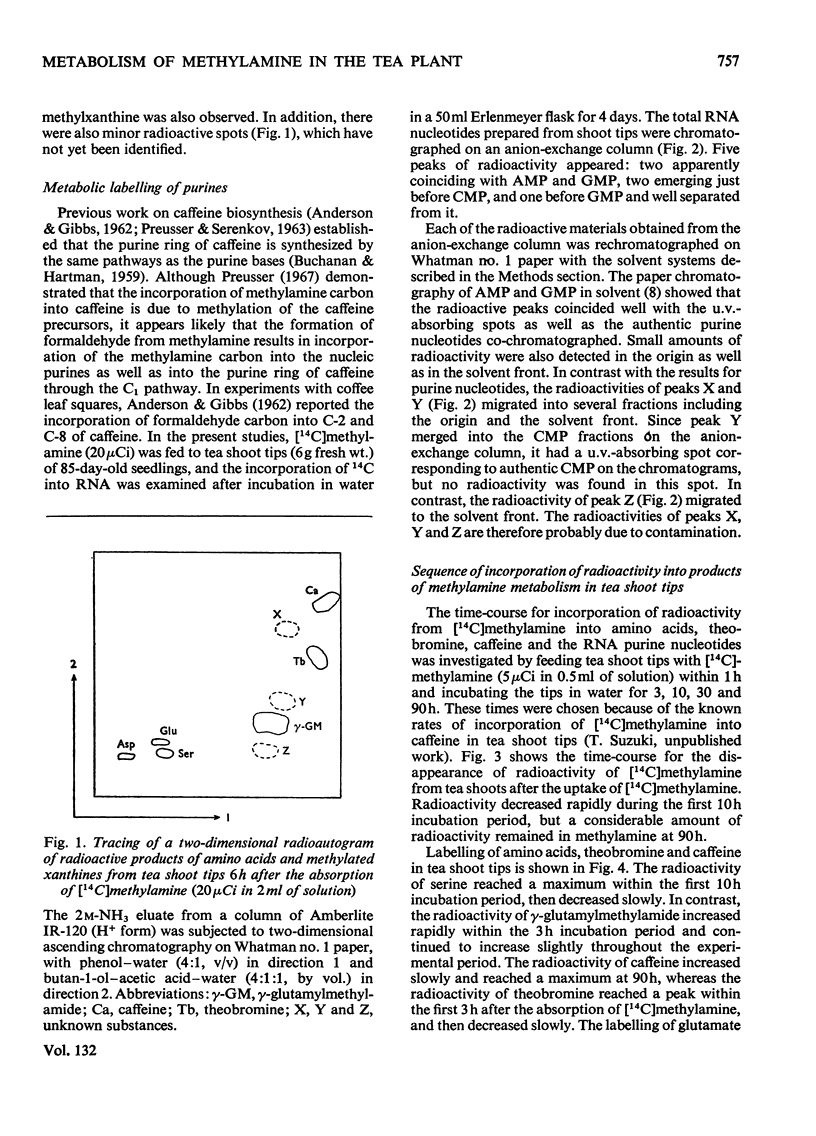
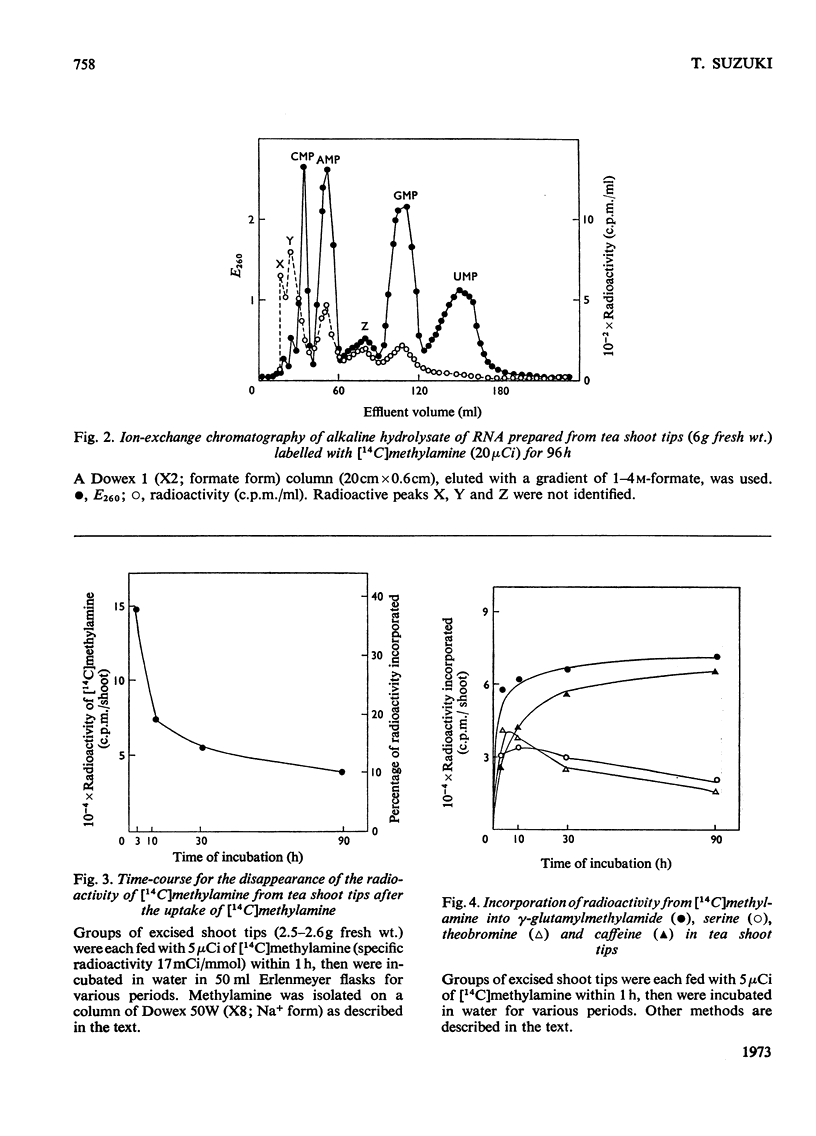
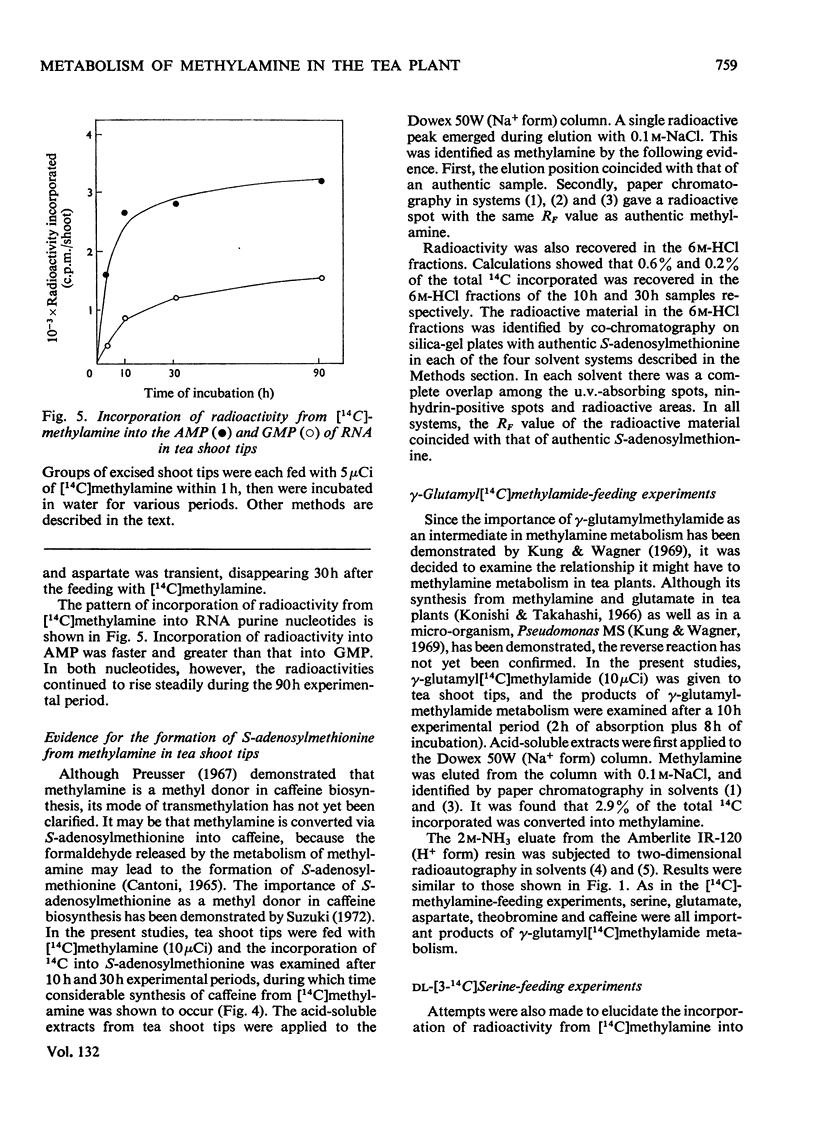




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON L., GIBBS M. The biosynthesis of caffeine in the coffee plant. J Biol Chem. 1962 Jun;237:1941–1944. [PubMed] [Google Scholar]
- Burton E. G., Sakami W. The formation of methionine from the monoglutamate form of methyltetrahydrofolate by higher plants. Biochem Biophys Res Commun. 1969 Jul 23;36(2):228–234. doi: 10.1016/0006-291x(69)90319-2. [DOI] [PubMed] [Google Scholar]
- Cossins E. A., Sinha S. K. The interconversion of glycine and serine by plant tissue extracts. Biochem J. 1966 Nov;101(2):542–549. doi: 10.1042/bj1010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Sinha S. K. The utilization of carbon-1 compounds by plants. II. The formation and metabolism of formate by higher plant tissues. Can J Biochem. 1965 Jun;43(6):685–698. doi: 10.1139/o65-079. [DOI] [PubMed] [Google Scholar]
- DAVISON D. C. Studies on plant formic dehydrogenase. Biochem J. 1951 Sep;49(4):520–526. doi: 10.1042/bj0490520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIKSTEIN S., BERGMANN F., CHAIMOVITZ M. Studies on uric acid and related compounds. II. Paper chromatography of substituted xanthines and uric acids. J Biol Chem. 1956 Jul;221(1):239–251. [PubMed] [Google Scholar]
- Dodd W. A., Cossins E. A. Homocysteine-dependent transmethylases catalyzing the synthesis of methionine in germinating pea seeds. Biochim Biophys Acta. 1970 Mar 24;201(3):461–470. doi: 10.1016/0304-4165(70)90166-2. [DOI] [PubMed] [Google Scholar]
- Dodd W. A., Cossins E. A. Metabolism of S-adenosylmethionine in germinating pea seeds: turnover and possible relationships between recycling of sulfur and transmethylation reactions. Arch Biochem Biophys. 1969 Sep;133(2):216–223. doi: 10.1016/0003-9861(69)90448-2. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Hill J. M., Mann P. J. Further properties of the diamine oxidase of pea seedlings. Biochem J. 1964 Apr;91(1):171–182. doi: 10.1042/bj0910171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller-Adler R. Determination of amine oxidases. Methods Biochem Anal. 1971;(Suppl):35–87. doi: 10.1002/9780470110409.ch2. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Wagner C. Gamma-glutamylmethylamide. A new intermediate in the metabolism of methylamine. J Biol Chem. 1969 Aug 10;244(15):4136–4140. [PubMed] [Google Scholar]
- Kung H. F., Wagner C. Oxidation of C-1 compounds by Pseudomonas sp. MS. Biochem J. 1970 Feb;116(3):357–365. doi: 10.1042/bj1160357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAO A., GREENBERG D. M. Studies on the incorporation of isotope from formaldehyde-C14 and serine-3-C14 into the methyl group of methionine. J Biol Chem. 1958 Feb;230(2):603–620. [PubMed] [Google Scholar]
- OSAWA S., TAKATA K., HOTTA Y. Nuclear and cytoplasmic ribonucleic acids of calf thymus. Biochim Biophys Acta. 1958 May;28(2):271–277. doi: 10.1016/0006-3002(58)90473-6. [DOI] [PubMed] [Google Scholar]
- Ogutuga D. B., Northcote D. H. Biosynthesis of caffeine in tea callus tissue. Biochem J. 1970 May;117(4):715–720. doi: 10.1042/bj1170715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROISER E., SERENKOV G. P. O BIOSINTEZE KOFEINA V LIST'IAKH CHAIA. Biokhimiia. 1963 Sep-Oct;28:857–861. [PubMed] [Google Scholar]
- Roberts G. R., Sanderson G. W. Changes undergone by free amino-acids during the manufacture of black tea. J Sci Food Agric. 1966 Apr;17(4):182–188. doi: 10.1002/jsfa.2740170409. [DOI] [PubMed] [Google Scholar]
- SIEGEL I., LAFAYE J. Formation of the beta-carbon of serine from formaldehyde. Proc Soc Exp Biol Med. 1950 Jul;74(3):620–623. doi: 10.3181/00379727-74-17995. [DOI] [PubMed] [Google Scholar]
- Shah S. P.J., Cossins E. A. Pteroylglutamates and methionine biosynthesis in isolated chloroplasts. FEBS Lett. 1970 Apr 16;7(3):267–270. doi: 10.1016/0014-5793(70)80177-6. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Ehninger D. J. Methods for the analysis and preparation of adenosylmethionine and adenosylhomocysteine. Anal Biochem. 1966 May;15(2):323–333. doi: 10.1016/0003-2697(66)90038-8. [DOI] [PubMed] [Google Scholar]
- Zappia V., Zydek-Cwick R., Schlenk F. The specificity of S-adenosylmethionine derivatives in methyl transfer reactions. J Biol Chem. 1969 Aug 25;244(16):4499–4509. [PubMed] [Google Scholar]


