Abstract
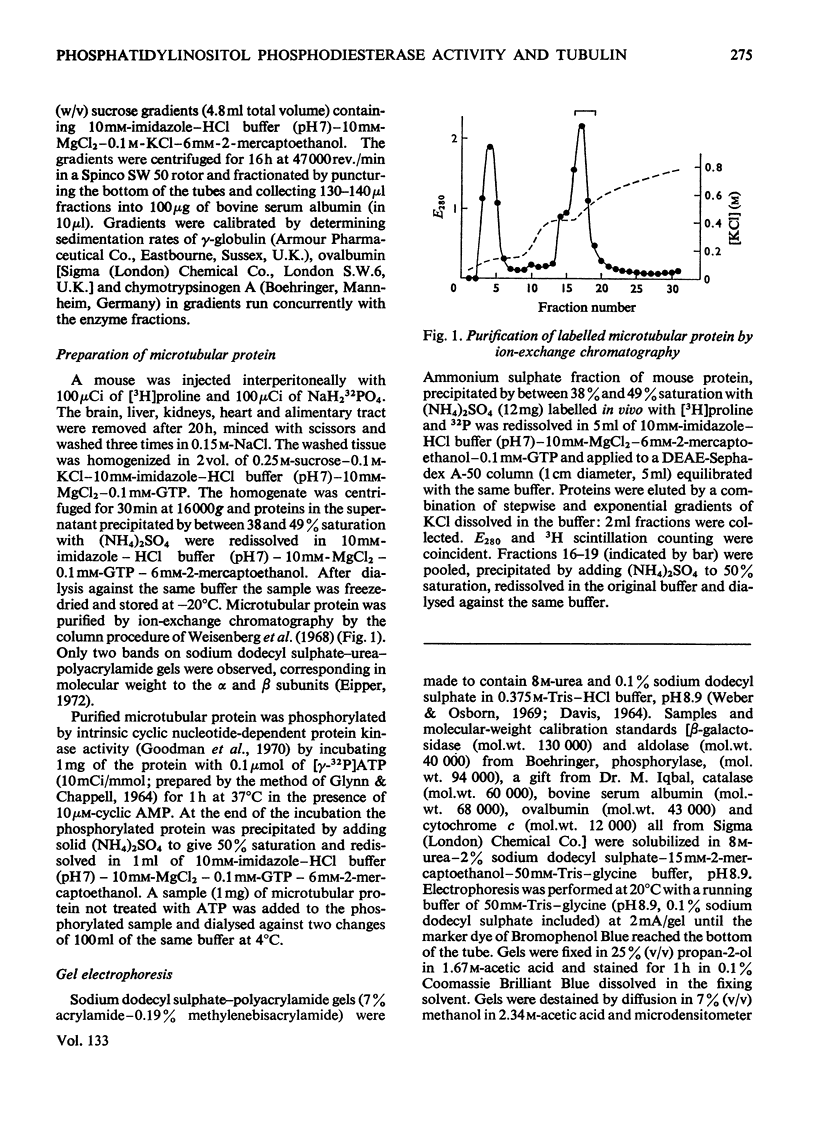
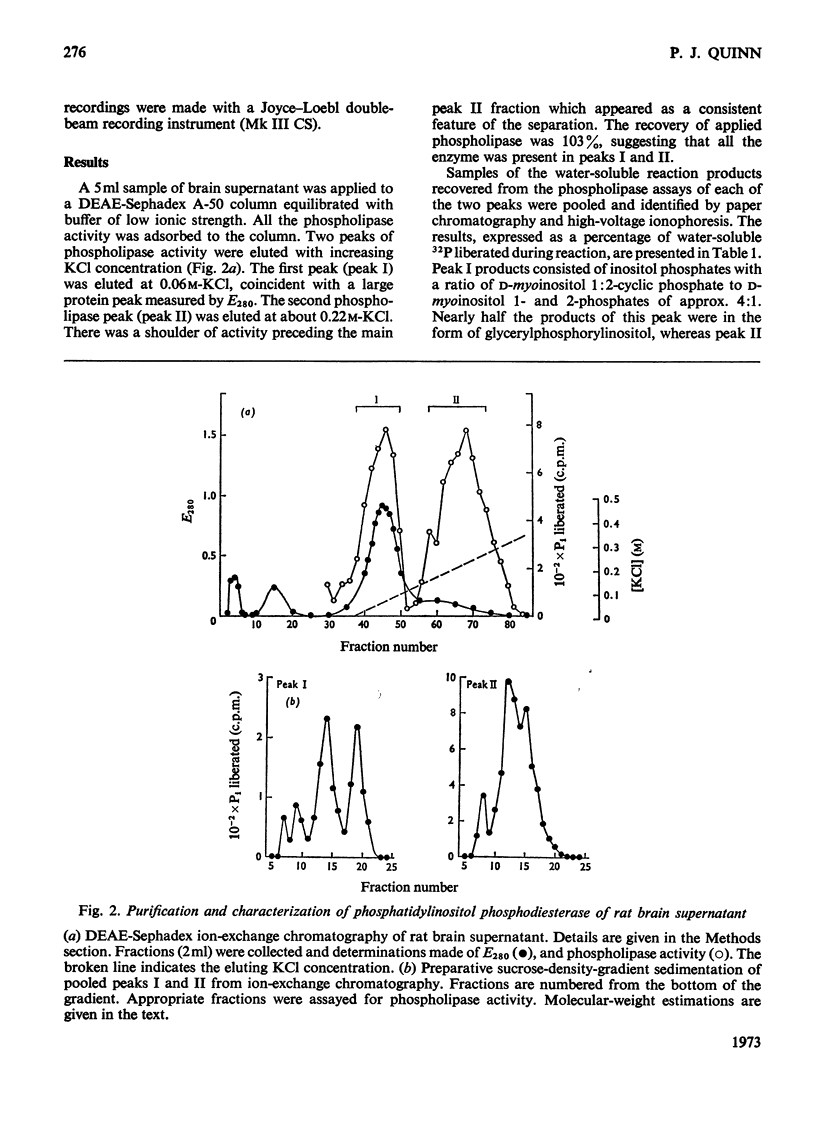
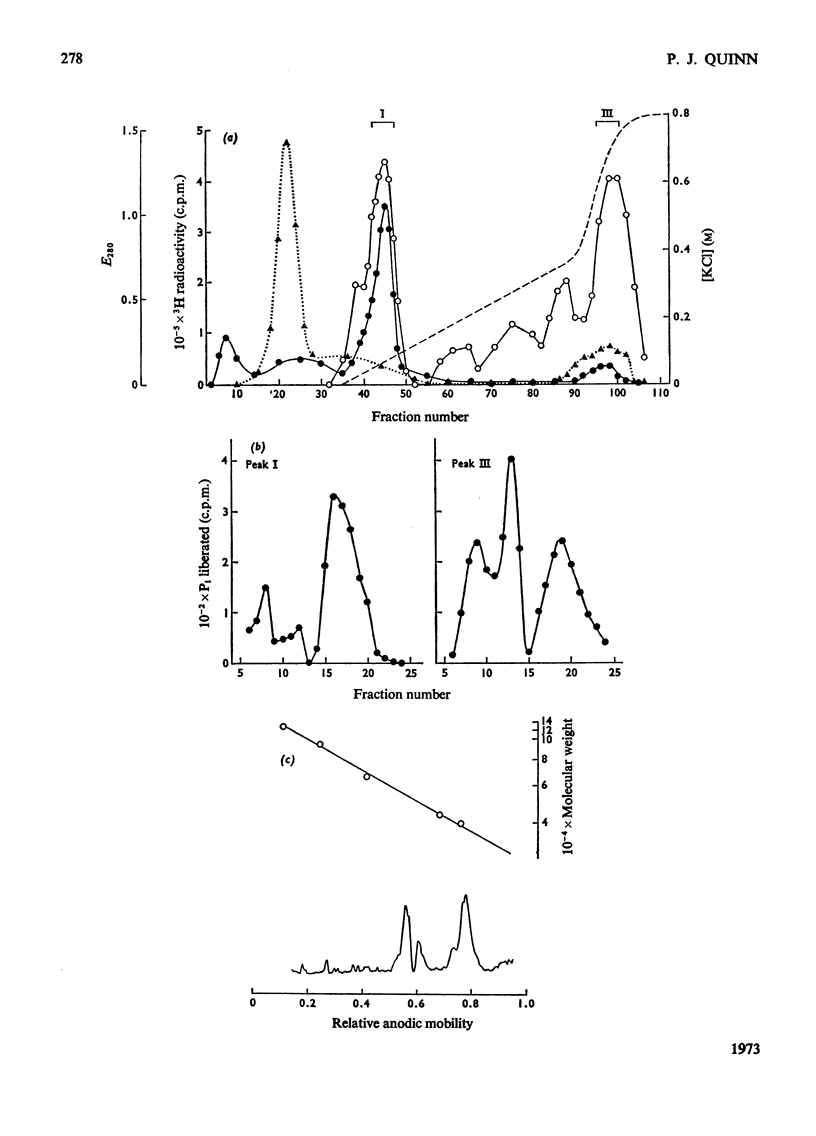
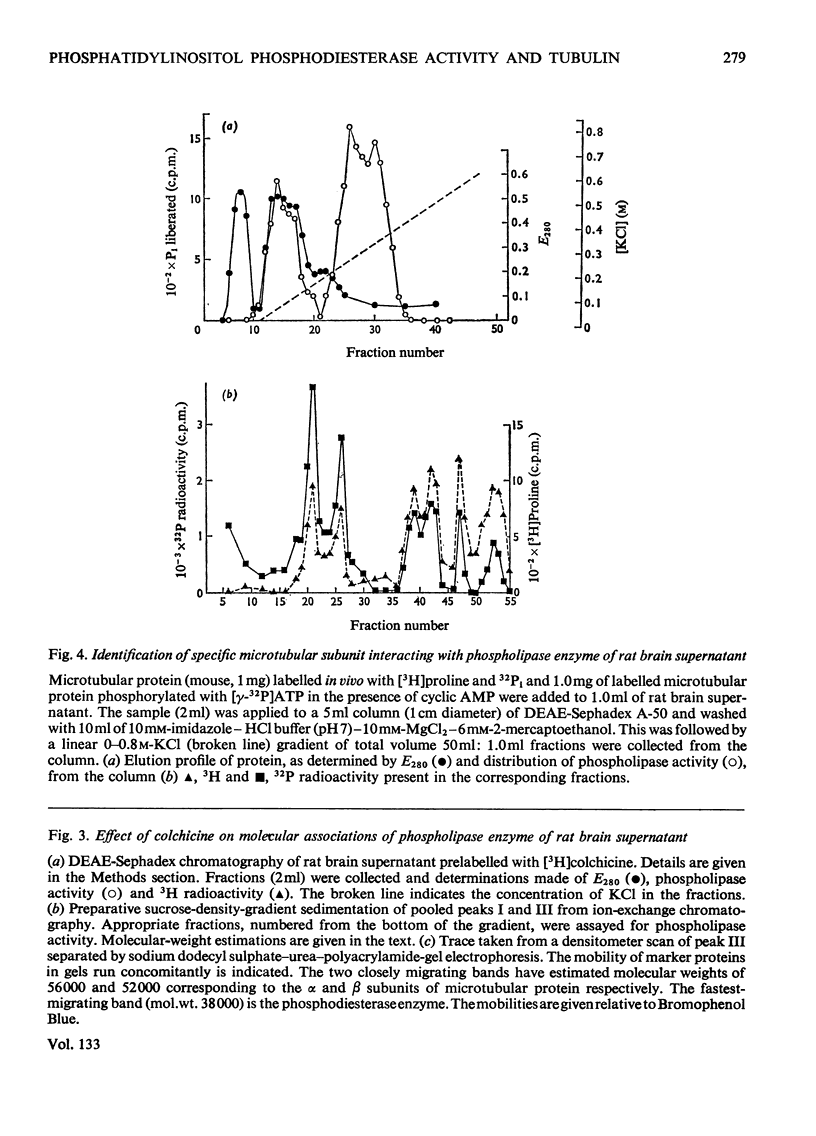
1. Supernatant proteins from rat brain were separated into two fractions containing phosphatidylinositol phosphodiesterase activity by chromatography on DEAE-Sephadex A-50. 2. The first fraction sediments in linear sucrose density gradients in two bands corresponding to molecular weights of 66000 and 36000. There was presumptive evidence that the lighter protein constituted the monomeric form of the enzyme. The second fraction sediments predominantly as a single protein of molecular weight 86000. 3. Treatment of rat brain supernatant with [3H]colchicine abolished the second DEAE-Sephadex peak and removed the lighter protein from the first peak. These proteins emerged in the same position as the protein binding [3H]colchicine at high salt concentration; phospholipase activity was recovered from linear sucrose density gradients in positions corresponding to molecular weights 88000 and 43000, together with an aggregate of molecular weight 140000. Electrophoresis on sodium dodecyl sulphate–urea–polyacrylamide gels of this fraction revealed only three proteins: the α and β-subunits of microtubular protein, of molecular weights 56000 and 52000 respectively, and a protein of molecular weight 38000. 4. A sample of microtubular protein from mouse, labelled in vivo with [3H]proline and 32Pi, was added to rat brain supernatant together with an equal amount of the same microtubular protein treated with cyclic AMP and [γ-32P]ATP and the mixture subsequently characterized by ion-exchange chromatography. Some phospholipase activity characteristic of the second peak from DEAE-Sephadex was associated with one fraction of added microtubular protein. This fraction was identified on the basis of the 3H:32P ratio as the β subunit of the protein treated with ATP and cyclic AMP. The subunit of added microtubular protein untreated with nucleotides was not associated with phospholipase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Borisy G. G., Shelanski M. L., Weisenberg R. C., Taylor E. W. Cytoplasmic filaments and tubules. Fed Proc. 1968 Sep-Oct;27(5):1186–1193. [PubMed] [Google Scholar]
- Atherton R. S., Hawthorne J. N. The phosphoinositide inositolphosphohydrolase of guinea-pig intestinal mucosa. Eur J Biochem. 1968 Mar;4(1):68–75. doi: 10.1111/j.1432-1033.1968.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Bryan J., Wilson L. Are cytoplasmic microtubules heteropolymers? Proc Natl Acad Sci U S A. 1971 Aug;68(8):1762–1766. doi: 10.1073/pnas.68.8.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa de Scarnati O., Rodriguez de Lores Arna Acetylcholine stimulation on phosphatidylinositolinositol phosphohydrolase of rat brain cortex. Biochim Biophys Acta. 1972 Jun 19;270(2):218–225. doi: 10.1016/0005-2760(72)90233-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., DAVENPORT J. B. Improvements in the method of determining individual phospholipids in a complex mixture by successive chemical hydrolyses. Biochem J. 1962 Sep;84:497–501. doi: 10.1042/bj0840497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. Studies on the enzymic hydrolysis of monophosphoinositide by phospholipase preparations from P. notatum and ox pancreas. Biochim Biophys Acta. 1959 May;33(1):68–77. doi: 10.1016/0006-3002(59)90499-8. [DOI] [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Torrontegui G., Berthet J. The action of insulin on the incorporation of [32P]phosphate in the phospholipids of rat adipose tissue. Biochim Biophys Acta. 1966 Jun 1;116(3):477–481. doi: 10.1016/0005-2760(66)90117-2. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fine R. E. Heterogeneity of tubulin. Nat New Biol. 1971 Oct 27;233(43):283–284. doi: 10.1038/newbio233283a0. [DOI] [PubMed] [Google Scholar]
- Fisher B. D., Mueller G. C. Gamma-hexachlorocyclohexane inhibits the initiation of lymphocyte growth by phytohemagglutinin. Biochem Pharmacol. 1971 Sep;20(9):2515–2518. doi: 10.1016/0006-2952(71)90255-3. [DOI] [PubMed] [Google Scholar]
- Fisher D. B., Mueller G. C. An early alteration in the phospholipid metabolism of lymphocytes by phytohemagglutinin. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1396–1402. doi: 10.1073/pnas.60.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedel R. O., Brown J. D., Durell J. The enzymic hydrolysis of phosphatidyl inositol by guinea pig brain: Sub-cellular distribution and hydrolysis products. J Neurochem. 1969 Mar;16(3):371–378. doi: 10.1111/j.1471-4159.1969.tb10376.x. [DOI] [PubMed] [Google Scholar]
- Gaut Z. N., Huggins C. G. Effect of epinephrine on the metabolism of the inositol phosphatides in rat heart in vivo. Nature. 1966 Nov 5;212(5062):612–613. doi: 10.1038/212612a0. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. L., Hawthorne J. N. Metabolism of the phosphoinositides in guinea-pig brain synaptosomes. J Neurochem. 1969 Sep;16(9):1377–1387. doi: 10.1111/j.1471-4159.1969.tb05989.x. [DOI] [PubMed] [Google Scholar]
- Hokin L. E. Dynamic aspects of phospholipids during protein secretion. Int Rev Cytol. 1968;23:187–208. doi: 10.1016/s0074-7696(08)60272-7. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Freinkel N., Dawson R. M. The metabolism of phosphatidylinositol in the thyroid gland of the pig. Biochem J. 1971 Jun;123(1):19–33. doi: 10.1042/bj1230019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. L., WALLACH D. F. The metabolic basis of phagocytosis. III. Incorporation of inorganic phosphate into various classes of phosphatides during phagocytosis. J Biol Chem. 1961 Jul;236:1895–1901. [PubMed] [Google Scholar]
- KEMP P., HUBSCHER G., HAWTHORNE J. N. Phosphoinositides. 3. Enzymic hydrolysis of inositol-containing phospholipids. Biochem J. 1961 Apr;79:193–200. doi: 10.1042/bj0790193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai M., White G. L., Hawthorne J. N. The phosphatidylinositol kinase of rat brain. Biochem J. 1966 Nov;101(2):328–337. doi: 10.1042/bj1010328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keough K. M., Thompson W. Soluble and particulate forms of phosphoinositide phosphodiesterase in ox brain. Biochim Biophys Acta. 1972 Jul 7;270(3):324–336. doi: 10.1016/0005-2760(72)90197-x. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., LEICHT W. S. METABOLISM OF PHOSPHATIDYL INOSITOL AND OTHER LIPIDS IN ACTIVE NEURONES OF SYMPATHETIC GANGLIA AND OTHER PERIPHERAL NERVOUS TISSUES. THE SITE OF THE INOSITIDE EFFECT. J Neurochem. 1965 Jan;12:1–13. doi: 10.1111/j.1471-4159.1965.tb10245.x. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Froscio M. Cyclic adenosine 3':5'-monophosphate and microtubule function: specific interaction of the phosphorylated protein subunits with a soluble brain component. Biochem Biophys Res Commun. 1971 Sep;44(5):1089–1095. doi: 10.1016/s0006-291x(71)80197-3. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Friedrichs B. Turnover of mammalian phospholipids. Rates of turnover and metabolic heterogeneity in cultured human lymphocytes and in tissues of healthy, starved and vitamin A-deficient rats. Biochem J. 1970 Sep;119(3):481–488. doi: 10.1042/bj1190481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumphrey A. M. Incorporation of [32P]orthophosphate into brain-slice phospholipids and their precursors. Effects of electrical stimulation. Biochem J. 1969 Mar;112(1):61–70. doi: 10.1042/bj1120061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry P. S., Hokin L. E. Studies on the role of phospholipids in phagocytosis. J Biol Chem. 1966 Jul 25;241(14):3354–3361. [PubMed] [Google Scholar]
- Schacht J., Agranoff B. W. Effects of acetylcholine on labeling of phosphatidate and phosphoinositides by ( 32 P) orthophosphate in nerve ending fractions of guinea pig cortex. J Biol Chem. 1972 Feb 10;247(3):771–777. [PubMed] [Google Scholar]
- Schneider P. B. Determination of specific activity of 32P-labeled compounds using Cerenkov counting. J Nucl Med. 1971 Jan;12(1):14–16. [PubMed] [Google Scholar]
- Scott T. W., Mills S. C., Freinkel N. The mechanism of thyrotrophin action in relation to lipid metabolism in thyroid tissue. Biochem J. 1968 Sep;109(3):325–332. doi: 10.1042/bj1090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Yagihara Y., Hawthrone J. N. Effects of acetylcholine on the incorporation of ( 32 P) orthophosphate in vitro into the phospholipids of nerve-ending particles from guinea pig brain. J Neurochem. 1972 Feb;19(2):355–367. doi: 10.1111/j.1471-4159.1972.tb01345.x. [DOI] [PubMed] [Google Scholar]


