Abstract
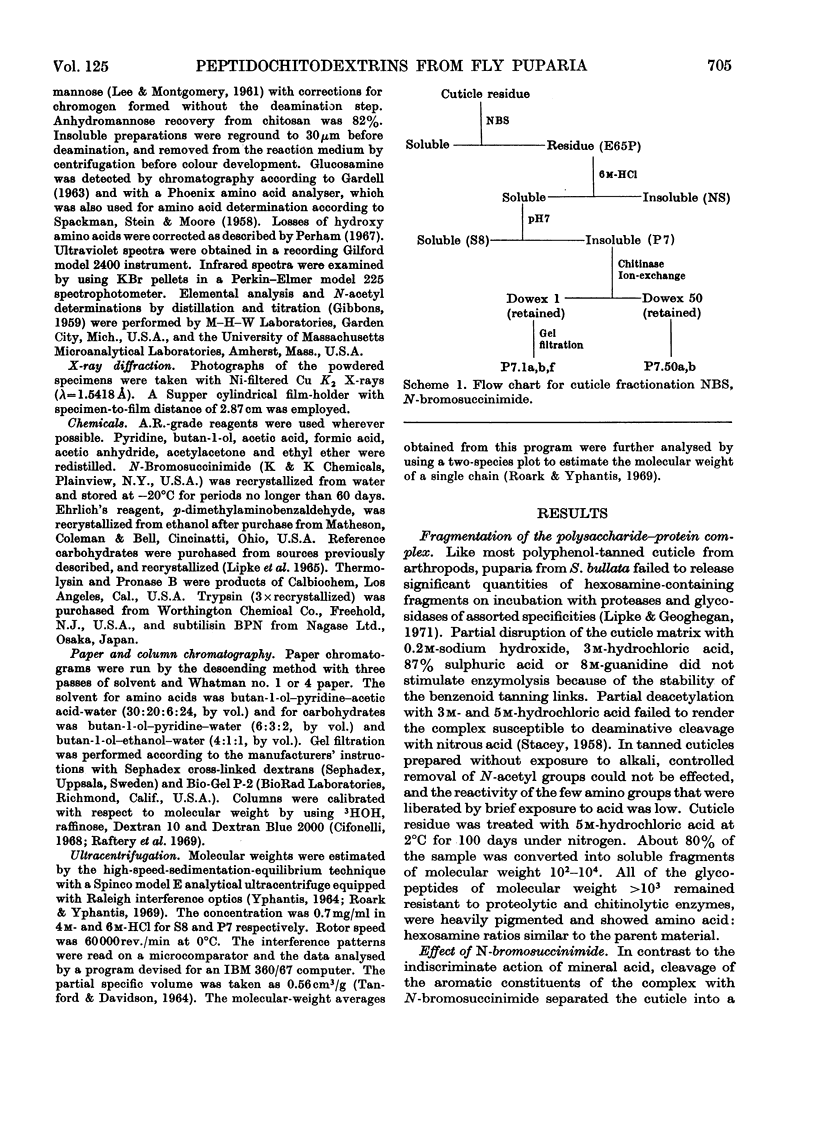
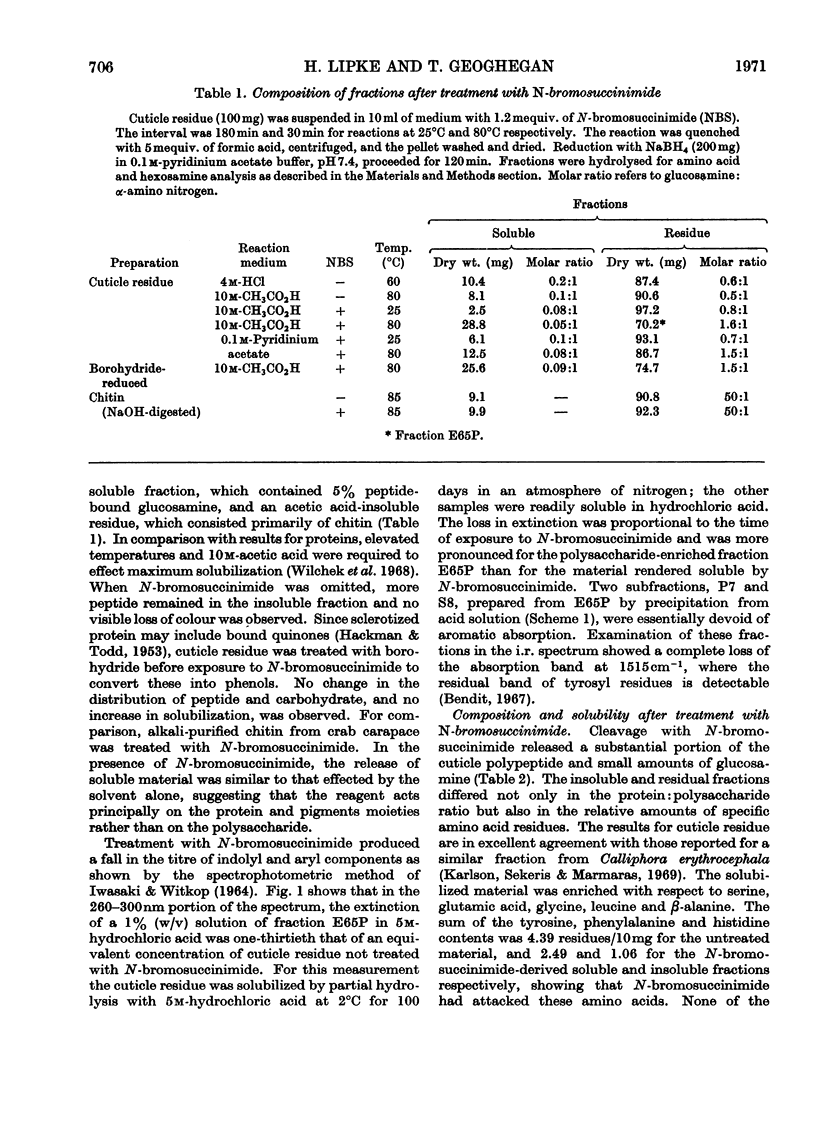
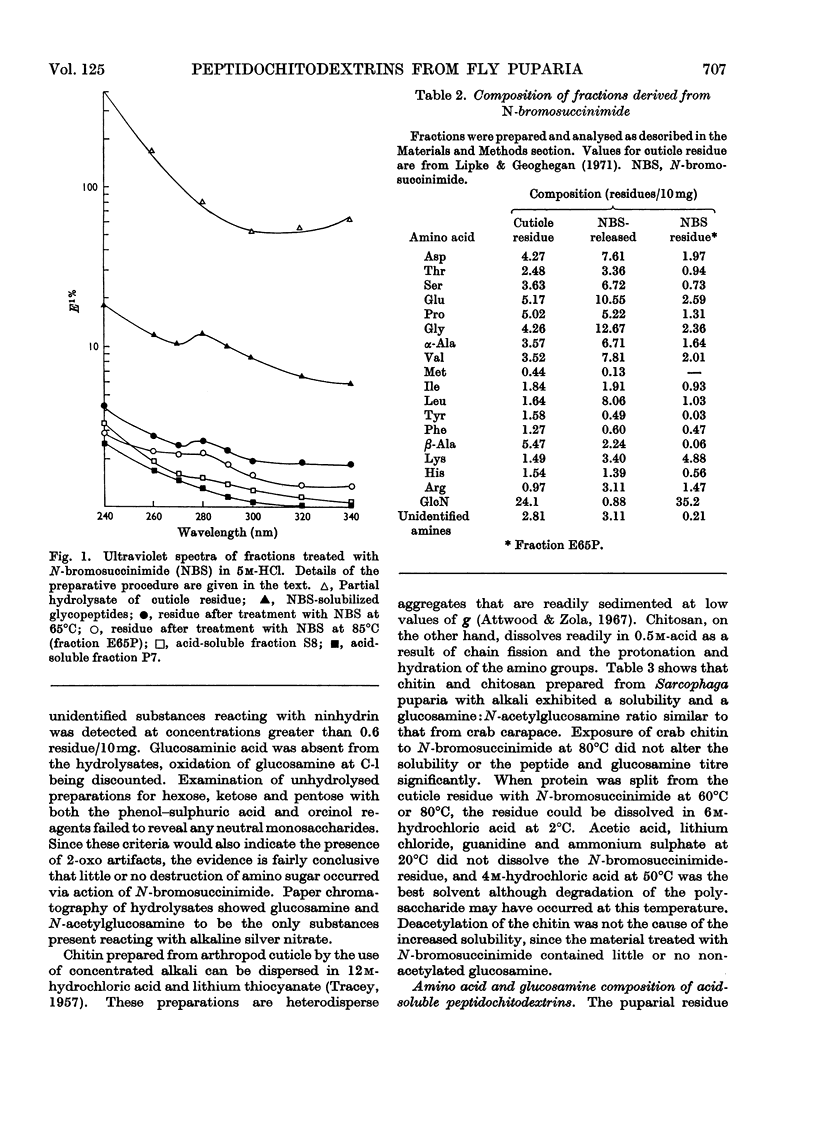
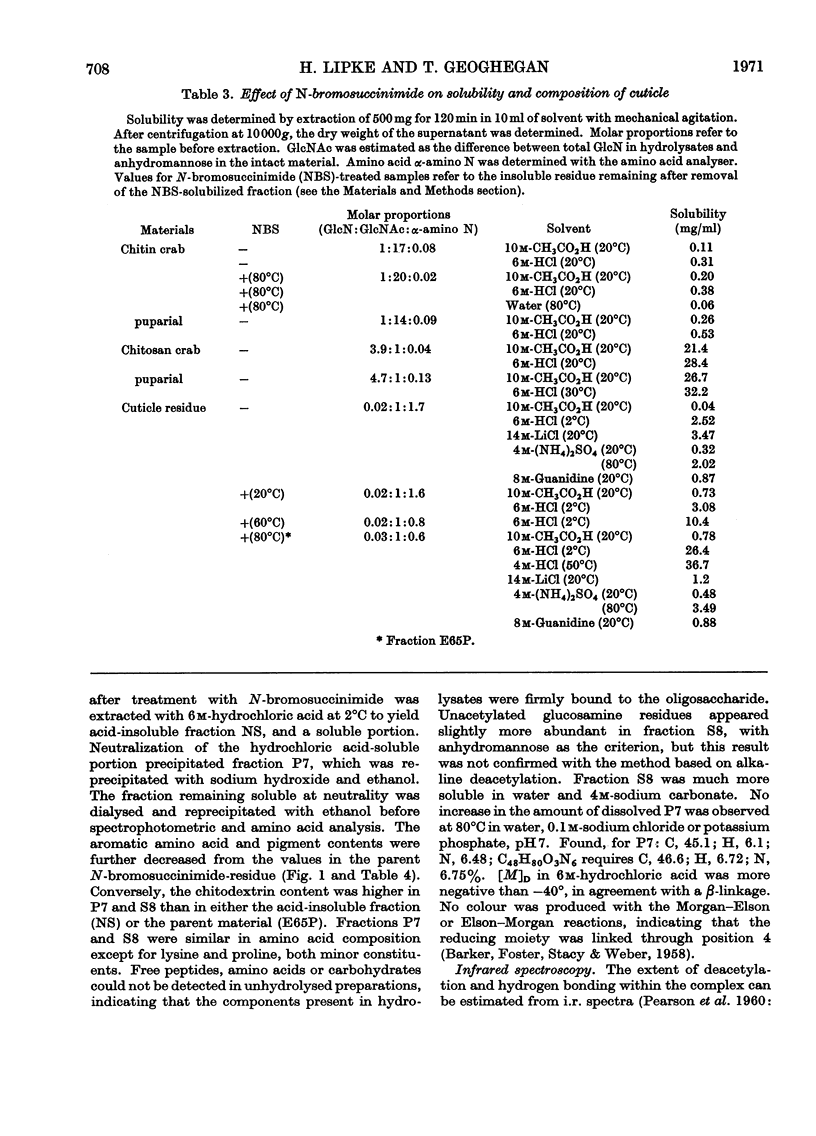
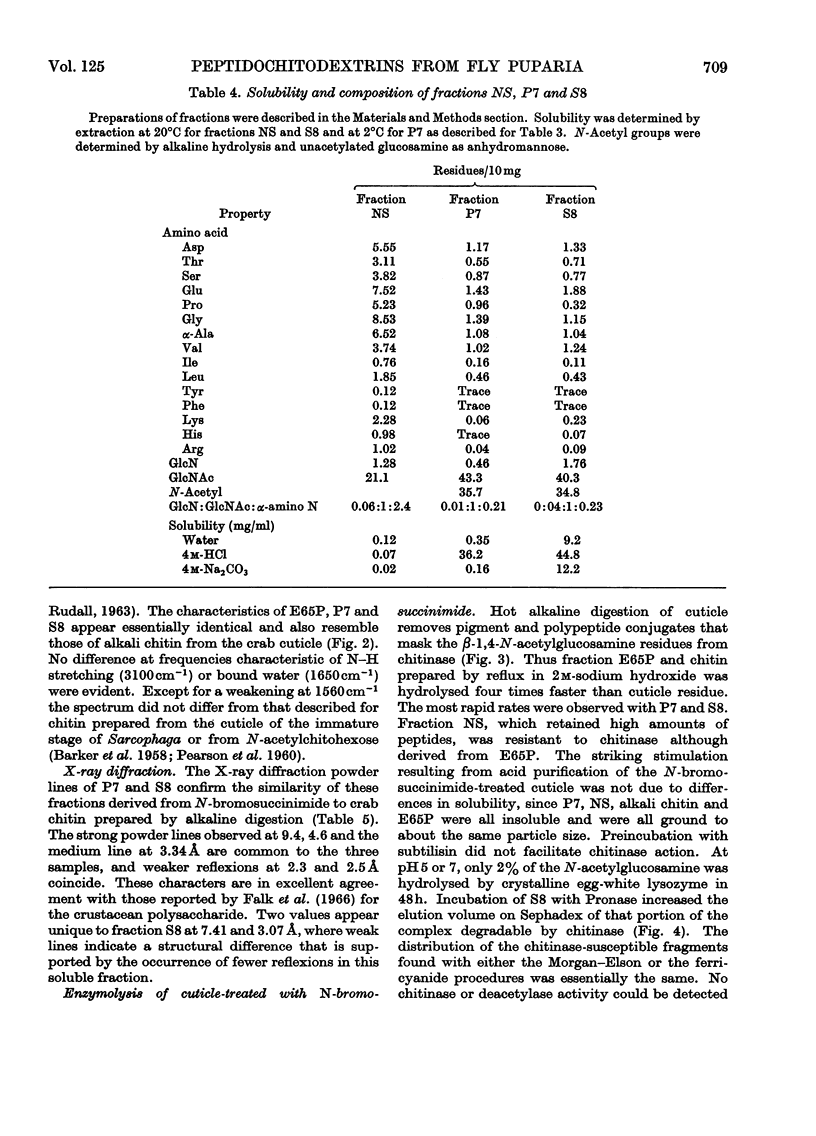
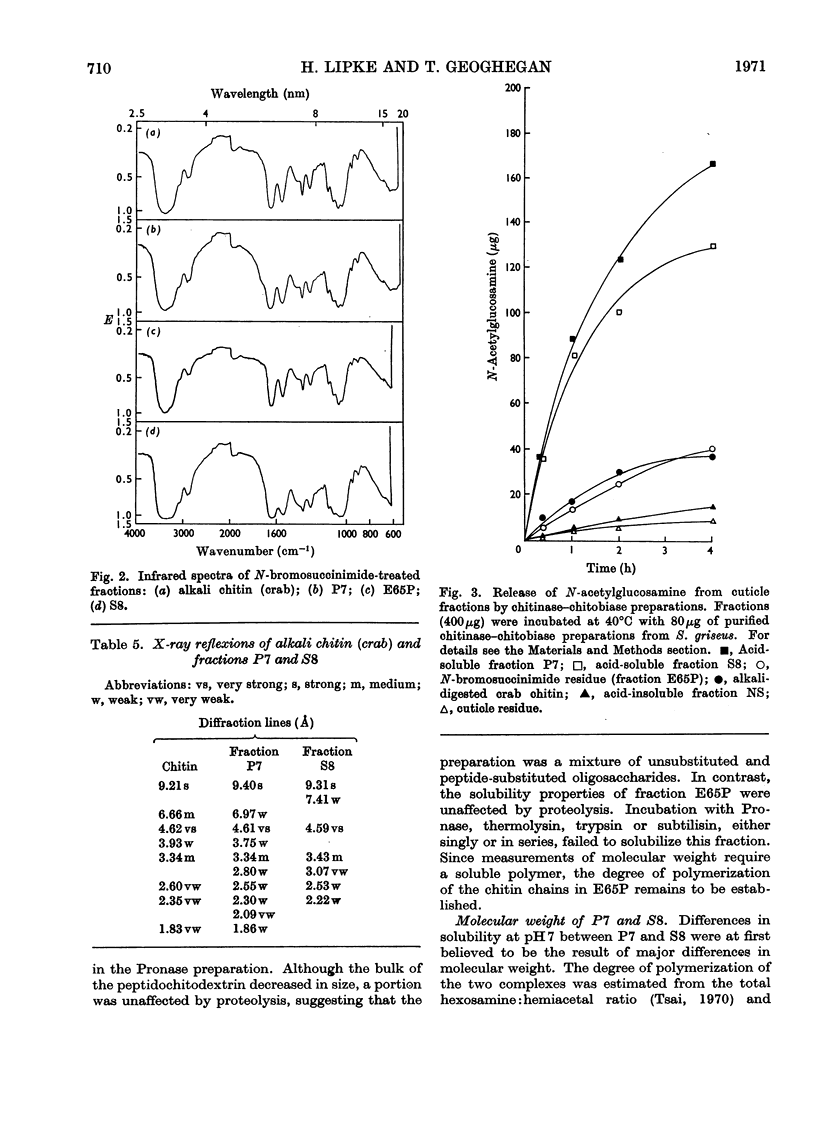
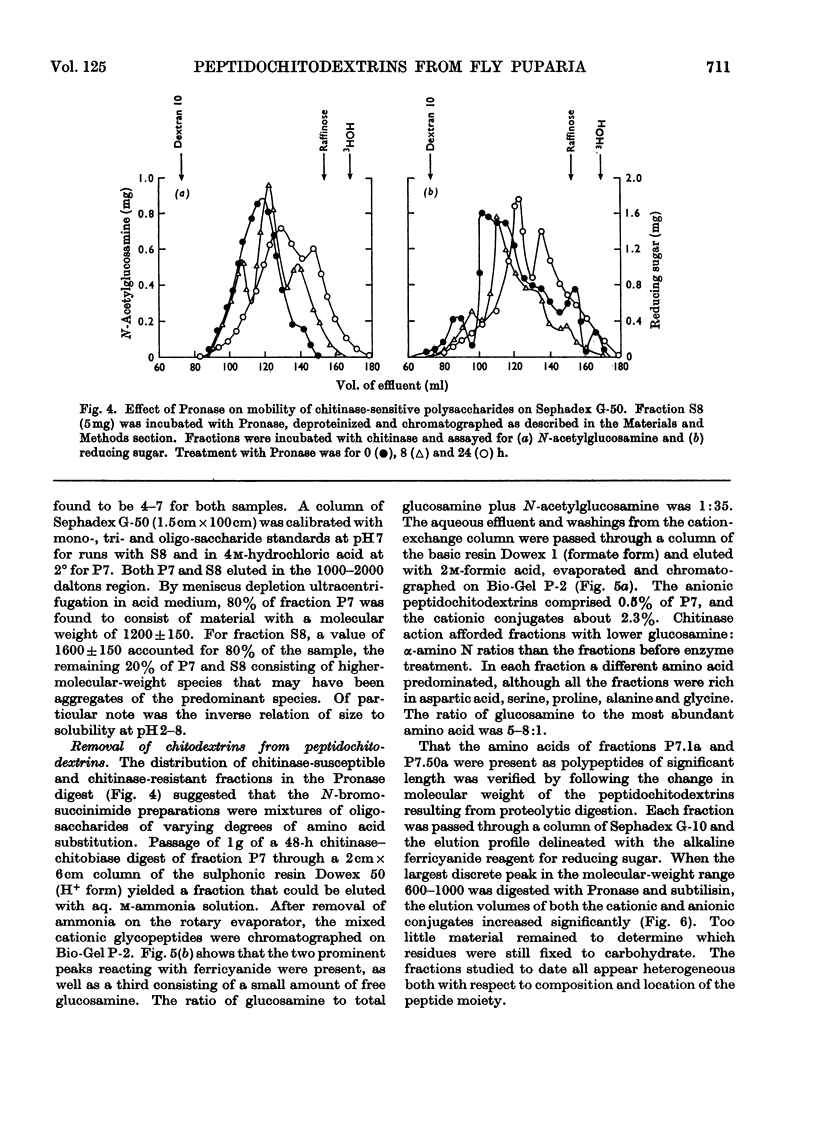
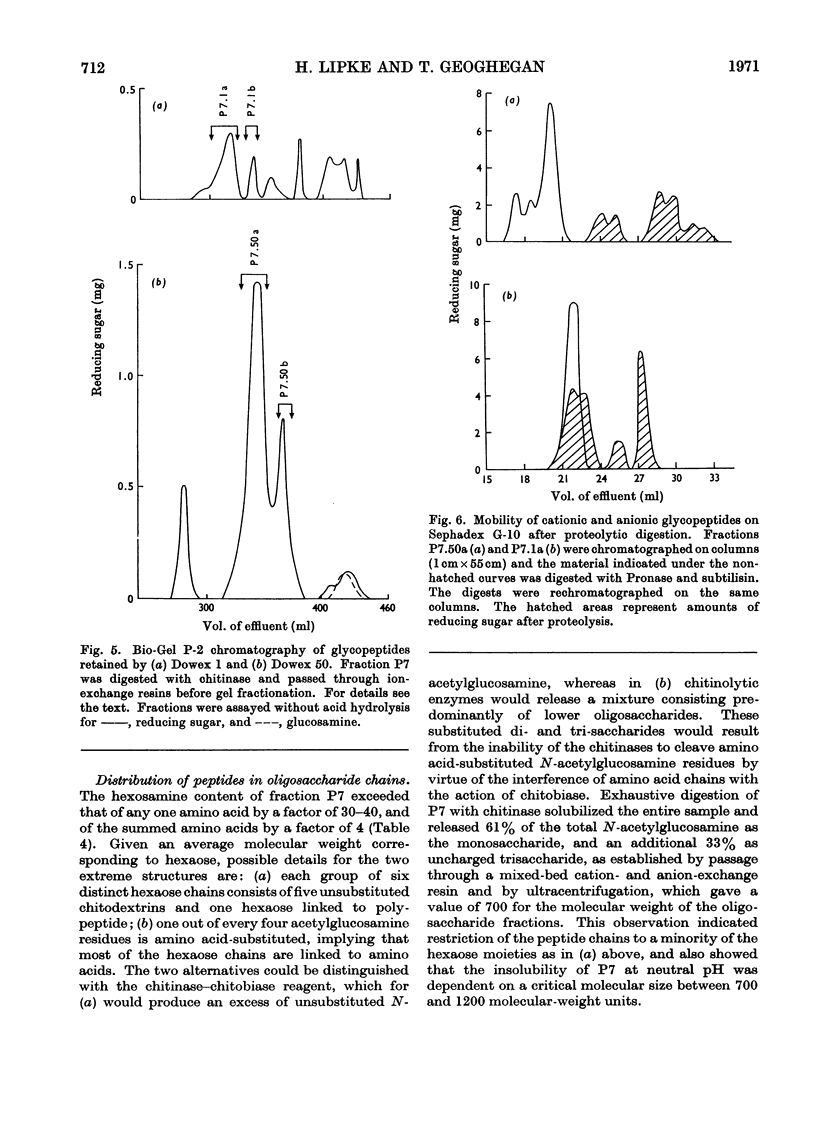
1. N-Bromosuccinimide cleaved proteins and pigments from fly puparia, increasing the chitin:protein ratio from 0.5 to 1.5. The product afforded subfractions (ratio 5:1) of molecular weights of 1200 and 1600 devoid of aromatic residues and N-terminal β-alanine, direct aryl links between polysaccharide chains being discounted. 2. The chitin–protein complex decreased in molecular weight when treated with Pronase, which suggested polypeptide bridges within the native chitin micelle. The limit dextrins generated by chitinase were mixtures of unsubstituted dextrins and peptidylated oligosaccharides, with the former predominating. 3. Peptidochitodextrins of similar molecular weight but markedly different solubility were prepared, which were indistinguishable with respect to amino acid, glucosamine, acetyl, X-ray or infrared characteristics. It is suggested that physical interactions contribute to the stability of the integument in addition to the covalent bonds that form during sclerotization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodnaryk R. P., Levenbook L. The role of beta-alanyl-L-tyrosine (sarcophagine) in puparium formation in the fleshfly Sarcophaga bullata. Comp Biochem Physiol. 1969 Sep 1;30(5):909–921. doi: 10.1016/0010-406x(69)90046-2. [DOI] [PubMed] [Google Scholar]
- Brunet P. C. The metabolism of aromatic compounds. Biochem Soc Symp. 1965;25:49–77. [PubMed] [Google Scholar]
- GIBBONS R. A. Chemical properties of two mucoids from bovine cervical mucin. Biochem J. 1959 Oct;73:209–217. doi: 10.1042/bj0730209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKMAN R. H., TODD A. R. Some observations on the reaction of catechol derivatives with amines and amino acids in presence of oxidizing agents. Biochem J. 1953 Nov;55(4):631–637. doi: 10.1042/bj0550631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman R. H., Goldberg M. Studies on chitin. VI. The nature of alpha- and beta-chitins. Aust J Biol Sci. 1965 Aug;18(4):935–946. doi: 10.1071/bi9650935. [DOI] [PubMed] [Google Scholar]
- Kuo M. J., Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967 Sep;94(3):624–629. doi: 10.1128/jb.94.3.624-629.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. C., MONTGOMERY R. Determination of hexosamines. Arch Biochem Biophys. 1961 May;93:292–296. doi: 10.1016/0003-9861(61)90265-x. [DOI] [PubMed] [Google Scholar]
- LIPKE H., GRAINGER M. M., SIAKOTOS A. N. POLYSACCHARIDE AND GLYCOPROTEIN FORMATION IN THE COCKROACH. I. IDENTITY AND TITER OF BOUND MONOSACCHARIDES. J Biol Chem. 1965 Feb;240:594–600. [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M. High recovery of tryptophan from acid hydrolysates of proteins. Biochem Biophys Res Commun. 1969 Apr 29;35(2):175–181. doi: 10.1016/0006-291x(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery M. A., Rand-Meir T., Dahlquist F. W., Parsons S. M., Borders C. L., Jr, Wolcott R. G., Beranek W., Jr, Jao L. Separation of glycosaminoglycan saccharide and glycoside mixtures by gel filtration. Anal Biochem. 1969 Sep;30(3):427–435. doi: 10.1016/0003-2697(69)90137-7. [DOI] [PubMed] [Google Scholar]
- Roark D. E., Yphantis D. A. Studies of self-associating systems by equilibrium ultracentrifugation. Ann N Y Acad Sci. 1969 Nov 7;164(1):245–278. doi: 10.1111/j.1749-6632.1969.tb14043.x. [DOI] [PubMed] [Google Scholar]
- Rudall K. M. Skeletal structure in insects. Biochem Soc Symp. 1965;25:83–92. [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]
- Tsai C. S. Determination of degree of polymerization of N-acetyl chitooligoses by chromatographic methods. Anal Biochem. 1970 Jul;36(1):114–122. doi: 10.1016/0003-2697(70)90338-6. [DOI] [PubMed] [Google Scholar]
- Wilchek M., Spande T., Milne G., Witkop B. The nonenzymatic conversion of tyrosine into mono- and dihydroxyindoles. Biochemistry. 1968 May;7(5):1777–1786. doi: 10.1021/bi00845a023. [DOI] [PubMed] [Google Scholar]
- Witkop B. Chemical cleavage of proteins. Selective fragmentations and modifications reveal structure. Science. 1968 Oct 18;162(3851):318–326. doi: 10.1126/science.162.3851.318. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


