Abstract
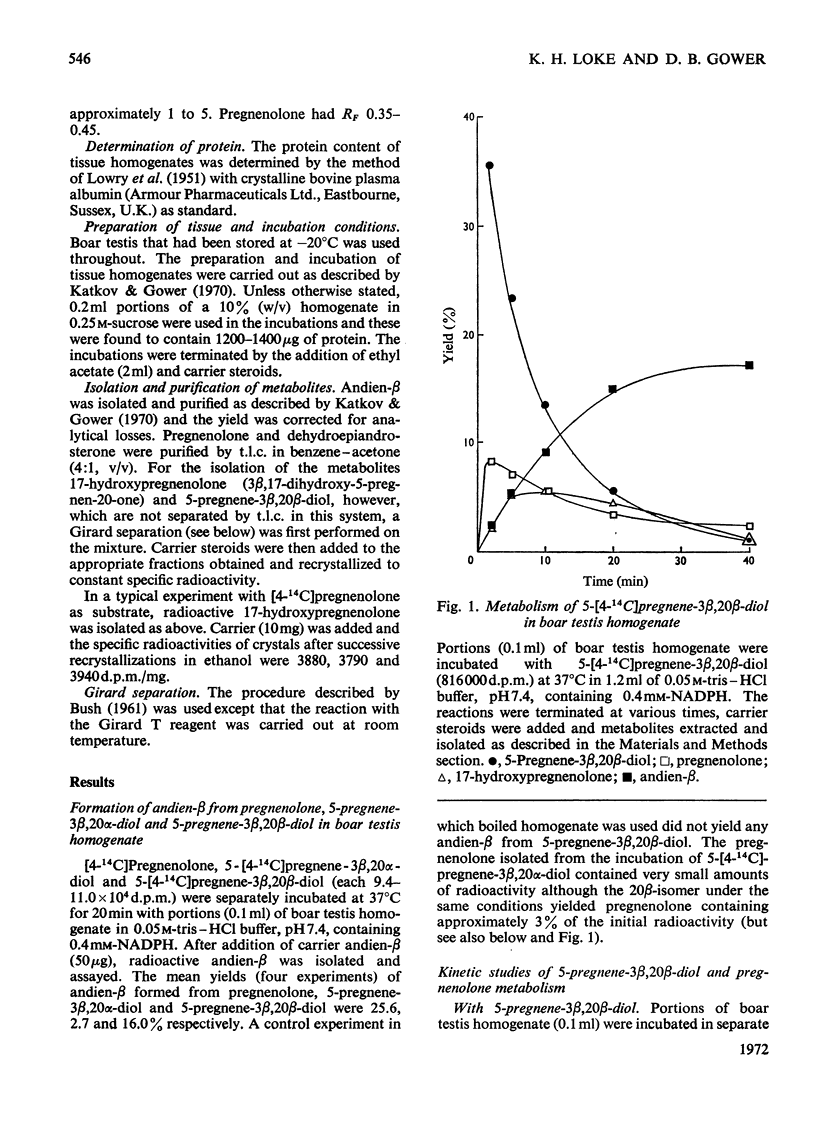
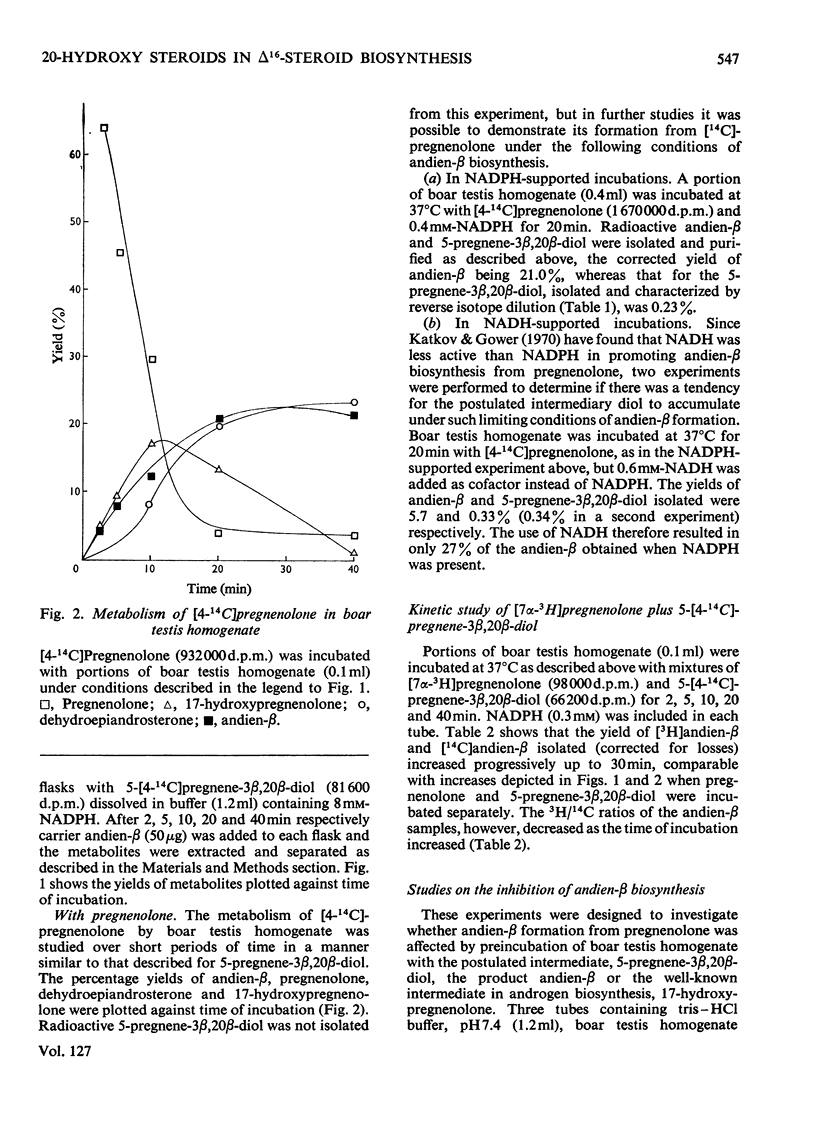
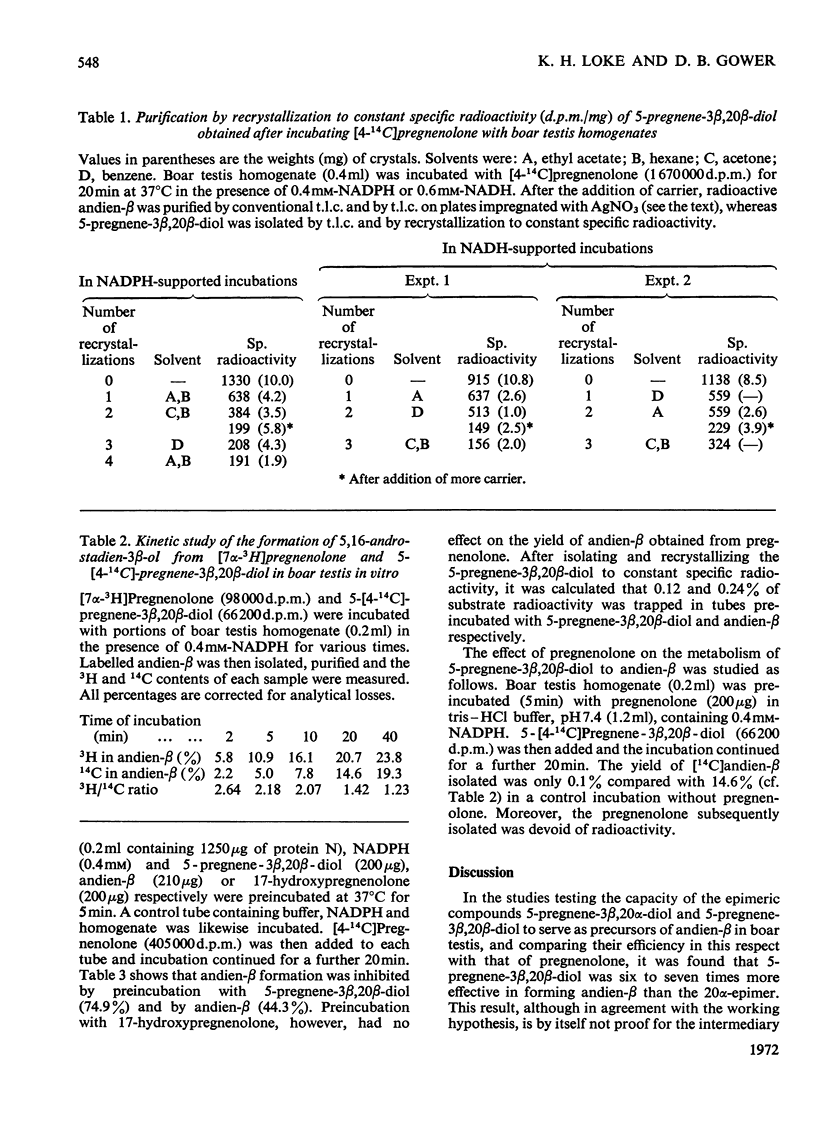
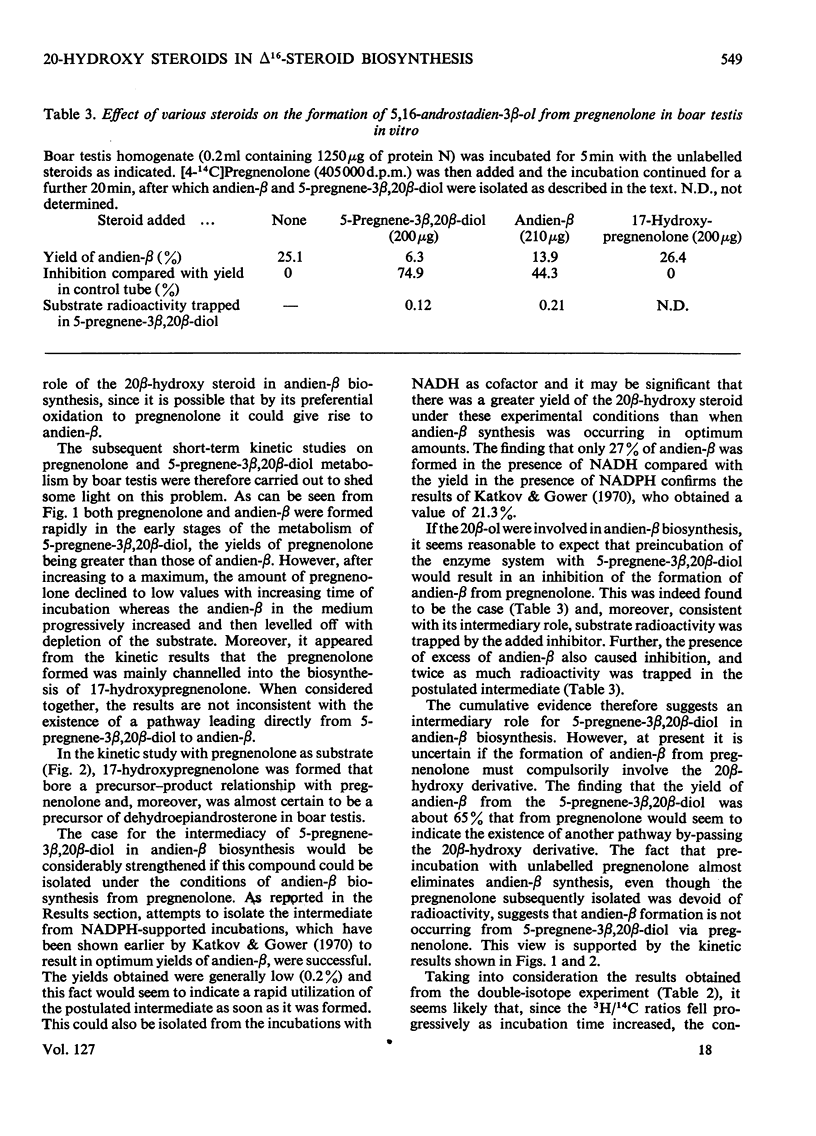
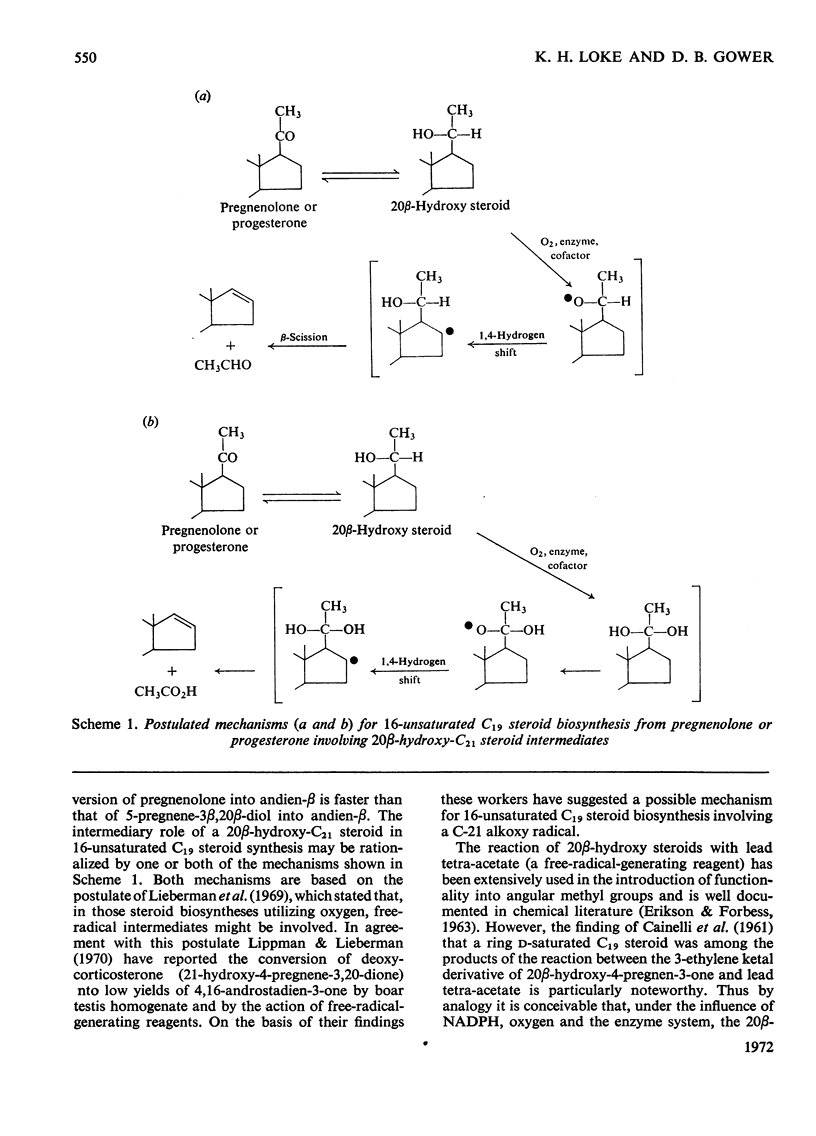
1. The possible involvement of 5-pregnene-3β,20β-diol in 16-unsaturated C19 steroid biosynthesis has been investigated. 2. 5,16-Androstadien-3β-ol (andien-β) formation from [4-14C]pregnenolone (3β-hydroxy-5-pregnen-20-one), 5-pregnene-3β,20α-diol and 5-pregnene-3β,20β-diol was studied in homogenates of boar testis and the mean yields obtained were 25.6, 2.7 and 16.0% respectively. 3. Short-term kinetic studies with pregnenolone and 5-pregnene-3β,20β-diol separately and together suggested that the latter compound might be an intermediate in the biosynthesis of andien-β. 4. In agreement with this interpretation, radioactive 5-pregnene-3β,20β-diol has been isolated during andien-β biosynthesis from [4-14C]pregnenolone in the presence of NADPH, more radioactivity being trapped under limiting conditions of andien-β formation with NADH present as cofactor. 5. Further, 5-pregnene-3β,20β-diol and andien-β have been shown to inhibit the formation of the 16-unsaturated C19 steroid from [4-14C]pregnenolone, the yield of radioactive 5-pregnene-3β,20β-diol increasing in the presence of added unlabelled andien-β. 6. It is concluded that there may be two pathways leading to 16-unsaturated C19 steroid formation from pregnenolone, one of these involving 5-pregnene-3β,20β-diol as an intermediate. Possible mechanisms are presented and discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N., Gower D. B. The biosynthesis of some androst-16-enes from C21 and C19 steroids in boar testicular and adrenal tissue. Biochem J. 1968 Jun;108(2):233–241. doi: 10.1042/bj1080233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Skinner S. J. The intermediary role of a 19-oxoandrogen in the biosynthesis of oestrogen. Biochem J. 1968 Sep;109(2):318–321. doi: 10.1042/bj1090318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower D. B., Ahmad N. Studies on the biosynthesis of 16-dehydro steroids. The metabolism of [4-14C]pregnenolone by boar adrenal and testis tissue in vitro. Biochem J. 1967 Aug;104(2):550–556. doi: 10.1042/bj1040550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower D. B., Thomas B. S. Gas-liquid chromatography of androst-16-enes as trimethylsilyl and chloromethyldimethylsilyl ethers. J Chromatogr. 1968 Aug 27;36(3):338–340. doi: 10.1016/s0021-9673(01)92951-6. [DOI] [PubMed] [Google Scholar]
- Katkov T., Gower D. B. The biosynthesis of 5 alpha-androst-16-en-3-one from progesterone by boar testis homogenate. Biochim Biophys Acta. 1968 Sep 2;164(1):134–136. doi: 10.1016/0005-2760(68)90082-9. [DOI] [PubMed] [Google Scholar]
- Katkov T., Gower D. B. The biosynthesis of androst-16-enes in boar testis tissue. Biochem J. 1970 Apr;117(3):533–538. doi: 10.1042/bj1170533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lieberman S., Bandy L., Lippman V., Roberts K. D. Sterol intermediates in the conversion of cholesterol into pregnenolone. Biochem Biophys Res Commun. 1969 Feb 21;34(4):367–371. doi: 10.1016/0006-291x(69)90389-1. [DOI] [PubMed] [Google Scholar]
- Lippman V., Lieberman S. Steroidal free radicals as possible intermediates in the biosynthesis of C19-delta 16-steroids. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1754–1760. doi: 10.1073/pnas.67.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Fukushima D. K. Studies on the heterolytic fragmentation of pregnane-16,20-diol derivatives to androst-16-enes. J Org Chem. 1970 Mar;35(3):561–564. doi: 10.1021/jo00828a004. [DOI] [PubMed] [Google Scholar]
- Skinner S. J., Akhtar M. The stereospecific removal of a C-19 hydrogen atom in oestrogen biosynthesis. Biochem J. 1969 Aug;114(1):75–81. doi: 10.1042/bj1140075. [DOI] [PMC free article] [PubMed] [Google Scholar]


