Abstract
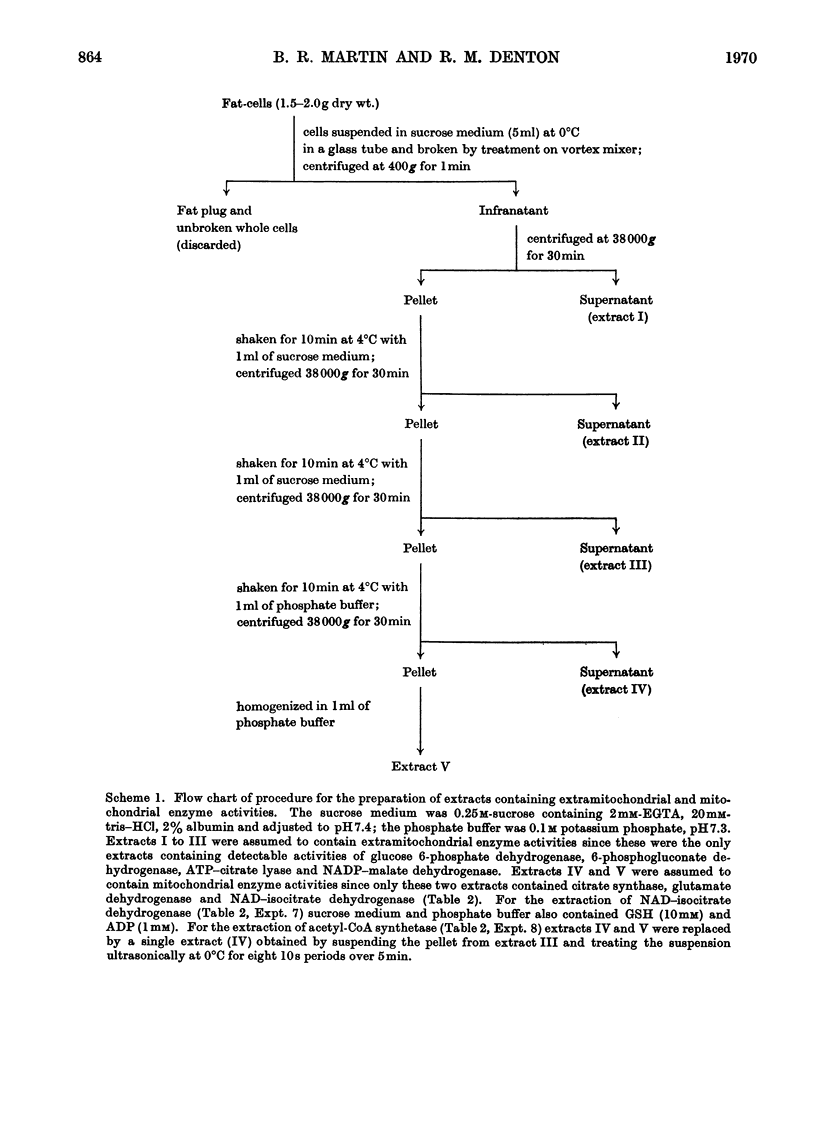
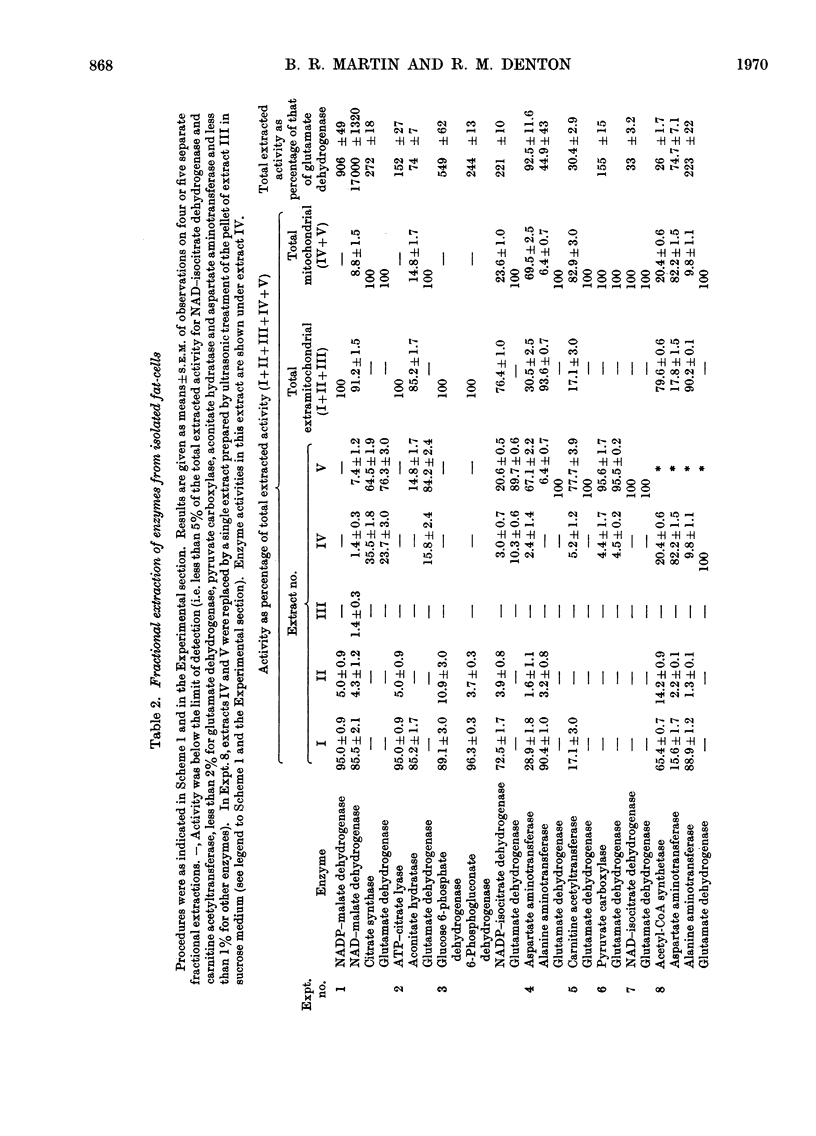
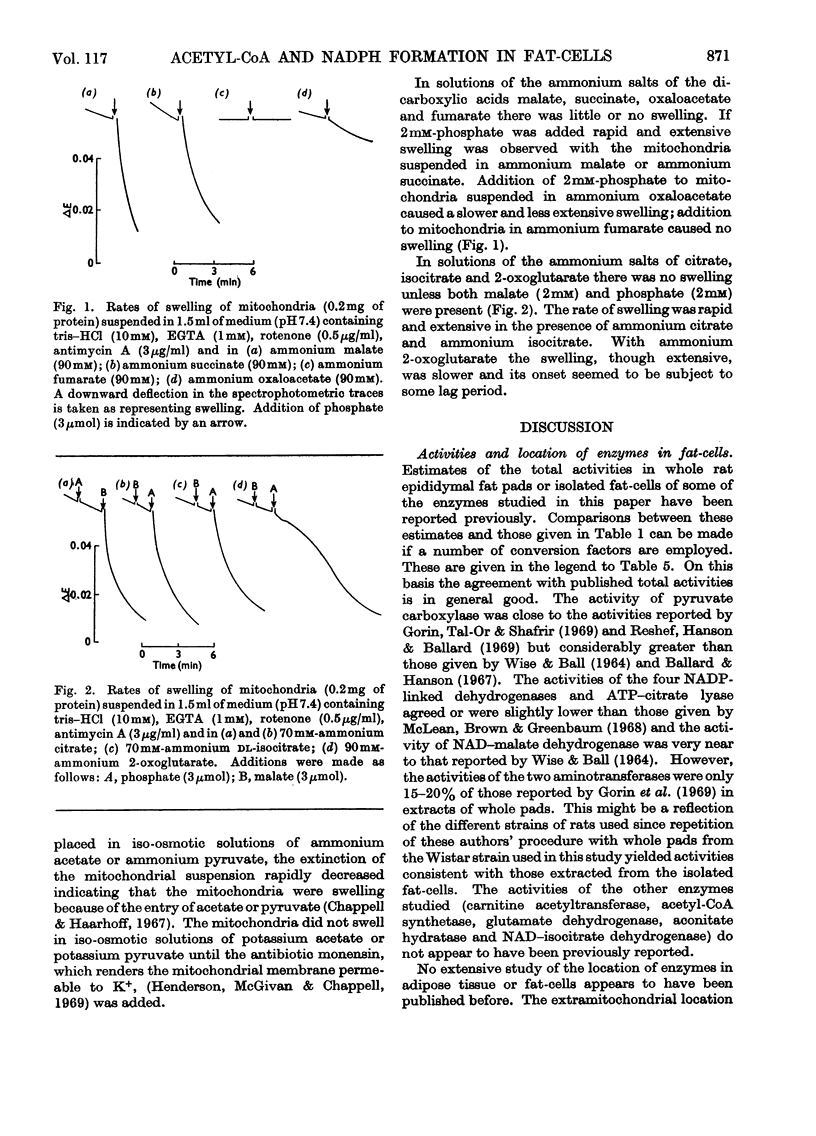
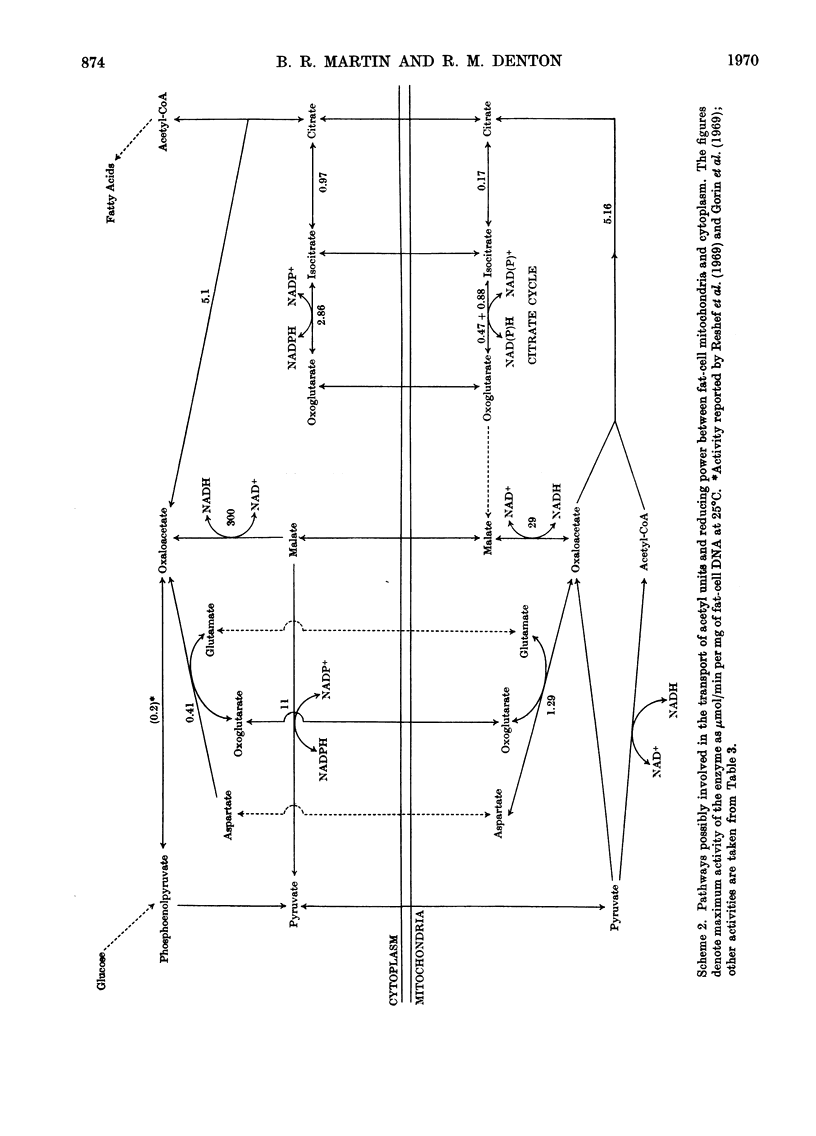
1. A method is described for extracting separately mitochondrial and extramitochondrial enzymes from fat-cells prepared by collagenase digestion from rat epididymal fat-pads. The following distribution of enzymes has been observed (with the total activities of the enzymes as units/mg of fat-cell DNA at 25°C given in parenthesis). Exclusively mitochondrial enzymes: glutamate dehydrogenase (1.8), NAD–isocitrate dehydrogenase (0.5), citrate synthase (5.2), pyruvate carboxylase (3.0); exclusively extramitochondrial enzymes: glucose 6-phosphate dehydrogenase (5.8), 6-phosphogluconate dehydrogenase (5.2), NADP–malate dehydrogenase (11.0), ATP–citrate lyase (5.1); enzymes present in both mitochondrial and extramitochondrial compartments: NADP–isocitrate dehydrogenase (3.7), NAD–malate dehydrogenase (330), aconitate hydratase (1.1), carnitine acetyltransferase (0.4), acetyl-CoA synthetase (1.0), aspartate aminotransferase (1.7), alanine aminotransferase (6.1). The mean DNA content of eight preparations of fat-cells was 109μg/g dry weight of cells. 2. Mitochondria showing respiratory control ratios of 3–6 with pyruvate, about 3 with succinate and P/O ratios of approaching 3 and 2 respectively have been isolated from fat-cells. From studies of rates of oxygen uptake and of swelling in iso-osmotic solutions of ammonium salts, it is concluded that fat-cell mitochondria are permeable to the monocarboxylic acids, pyruvate and acetate; that in the presence of phosphate they are permeable to malate and succinate and to a lesser extent oxaloacetate but not fumarate; and that in the presence of both malate and phosphate they are permeable to citrate, isocitrate and 2-oxoglutarate. In addition, isolated fat-cell mitochondria have been found to oxidize acetyl l-carnitine and, slowly, l-glycerol 3-phosphate. 3. It is concluded that the major means of transport of acetyl units into the cytoplasm for fatty acid synthesis is as citrate. Extensive transport as glutamate, 2-oxoglutarate and isocitrate, as acetate and as acetyl l-carnitine appears to be ruled out by the low activities of mitochondrial aconitate hydratase, mitochondrial acetyl-CoA hydrolyase and carnitine acetyltransferase respectively. Pathways whereby oxaloacetate generated in the cytoplasm during fatty acid synthesis by ATP–citrate lyase may be returned to mitochondria for further citrate synthesis are discussed. 4. It is also concluded that fat-cells contain pathways that will allow the excess of reducing power formed in the cytoplasm when adipose tissue is incubated in glucose and insulin to be transferred to mitochondria as l-glycerol 3-phosphate or malate. When adipose tissue is incubated in pyruvate alone, reducing power for fatty acid, l-glycerol 3-phosphate and lactate formation may be transferred to the cytoplasm as citrate and malate.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., GREEN D. E., HELE P., HIFT H., VON KORFF R. W., RAMAKRISHNAN C. V. The acetate activating enzyme system of heart muscle. J Biol Chem. 1953 Jul;203(1):35–45. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Hanson R. W. The citrate cleavage pathway and lipogenesis in rat adipose tissue: replenishment of oxaloacetate. J Lipid Res. 1967 Mar;8(2):73–79. [PubMed] [Google Scholar]
- Bartley J., Abraham S., Chaikoff I. L. Concerning the form in which acetyl units produced in mitochondria are transferred to the site of de novo fatty acid synthesis in the cell. Biochem Biophys Res Commun. 1965 Jun 9;19(6):770–776. doi: 10.1016/0006-291x(65)90326-8. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., PLAUT G. W. ACTIVATION AND INHIBITION OF DPN-LINKED ISOCITRATE DEHYDROGENASE OF HEART BY CERTAIN NUCLEOTIDES. Biochemistry. 1963 Sep-Oct;2:1023–1032. doi: 10.1021/bi00905a020. [DOI] [PubMed] [Google Scholar]
- Cañas-Rodriguez A., Smith H. W. The identification of the antimicrobial factors of the stomach contents of sucking rabbits. Biochem J. 1966 Jul;100(1):79–82. doi: 10.1042/bj1000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J Biol Chem. 1966 May 25;241(10):2413–2420. [PubMed] [Google Scholar]
- Chappell J. B., Robinson B. H. Penetration of the mitochondrial membrane by tricarboxylic acid anions. Biochem Soc Symp. 1968;27:123–133. [PubMed] [Google Scholar]
- Chase J. F., Tubbs P. K. Some kinetic studies on the mechanism of action of carnitine acetyltransferase. Biochem J. 1966 Apr;99(1):32–40. doi: 10.1042/bj0990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D ADAMO A. F., Jr, HAFT D. E. AN ALTERNATE PATHWAY OF ALPHA-KETOGLUTARATE CATABOLISM IN THE ISOLATED, PERFUSED RAT LIVER. I. STUDIES WITH DL-GLUTAMATE-2- AND -5-14C. J Biol Chem. 1965 Feb;240:613–617. [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton R. M., Randle P. J. Measurement of flow of carbon atoms from glucose and glycogen glucose to glyceride glycerol and glycerol in rat heart and epididymal adipose tissue. Effects of insulin, adrenaline and alloxan-diabetes. Biochem J. 1967 Aug;104(2):423–434. doi: 10.1042/bj1040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- FRAENKEL G., FRIEDMAN S. Carnitine. Vitam Horm. 1957;15:73–118. doi: 10.1016/s0083-6729(08)60508-7. [DOI] [PubMed] [Google Scholar]
- FRIEDEN C. Glutamic dehydrogenase. I. The effect of coenzyme on the sedimentation velocity and kinetic behavior. J Biol Chem. 1959 Apr;234(4):809–814. [PubMed] [Google Scholar]
- FRITZ I. B., SCHULTZ S. K., SRERE P. A. Properties of partially purified carnitine acetyltransferase. J Biol Chem. 1963 Jul;238:2509–2517. [PubMed] [Google Scholar]
- Gorin E., Tal-Or Z., Shafrir E. Glyceroneogenesis in adipose tissue of fasted, diabetic and triamcinolone treated rats. Eur J Biochem. 1969 Apr;8(3):370–375. doi: 10.1111/j.1432-1033.1969.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Gul B., Dils R. Pyruvate carboxylase in lactating rat and rabbit mammary gland. Biochem J. 1969 Feb;111(3):263–271. doi: 10.1042/bj1110263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin M. L., Robinson B. H., Martin B. R., Dentron R. M. Permeability of rat adipose tissue mitochondria to citrate, isocitrate and 2-oxoglutarate. Nature. 1969 Sep 27;223(5213):1369–1371. doi: 10.1038/2231369a0. [DOI] [PubMed] [Google Scholar]
- Henderson P. J., McGivan J. D., Chappell J. B. The action of certain antibiotics on mitochondrial, erythrocyte and artificial phospholipid membranes. The role of induced proton permeability. Biochem J. 1969 Feb;111(4):521–535. doi: 10.1042/bj1110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning H. V., Stumpf B., Ohly B., Seubert W. On the mechanism of gluconeogenesis and its regulation. 3. The glucogenic capacity and the activities of pyruvate carboxylase and PEP-carboxylase of rat kidney and rat liver after cortisol treatment and starvation. Biochem Z. 1966 Apr 27;344(3):274–288. [PubMed] [Google Scholar]
- JEANRENAUD B., RENOLD A. E. Studies on rat adipose tissue in vitro. 7. Effects of adrenal cortical hormones. J Biol Chem. 1960 Aug;235:2217–2223. [PubMed] [Google Scholar]
- KARMEN A., WROBLEWSKI F., LADUE J. S. Transaminase activity in human blood. J Clin Invest. 1955 Jan;34(1):126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDATE INTO FAT. ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF NORMAL AND DIABETIC RATS. Biochem J. 1965 Jun;95:832–837. doi: 10.1042/bj0950832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The metabolism of tritiated glucose by rat adipose tissue. J Biol Chem. 1966 Aug 10;241(15):3600–3610. [PubMed] [Google Scholar]
- Kneer P., Ball E. G. Studies on the metabolism of adipose tissue. XXI. An evaluation of the major pathways of pyruvate metabolism. J Biol Chem. 1968 Jun 10;243(11):2863–2870. [PubMed] [Google Scholar]
- Kornacker M. S., Ball E. G. Citrate cleavage in adipose tissue. Proc Natl Acad Sci U S A. 1965 Sep;54(3):899–904. doi: 10.1073/pnas.54.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leveille G. A., Hanson R. W. Adaptive changes in enzyme activity and metabolic pathways in adipose tissue from meal-fed rats. J Lipid Res. 1966 Jan;7(1):46–55. [PubMed] [Google Scholar]
- Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. Biochem Soc Symp. 1968;27:61–86. [PubMed] [Google Scholar]
- Norum K. R., Bremer J. The localization of acyl coenzyme A-carnitine acyltransferases in rat liver cells. J Biol Chem. 1967 Feb 10;242(3):407–411. [PubMed] [Google Scholar]
- Plaut G. W., Aogaichi T. The separation of DPN-linked and TPN-linked isocitrate dehydrogenase activities of mammalian liver. Biochem Biophys Res Commun. 1967 Aug 23;28(4):628–634. doi: 10.1016/0006-291x(67)90360-9. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reshef L., Hanson R. W., Ballard F. J. Glyceride-glycerol synthesis from pyruvate. Adaptive changes in phosphoenolpyruvate carboxykinase and pyruvate carboxylase in adipose tissue and liver. J Biol Chem. 1969 Apr 25;244(8):1994–2001. [PubMed] [Google Scholar]
- Rognstad R., Katz J. Acetyl group transfer in lipogenesis. II. Fatty acid synthesis from intra- and extramitochondrial acetyl CoA. Arch Biochem Biophys. 1968 Sep 20;127(1):437–444. doi: 10.1016/0003-9861(68)90248-8. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. The balance of pyridine nucleotides and ATP in adipose tissue. Proc Natl Acad Sci U S A. 1966 May;55(5):1148–1156. doi: 10.1073/pnas.55.5.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCRUTTON M. C., UTTER M. F. PYRUVATE CARBOXYLASE. 3. SOME PHYSICAL AND CHEMICAL PROPERTIES OF THE HIGHLY PURIFIED ENZYME. J Biol Chem. 1965 Jan;240:1–9. [PubMed] [Google Scholar]
- SPENCER A. F., LOWENSTEIN J. M. The supply of precursors for the synthesis of fatty acids. J Biol Chem. 1962 Dec;237:3640–3648. [PubMed] [Google Scholar]
- SRERE P. A., BHADURI A. Incorporation of radioactive citrate into fatty acids. Biochim Biophys Acta. 1962 May 21;59:487–489. doi: 10.1016/0006-3002(62)90205-6. [DOI] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Schmidt K., Katz J. Metabolism of pyruvate and L-lactate by rat adipose tissue. J Biol Chem. 1969 Apr 25;244(8):2125–2131. [PubMed] [Google Scholar]
- Seubert W., Henning H. V., Schoner W., L'age M. Effects of cortisol on the levels of metabolites and enzymes controlling glucose production from pyruvate. Adv Enzyme Regul. 1968;6:153–187. doi: 10.1016/0065-2571(68)90012-5. [DOI] [PubMed] [Google Scholar]
- Spencer A., Corman L., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. A comparison of citrate and acetate incorporation into fatty acids. Biochem J. 1964 Nov;93(2):378–388. doi: 10.1042/bj0930378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Membranes and fatty acid metabolism. Br Med Bull. 1968 May;24(2):158–164. doi: 10.1093/oxfordjournals.bmb.a070619. [DOI] [PubMed] [Google Scholar]
- WISE E. M., Jr, BALL E. G. MALIC ENZYME AND LIPOGENESIS. Proc Natl Acad Sci U S A. 1964 Nov;52:1255–1263. doi: 10.1073/pnas.52.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Serum glutamic pyruvic transaminase in cardiac with hepatic disease. Proc Soc Exp Biol Med. 1956 Apr;91(4):569–571. doi: 10.3181/00379727-91-22330. [DOI] [PubMed] [Google Scholar]
- de Haan E. J., Tager J. M. Evidence for a permeability barrier for alpha-oxoglutarate in rat-liver mitochondria. Biochim Biophys Acta. 1968 Jan 15;153(1):98–112. doi: 10.1016/0005-2728(68)90150-3. [DOI] [PubMed] [Google Scholar]


