Abstract
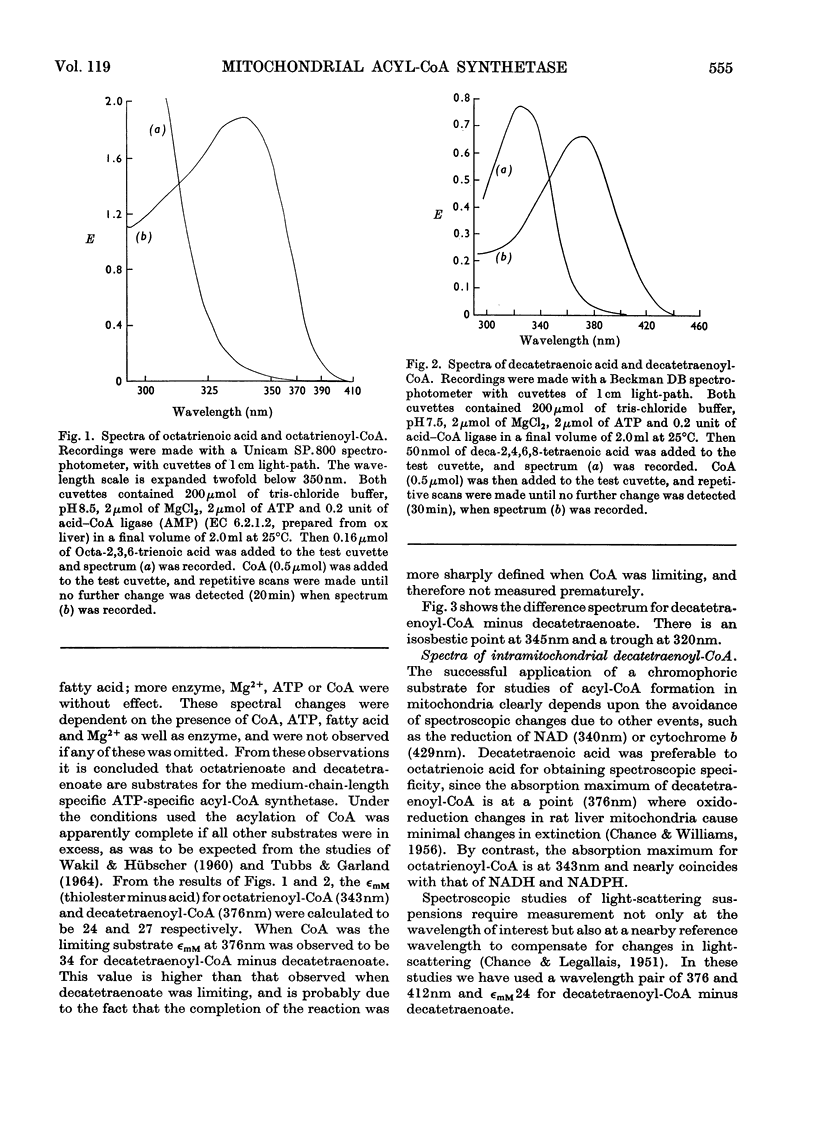
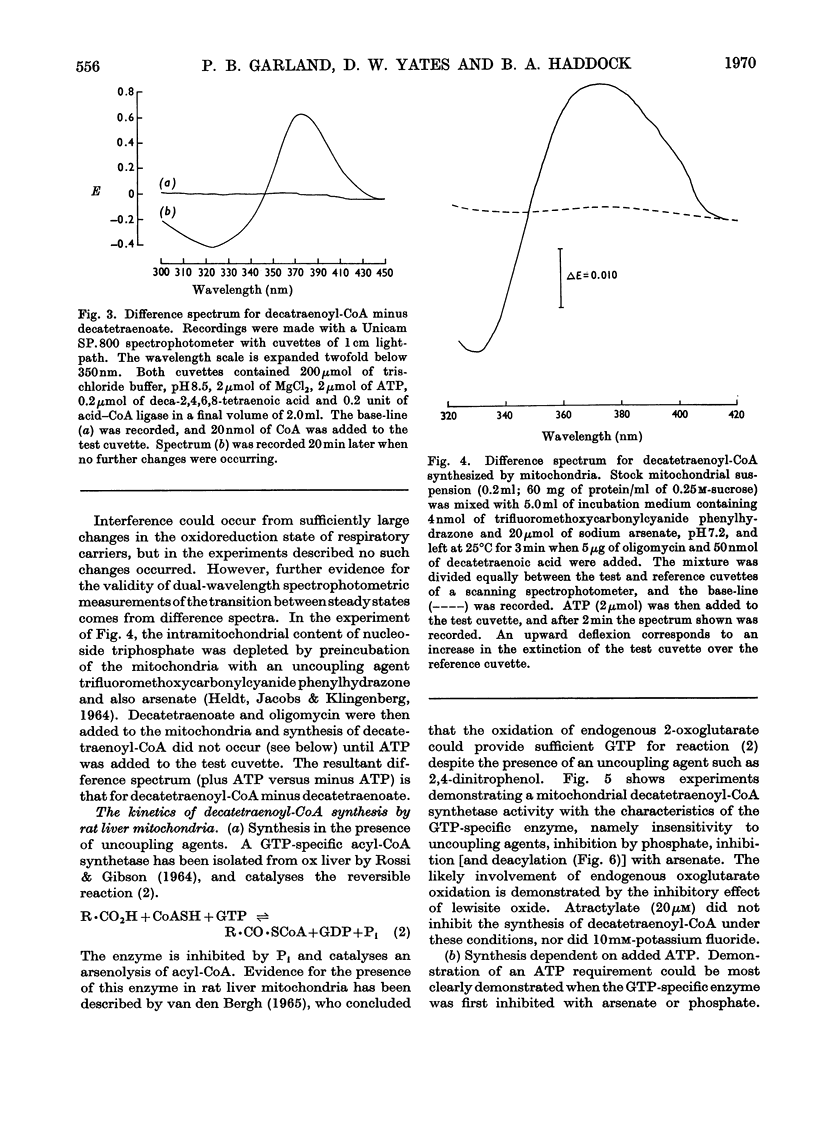
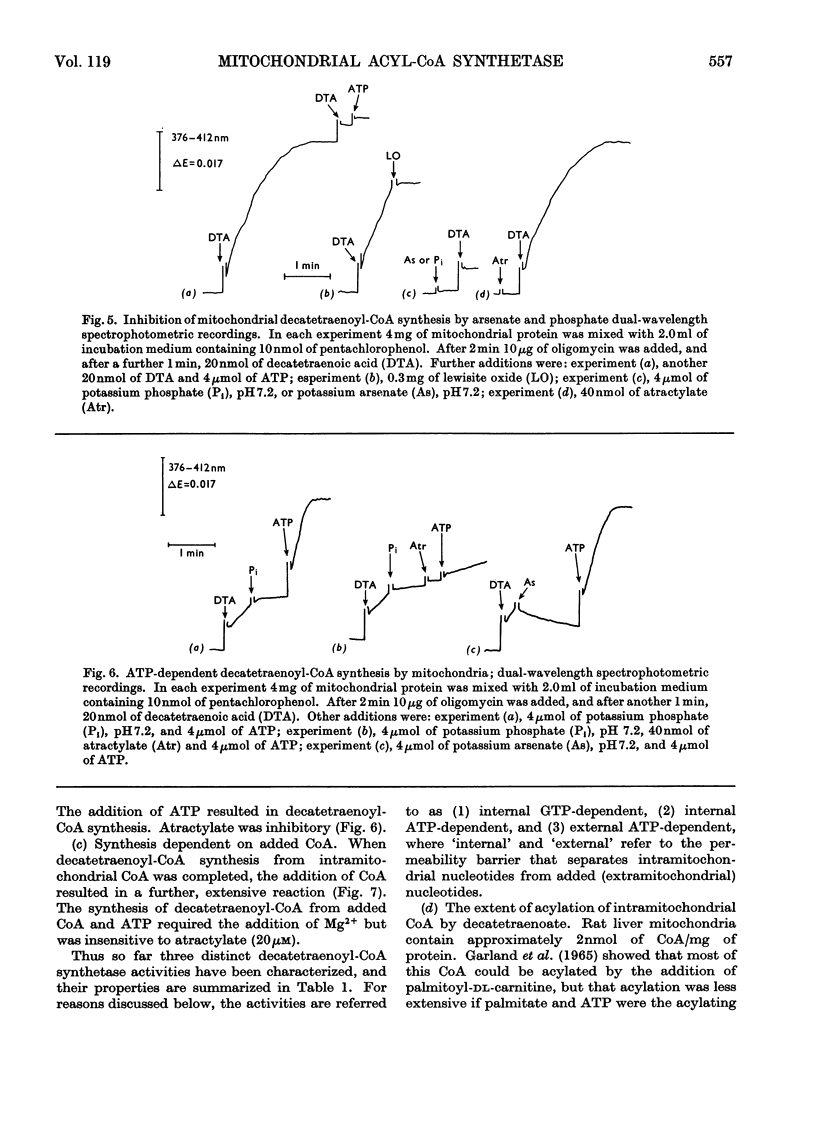
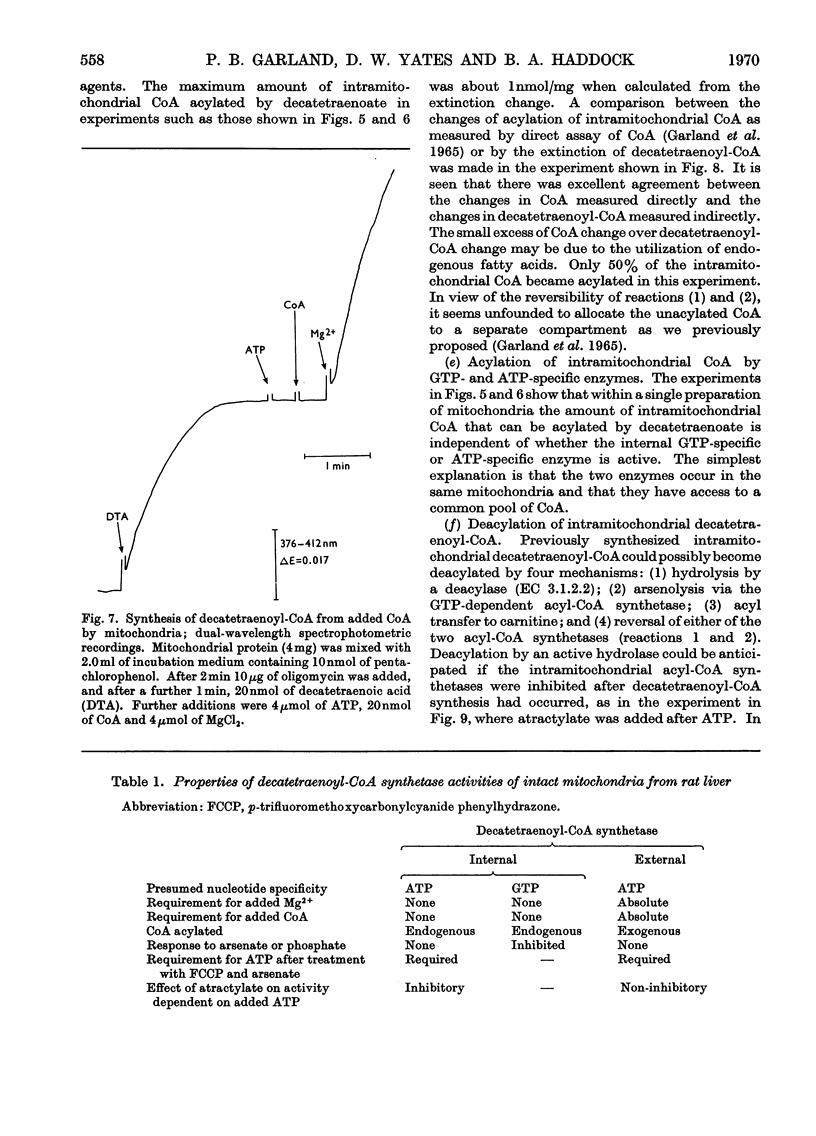
1. Deca-2,4,6,8-tetraenoic acid is a substrate for both ATP-specific (EC 6.2.1.2 or 3) and GTP-specific (EC 6.2.1.–) acyl-CoA synthetases of rat liver mitochondria. The enzymic synthesis of decatetraenoyl-CoA results in new spectral characteristics. The difference spectrum for the acyl-CoA minus free acid has a maximum at 376nm with εmM 34. Isosbestic points are at 345nm and 440nm. 2. The acylation of CoA by decatetraenoate in mitochondrial suspensions can be continuously measured with a dual-wavelength spectrophotometer. 3. By using this technique, three distinct types of acyl-CoA synthetase activity were demonstrated in rat liver mitochondria. One of these utilized added CoA and ATP, required added Mg2+ and corresponded to a previously described `external' acyl-CoA synthetase. The other two acyl-CoA synthetase activities utilized intramitochondrial CoA and did not require added Mg2+. Of these two `internal' acyl-CoA synthetases, one was insensitive to uncoupling agents, was inhibited by phosphate or arsenate, and corresponded to the GTP-specific enzyme. The other corresponded to the ATP-specific enzyme. 4. Atractylate inhibited the activity of the two internal acyl-CoA synthetases only when the energy source was added ATP. 5. The amount of intramitochondrial CoA acylated by decatetraenoate was independent of whether the internal ATP-specific or GTP-specific acyl-CoA synthetase was active. It is concluded that these two internal acyl-CoA synthetases have access to the same intramitochondrial pool of CoA. 6. The amount of intramitochondrial CoA that could be acylated with decatetraenoate was decreased by the addition of palmitoyl-dl-carnitine, 2-oxoglutarate, or pyruvate. These observations indicated that pyruvate dehydrogenase (EC 1.2.4.1), oxoglutarate dehydrogenase (EC 1.2.4.2), carnitine palmitoyltransferase (EC 2.3.1.–), citrate synthase (EC 4.1.3.7), and succinyl-CoA synthetase (EC 6.2.1.4) all have access to the same intramitochondrial pool of CoA as do the two internal acyl-CoA synthetases.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Galzigna L., Rossi C. R., Sartorelli L., Gibson D. M. A guanosine triphosphate-dependent acyl coenzyme A synthetase from rat liver mitochondria. J Biol Chem. 1967 May 10;242(9):2111–2115. [PubMed] [Google Scholar]
- Garland P. B., Shepherd D., Yates D. W. Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem J. 1965 Nov;97(2):587–594. doi: 10.1042/bj0970587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Yates D. W., Garland P. B. The localization of some coenzyme A-dependent enzymes in rat liver mitochondria. Biochem J. 1970 Sep;119(3):565–573. doi: 10.1042/bj1190565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER H. R., WAKIL S. J., BOCK R. M. Studies on fatty acid oxidation. I. Enzymatic activation of fatty acids. J Biol Chem. 1953 Sep;204(1):453–468. [PubMed] [Google Scholar]
- Nicholls DG RAND P. B. Th control of isocitrate oxidation by rat liver mitochondria. Biochem J. 1969 Sep;114(2):215–225. doi: 10.1042/bj1140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C. R., Galzigna L., Alexandre A., Gibson D. M. Oxidation of long chain fatty acids by rat liver mitochondria. J Biol Chem. 1967 May 10;242(9):2102–2110. [PubMed] [Google Scholar]
- Tubbs P. K., Garland P. B. Variations in tissue contents of coenzyme A thio esters and possible metabolic implications. Biochem J. 1964 Dec;93(3):550–557. doi: 10.1042/bj0930550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. W., Garland P. B. The partial latency and intramitochondrial distribution of carnitine-palmitoyltransferase (e.c.2.3.1.-), and the CoASH and carnitine permeable space of rat liver mitochondria. Biochem Biophys Res Commun. 1966 May 25;23(4):460–465. doi: 10.1016/0006-291x(66)90750-9. [DOI] [PubMed] [Google Scholar]
- Yates D. W., Shepherd D., Garland P. B. Organization of fatty-acid activation in rat liver mitochondria. Nature. 1966 Mar 19;209(5029):1213–1215. doi: 10.1038/2091213a0. [DOI] [PubMed] [Google Scholar]


