Abstract
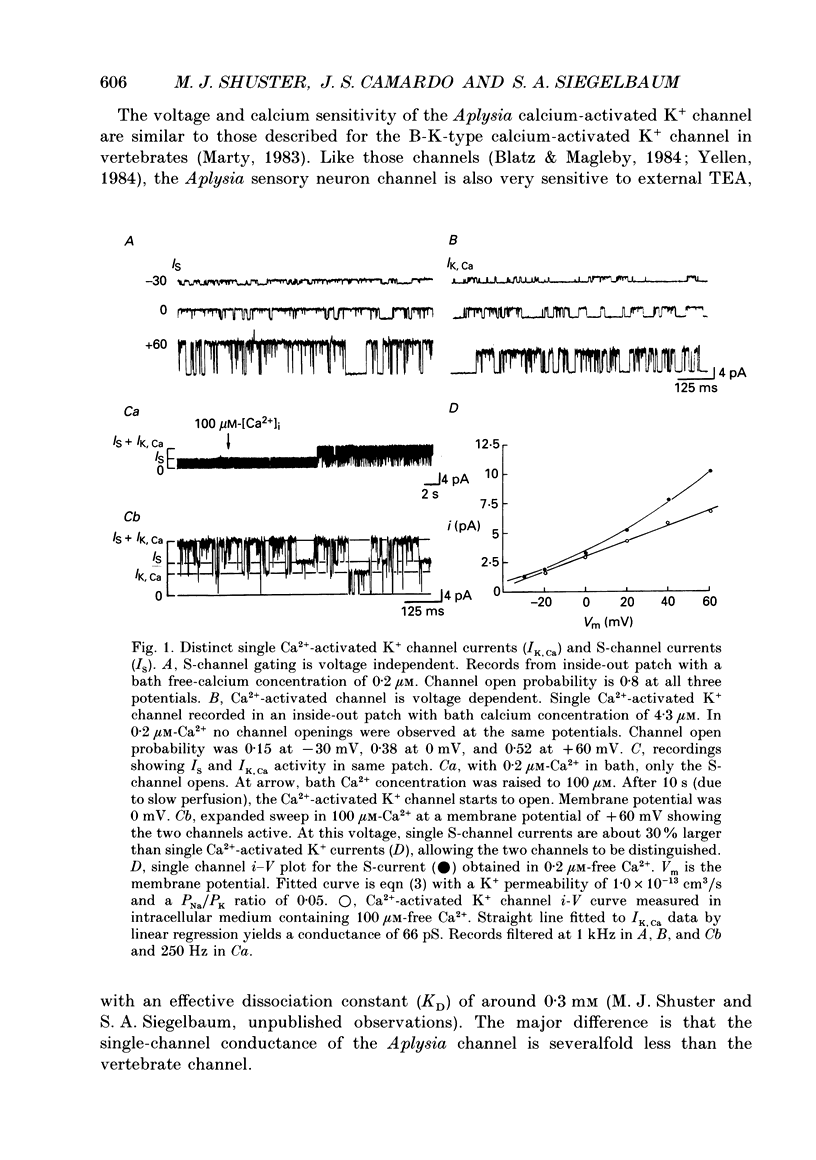
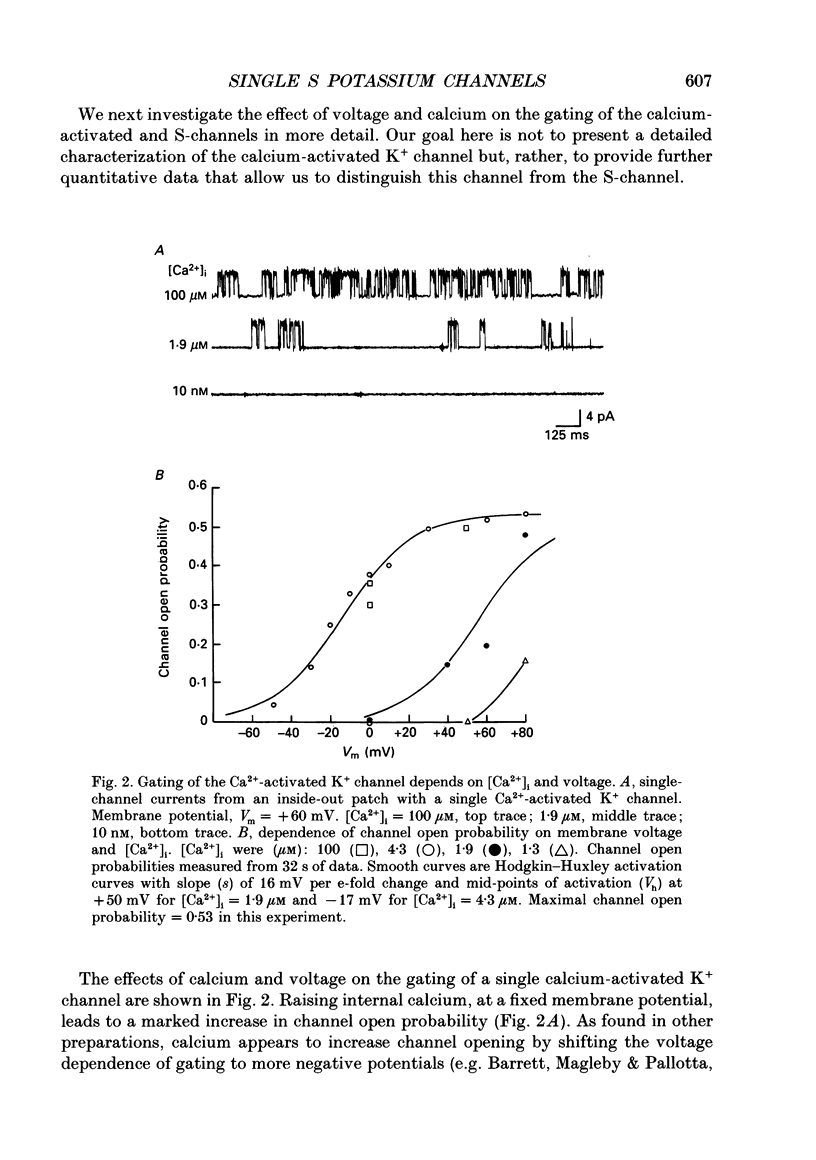
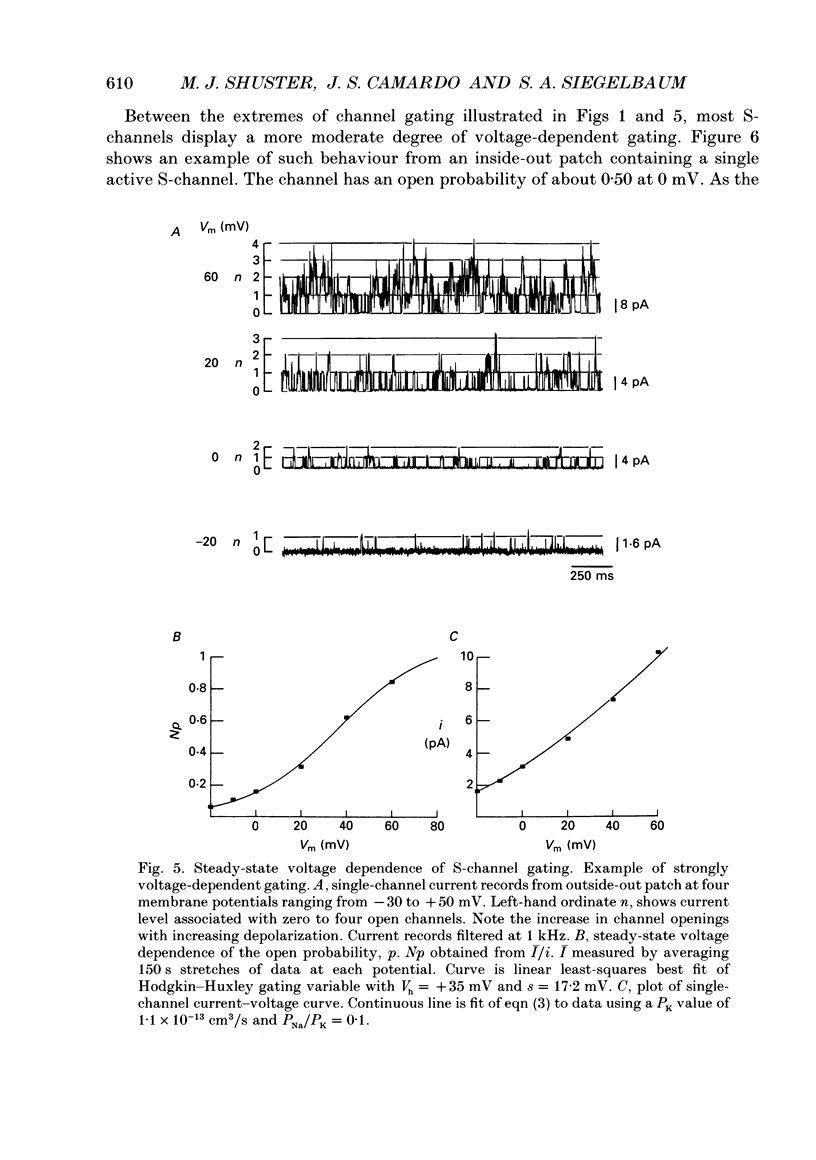
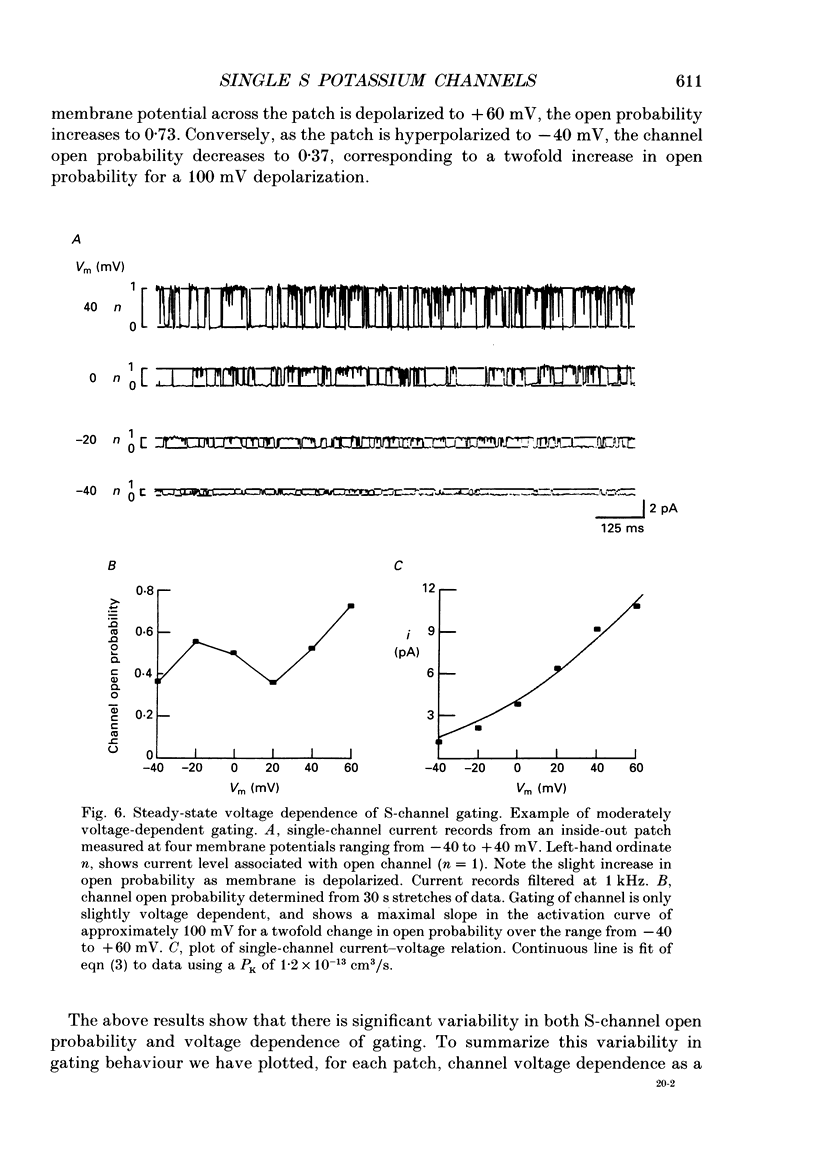
1. Single potassium channel currents were recorded in cell-attached and cell-free patches from Aplysia sensory neurons. Two prominent classes of K+ channels were identified that have similar single-channel current amplitude at 0 mV: (1) the resting conductance serotonin-sensitive K+ channels (S-channels) previously described in these neurons; and (2) a calcium-activated K+ channel. A series of experiments were carried out which enable these channels to be distinguished on the basis of their biophysical properties. These experiments also provide further insight into the gating and ionic selectivity of the S-channel. 2. In inside-out patches, single calcium-activated K+ channel currents (IK,Ca) show a linear i-V curve with a slope conductance of 66 pS (normal sea water outside, 360 mM-KCl inside) whereas single S-channels display an outwardly rectifying i-V curve with a slope conductance of 90 pS at 0 mV. 3. The gating of IK,Ca has a steep voltage dependence, with open probability showing an e-fold increase for a 16 mV depolarization. Increasing internal calcium concentration from 0.2 to 10 microM shifts the activation curve by 60 mV in the hyperpolarizing direction. 4. S-channel gating is independent of internal calcium (from less than 10 nM up to 100 microM). Steady-state open probability of the S-channel generally shows a weak dependence on membrane potential, with open probability increasing twofold for a 30-100 mV depolarization. Occasional patches were observed with S-channels displaying a much greater voltage sensitivity, with open probability increasing e-fold for a 16-20 mV depolarization. 5. S-channels are selective for K+ over Na+. The selectivity ratio depends on the ratio of Na+ to K+ concentration on the same side of the membrane. Increasing K+ concentration appears to increase relative Na+ permeability, suggesting ion-ion interactions within the channel. 6. We conclude that Aplysia sensory neurons contain two prominent distinguishable classes of K+ channels, the Ca(2+)-independent S-channel and a Ca(2+)-activated channel. The gating properties of the S-channels allow them to contribute outward repolarizing current over a wide range of membrane potentials so that their modulation by neurotransmitters contributes to changes in both resting potential and action potential duration.
Full text
PDF






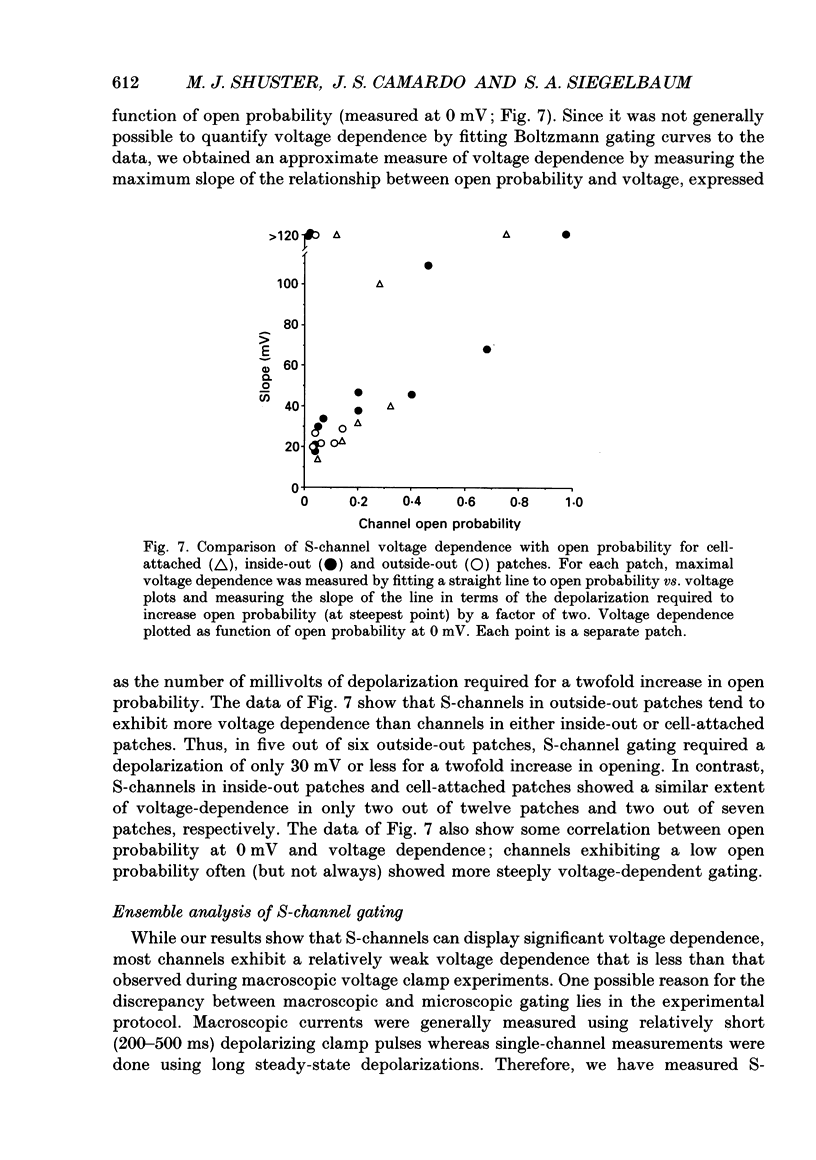
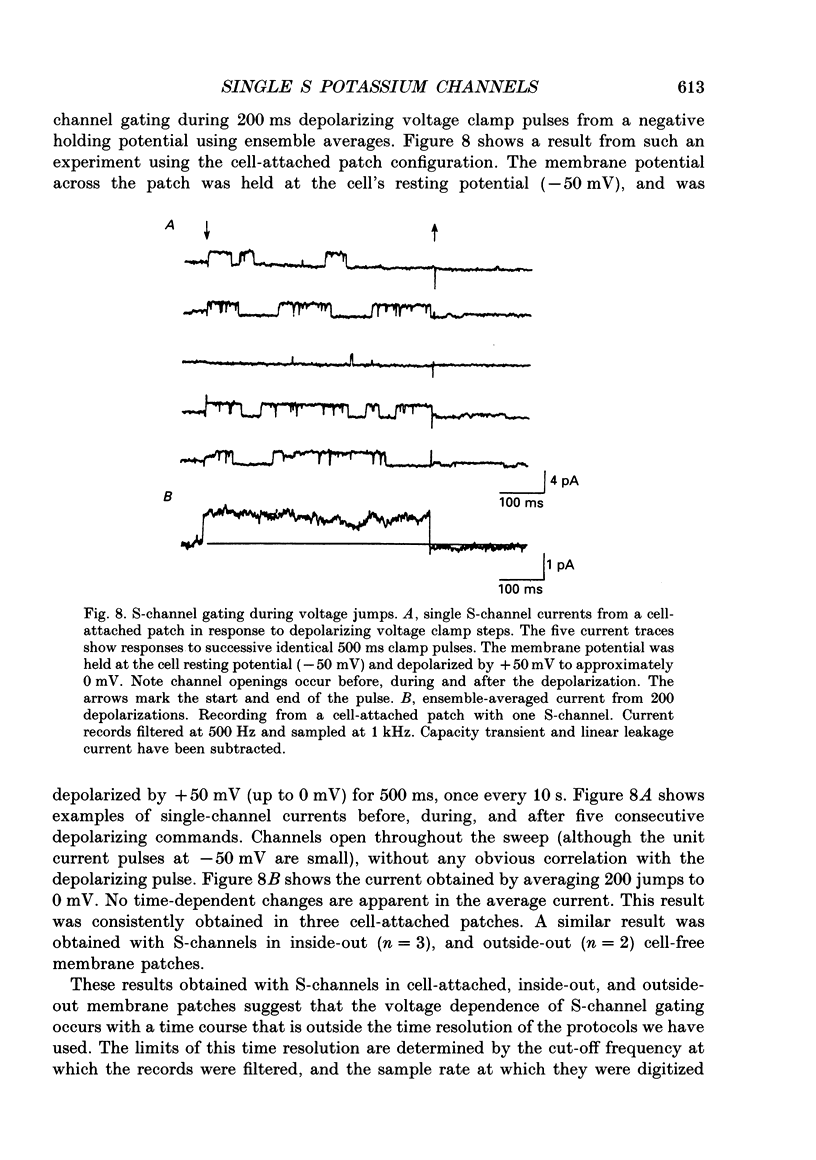
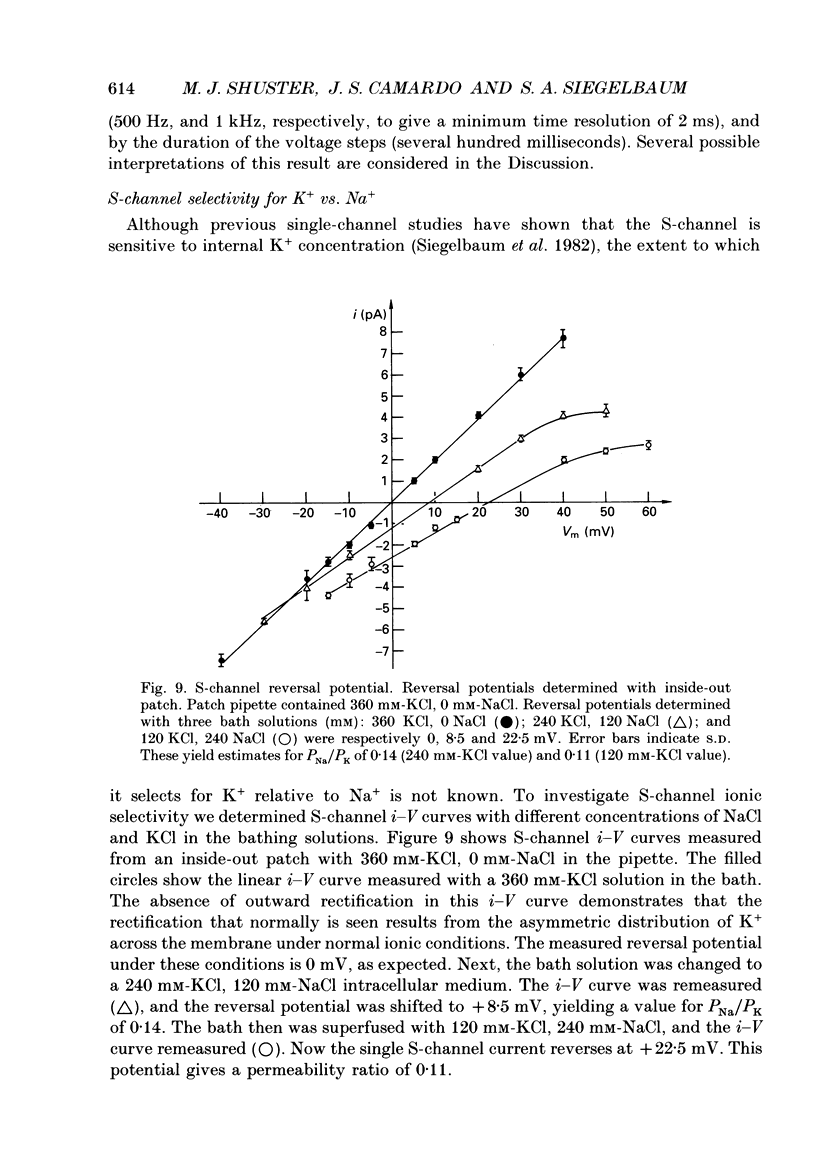
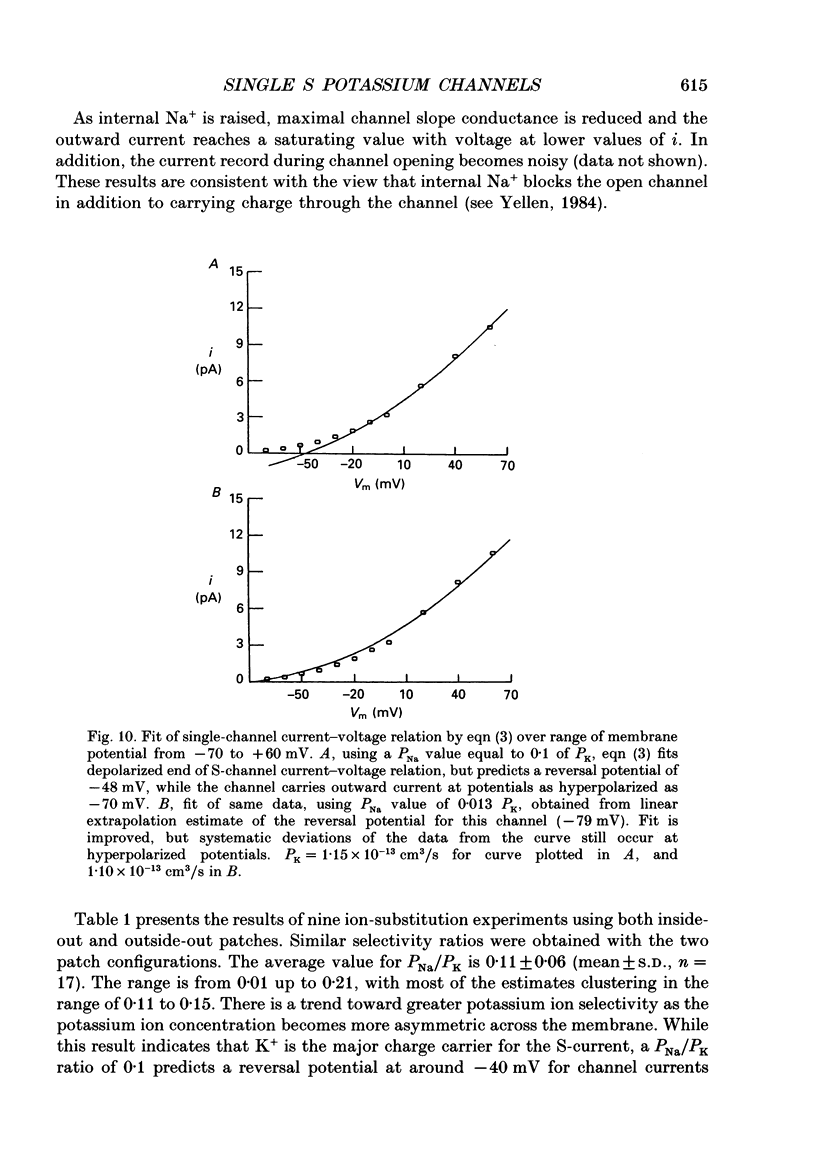





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter D. A., Byrne J. H. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989 Sep;62(3):665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. II: Non-independence and current-voltage relations. J Physiol. 1980 Oct;307:243–257. doi: 10.1113/jphysiol.1980.sp013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardetti F., Campbell W. B., Falck J. R., Demontis G., Rosolowsky M. Products of heme-catalyzed transformation of the arachidonate derivative 12-HPETE open S-type K+ channels in Aplysia. Neuron. 1989 Oct;3(4):497–505. doi: 10.1016/0896-6273(89)90208-0. [DOI] [PubMed] [Google Scholar]
- Belardetti F., Kandel E. R., Siegelbaum S. A. Neuronal inhibition by the peptide FMRFamide involves opening of S K+ channels. Nature. 1987 Jan 8;325(7000):153–156. doi: 10.1038/325153a0. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Ion conductance and selectivity of single calcium-activated potassium channels in cultured rat muscle. J Gen Physiol. 1984 Jul;84(1):1–23. doi: 10.1085/jgp.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina V., Eckert R., Erxleben C. Suppression of calcium current by an endogenous neuropeptide in neurones of Aplysia californica. J Physiol. 1987 Jul;388:565–595. doi: 10.1113/jphysiol.1987.sp016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttner N., Siegelbaum S. A., Volterra A. Direct modulation of Aplysia S-K+ channels by a 12-lipoxygenase metabolite of arachidonic acid. Nature. 1989 Nov 30;342(6249):553–555. doi: 10.1038/342553a0. [DOI] [PubMed] [Google Scholar]
- Cachelin A. B., De Peyer J. E., Kokubun S., Reuter H. Sodium channels in cultured cardiac cells. J Physiol. 1983 Jul;340:389–401. doi: 10.1113/jphysiol.1983.sp014768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camardo J. S., Shuster M. J., Siegelbaum S. A., Kandel E. R. Modulation of a specific potassium channel in sensory neurons of Aplysia by serotonin and cAMP-dependent protein phosphorylation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):213–220. doi: 10.1101/sqb.1983.048.01.024. [DOI] [PubMed] [Google Scholar]
- Edmonds B., Klein M., Dale N., Kandel E. R. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990 Nov 23;250(4984):1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Krasne S., Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+-Tl+ mixtures. J Gen Physiol. 1977 Sep;70(3):269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Long-opening mode of gating of neuronal calcium channels and its promotion by the dihydropyridine calcium agonist Bay K 8644. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2178–2182. doi: 10.1073/pnas.82.7.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Gration K. A., Usherwood P. N. Single glutamate-activated channels in locust muscle. Nature. 1979 Apr 12;278(5705):643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Pollock J. D., Bernier L., Camardo J. S. Serotonin and cyclic adenosine 3':5'-monophosphate modulate the potassium current in tail sensory neurons in the pleural ganglion of Aplysia. J Neurosci. 1985 Jul;5(7):1862–1871. doi: 10.1523/JNEUROSCI.05-07-01862.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. D., Camardo J. S. Regulation of single potassium channels by serotonin in the cell bodies of the tail mechanosensory neurons of Aplysia californica. Brain Res. 1987 May 5;410(2):367–370. doi: 10.1016/0006-8993(87)90340-4. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Siegelbaum S. A. Pharmacological characterization of the serotonin-sensitive potassium channel of Aplysia sensory neurons. J Gen Physiol. 1987 Oct;90(4):587–608. doi: 10.1085/jgp.90.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegelbaum S. A., Camardo J. S., Kandel E. R. Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature. 1982 Sep 30;299(5882):413–417. doi: 10.1038/299413a0. [DOI] [PubMed] [Google Scholar]
- Walsh J. P., Byrne J. H. Modulation of a steady-state Ca2+-activated, K+ current in tail sensory neurons of Aplysia: role of serotonin and cAMP. J Neurophysiol. 1989 Jan;61(1):32–44. doi: 10.1152/jn.1989.61.1.32. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]


