Abstract
Background
Greater than half of cancer patients experience radiation therapy, for both radical and palliative objectives. It is well known that researches on radiation response mechanisms are conducive to improve the efficacy of cancer radiotherapy. p21 was initially identified as a widespread inhibitor of cyclin-dependent kinases, transcriptionally modulated by p53 and a marker of cellular senescence. It was once considered that p21 acts as a tumour suppressor mainly to restrain cell cycle progression, thereby resulting in growth suppression. With the deepening researches on p21, p21 has been found to regulate radiation responses via participating in multiple cellular processes, including cell cycle arrest, apoptosis, DNA repair, senescence and autophagy. Hence, a comprehensive summary of the p21’s functions in radiation response will provide a new perspective for radiotherapy against cancer.
Methods
We summarize the recent pertinent literature from various electronic databases, including PubMed and analyzed several datasets from Gene Expression Omnibus database. This review discusses how p21 influences the effect of cancer radiotherapy via involving in multiple signaling pathways and expounds the feasibility, barrier and risks of using p21 as a biomarker as well as a therapeutic target of radiotherapy.
Conclusion
p21’s complicated and important functions in cancer radiotherapy make it a promising therapeutic target. Besides, more thorough insights of p21 are needed to make it a safe therapeutic target.
Keywords: p21, Cell cycle, Apoptosis, DNA damage repair, Cancer radiotherapy
Introduction
Currently, radiotherapy is widely utilized in cancer treatment. The majority of cancer patients receive radiotherapy, aiming to cure or relieve the symptoms, and radiotherapy can be employed alone or together with any other treatment strategies, including surgery and chemotherapy (De Ruysscher et al. 2019). However, many cellular and molecular mechanisms responding to ionizing radiations exert an influence on the radiotherapy efficacy. Especially some radiation response mechanisms endowing with radioresistance to tumour cells result in the failure of radiotherapy. Therefore, it is absolutely necessary to explore the mechanisms further and provide a better understanding of radiotherapy.
p21, a low-molecular-weight molecule (21 kDa) transcribed from CDKN1A gene, was first identified as a cyclin-dependent kinase (CDK) regulator, which plays a significant role in controlling cell cycle progression (Harper et al. 1993). p21 stagnates cell cycle progression during G1 and S phases via binding to and inhibiting cyclin-CDK1,2,4,6 complexes (Bertoli et al. 2013; Georgakilas et al. 2017). If be elevated, p21 causes cell growth arrest at the G2 phase (Niculescu et al. 1998) and is necessary for maintaining G2 arrest following DNA damage in a p53-dependent pathway (Bunz et al. 1998). In line with this, in early days, p21 was deemed to be a tumour suppressor in brain, lung, and colon cancer cells and a target gene mediated by p53 (el-Deiry et al. 1993). However, as research continues, contrasting findings of p21 function emerged. The first discrepancy appeared in 1997. It was detected that low concentrations of p21 resulted in an augment in the recruitment of Cdk4/6 and cyclin D complexes (Cheng et al. 1999; LaBaer et al. 1997) indicating that p21 is needed for G1 → S phase progression and facilitates cell proliferation. What’s more, the expression and function of p21 varies in different tumour cells. Diminished expression of p21 was observed in non-small cell lung (Teramen et al. 2011) and prostate (Bott et al. 2005) cancer cells, high grade breast cancer (Askari et al. 2013) and acute lymphoblastic leukemia correlated with poor prognosis (Roman-Gomez et al. 2002), while its high expression level causing its oncogenic activity was discovered in various human cancers such as renal cell carcinoma, testicular cancer, breast cancer, hepatocellular carcinoma, gliomas, prostate cancer, multiple myeloma, cervical carcinoma, acute myeloid cancer, esophageal squamous cell carcinoma, ovarian cancer, and soft tissue sarcomas (Abbas and Dutta 2009; Liu et al. 2013). p21 acts either as a tumour suppressor or as an oncogene primarily depending on the cellular type, cellular context, its subcellular localization and post-translational modifications (Kreis et al. 2019). The “antagonistic duality” of p21 (Gartel and Tyner 2002) was also found in cancer cells in response to radiotherapy and p21 modulates these responses via miscellaneous cellular processes including cell cycle arrest, apoptosis, metabolism, DNA repair and autophagy. There is a growing body of evidence to indicate that p21 is closely linked to radiotherapy, but the results of these studies are somewhat perplexing and contradictory. In the present review, we summarize the mechanisms and functions of p21 participating in radiotherapy. Finally, given its dual role in response to radiotherapy, we highlight the feasibility, risks and prospect of using p21 as a biomarker as well as a radiotherapeutic target.
Function of p21 in cancer radiotherapy
p21 in response to radiation
Common view considers ionizing radiation can directly or indirectly cause DNA damage. In this process, various aspects of the signal pathways are activated in response to radiation such as p53, ATM, MAPK, mTOR and NF-κB affecting miscellaneous cellular processes including proliferation, cell cycle arrest, apoptosis, autophagy, senescence, DNA repair, differentiation, cellular transformation and endoplasmic reticulum stress.
Participation in the response to radiation is the foundation of modulating cancer cell responses to ionizing radiation. Since the discovery of p21 in 1993, there is an abundance of literature documenting the change level of p21 in normal and cancer cells after irradiation, listed in Table 1. Down-regulation of p21 after irradiation was detected in various cancer cell types (nasopharyngeal, lung, breast, prostrate, cervix, colon, glioma and squamous cancers) (Biswas et al. 2017; Soria et al. 2006; Tian et al. 2000; Wang et al. 1999a; Wu et al. 2020) and most of these down-regulations were caused by UV irradiation (Wang et al. 1999a). However, more studies have reported that p21 was able to be attracted by radiation via the p53-dependent or p53-independent pathway. Supporting this positive regulation in normal cells, p21 is radiation-elevated in brain (Liu et al. 2020), cardiac microvascular endothelial (Zeng et al. 2020), liver Kupffer cells (Soysa et al. 2019), lymphocytes (Nguyen et al. 2020) various fibroblasts (Al-Khalaf et al. 2012; Cao et al. 2014; Crochemore et al. 2019; Fournier et al. 2004; Poon et al. 1996; Wu and Levine 1997), astrocytes and mesenchymal stem cells (Bylicky et al. 2019). Of note, the augment of p21 level was observed after irradiation with X-rays in some cancer types mentioned above, including cervix (Furusawa et al. 2012; Niibe et al. 1999), breast (Nenoi et al. 2006) cancer and glioma (Shu et al. 1998). In Table 1, most studies detected the elevation of p21 after irradiation with different radiation types. The specific molecular mechanism has been reported that p53 is the main transcriptional modulator of p21. p21 contains two conserved p53 responsive elements (p53RE) in its promoter. DNA damage up-regulates the p53 activity and subsequently results in p21 expression (Jung et al. 2010). In addition, the regulatory elements of p21 at − 1.1 kb, − 1.4 kb, and − 1.8 kb involved in the augment of p21 in response to radiation and Oct-1 binds constitutively to the elements at − 1.1 kb and − 1.8 kb facilitating the transcription of p21 (Nenoi et al. 2009). Besides, NF-κB also promotes the transcription of p21 after radiation (Szoltysek et al. 2018). Therefore, the mutation status of p53 might impact partial functions of p21. Apart from these findings, another study showed that p21 mRNA splice variant 4 is preferentially translated following radiation-induced eIF2α-P by a mechanism mediated in part by upstream open reading frames situated in the 5′-leader of p21 mRNA (Collier et al. 2018). In light of these findings, we may conclude that p21’s over-expression post-irradiation is regulated mainly at the transcriptional level, including transcriptional activation and alternative splicing. Certainly, it is not difficult to find that there are some conflicting results in the literature even for the same type of cancer and irradiation mode (Tian et al. 2000; Wendt et al. 2006). At this point, the contents described in the literature are somewhat confusing, with different results being presented. Fortunately, Kraus et al. gave a more reasonable explanation about the p21 level variation from the background expression of glioma cells after irradiation. For primary glioblastoma multiforme (GBM) with low background expression of p21 mRNA, radiation activated p21 transcriptionally, while for GBM in which p21 was basally over-expressed, radiation led to a decrease of mRNA level or had no effect (Kraus et al. 2000). Besides, Fournier et al. considered that up-regulation of p21 induced by ionizing radiation depended on the dose and (linear energy transfer, LET) levels (Fournier et al. 2004). Additionally, Petragnano et al. found that high dose-rate more efficiently up-regulated the expression of p21 compared to low dose-rate (Petragnano et al. 2020b).
Table 1.
p21 in different biological materials in response to radiation
| Experimental material | Treatment | p21 status after irradiation | Study |
|---|---|---|---|
| Lung cancer cell lines A549, H1299, and H460 | Ionizing radiation | Down-regulation | Biswas et al. (2017) |
| Human colon carcinoma cells (HCT116/p21+/+) | γ-Irradiation from a 137Cs source | Down-regulation | Tian et al. (2000) |
| Human nasopharyngeal carcinoma cell lines | X-ray | Down-regulation | Wu et al. (2020) |
| Nasopharyngeal carcinoma cell line | X-ray | Down-regulation | Liu et al. (2006) |
| Cancer cell lines | UV irradiation | Down-regulation in various cancer cell types (breast, prostrate, cervix, colon, glioma, squamous cancers), independently of their p53 genetic and functional status | Wang et al. (1999a) |
| Parental H1299 human lung epithelial carcinoma cells (p53 null), HCT116 and RKO human colorectal cancer cell lines expressing wild-type p53 and WI38 human fibroblasts | UV irradiation | Down-regulation | Soria et al. (2006) |
| Brain cell PC12 | 60Co and UVA | Up-regulation | Liu et al. (2020) |
| Primary human fibroblasts | Chronic γ-Irradiation | Up-regulation by p53 | Cao et al. (2014) |
| Squamous carcinoma cells | γ-Irradiation from a 60Co source | Up-regulation by p53 | Graham et al. (2011) |
| MCF-7 and HCT116 cell lines | γ-Irradiation | Up-regulation | Wendt et al. (2006) |
| HCT116 cell line | γ-Irradiation | Up-regulation | Sohn et al. (2006) |
| CCR6 + Th17 lymphocytes | γ-Irradiation from a 137Cs source | Up-regulation | Nguyen et al. (2020) |
| Primary normal human skin fibroblast HFSN1 cells | UV irradiation | Up-regulation | Al-Khalaf et al. (2012) |
| Normal female human foetal lung IMR-90 fibroblasts | UV irradiation | Up-regulation | Crochemore et al. (2019) |
| Normal human foreskin AG1523 diploid fibroblasts | UV irradiation | Up-regulation | Poon et al. (1996) |
| Fibroblast | UVC irradiation | Up-regulation | Mirzayans et al. (2008) |
| Rat embryo fibroblasts (REF) and mouse embryo fibroblasts (MEF) | UV irradiation | Up-regulation | Wu and Levine 1997) |
| Normal volunteers | UV irradiation | Up-regulation | Murphy et al. (2002) |
| N-TERT keratinocytes | UVB irradiation | p21 mRNA splice variant 4 is preferentially translated after radiation | Collier et al. (2018) |
| GBM cell lines | X-ray | Up-regulation by p53 | Shu et al. (1998) |
| Cardiac microvascular endothelial cells | X-ray | Up-regulation | Zeng et al. (2020) |
| Human oral cancer cell lines | X-ray | Up-regulation | Ho et al. (2019) |
| Normal human foreskin fibroblasts | X-rays and low-energy heavy ions | Up-regulation depended on the dose and LET | Fournier et al. (2004) |
| HeLa cells | X-ray | Up-regulation | Furusawa et al. (2012) |
| Human breast adenocarcinoma cell line MCF-7 | X-ray | Up-regulation | Nenoi et al. (2006) |
| Human breast adenocarcinoma cell line MCF-7 | X-ray | Up-regulation | Nenoi et al. (2009) |
| Human osteosarcoma U2-OS cells | X-ray | Up-regulation | Szoltysek et al. (2018) |
| Human uveal melanoma 92–1 cells | X-ray and Fe ions | Up-regulation | Zhang et al. (2016) |
| Normal human astrocytes and human mesenchymal stem cells | X-ray | Up-regulation | Bylicky et al. (2019) |
| Human epidermoid carcinoma cells A431 | Photodynamic therapy | Up-regulation | Ahmad et al. (1998) |
| Human pancreatic cancer cell lines Capan‑1 and Panc‑1 | Proton beam | Up-regulation | Lee et al. (2019) |
| Embryonal rhabdomyosarcoma RD, prostate cancer cell lines PC3 | Electron beam | Up-regulation | Petragnano et al. (2020b) |
| Invasive cervical cancer clinical samples | Patients were treated with a combination of external whole pelvis and intracavitary irradiation | Up-regulation | Niibe et al. (1999) |
| Liver Kupffer cells | Irradiation | Up-regulation | Soysa et al. (2019) |
| Lung carcinoma xenografts | 125I | Up-regulation | Jin et al. (2020) |
| Prostate cancer patients | 192Ir | Up-regulation | Keam et al. (2018) |
| Human glial tumours | γ-Irradiation from a 60Co source | p21 level after irradiation varied from their background expression | Kraus et al. (2000) |
Meanwhile, we retrieved the Gene Expression Omnibus (GEO) database and analyzed several datasets of irradiated tumor cells to check the alteration of p21’s expression. As shown in Fig. 1a, p21 was up-regulated in three melanoma cell lines after irradiation with X-rays or carbon ions. Similar phenomenon has also been observed in squamous cell carcinoma, p21’s expression was promoted at 3 h and 24 h post-irradiation (Fig. 1b). However, p21 decreased in pancreatic cancer cells after irradiation with X-rays at 8 Gy (Fig. 1c). The analysis of the GEO database confirmed that radiation could cause the observed changes in p21 expression.
Fig. 1.
Expression of p21 in different tumor cell lines after irradiation. a Three melanoma cell lines 92-1, Colo679 and HMV-I after irradiation with 2 Gy of X-rays or carbon ions (dataset no. GSE6630). b Squamous cell carcinoma cell line after irradiation with X-rays (dataset no. GSE9716). c Pancreatic cancer cell line MIAPaCa-2 after irradiation with 8 Gy of X-rays (dataset no. GSE107443)
A series of evidence strongly highlights the notion that p21 tightly participates in radiation responses in both normal and cancer cells, while the changes of its expression level after irradiation depend on the genetic background of cells and irradiation conditions.
p21 and cell cycle
Cell proliferation depends on the propelling of cell cycle progression, which is regulated by cyclins and cyclin-dependent kinases (CDKs). Distinct cyclin/CDK complexes via sequential activation and inactivation modulate the initiation and transition of cell cycles from G1 to S and G2 to mitosis (Malumbres and Barbacid 2001). Among all identified CDKs, CDK1, CDK2, CDK3, CDK4 and CDK6 are devoted to cell cycle modulation (Lee et al. 2009; Pavlides et al. 2016). The abundance of cyclin varies at different phases of the cell cycle and cyclin binds with different CDKs to regulate their activity. Initially, cyclin D activates CDK4 and CDK6 at G1 phase in response to mitogenic stimuli. Analogously, CDK2 interacts with cyclin E1 and E2 and then contributes to G1/S transition. During S phase, cyclin E is degraded and replaced by cyclin A, resulting in the progress from S phase to mitosis, and finally, at the end of G2 phase, CDK2 binds with cyclin B (Malumbres and Barbacid 2005). Certainly, there are instances where inhibiting the activity of CDKs offers physiological benefits. The CDK activity is controlled by the binding of inhibitory proteins, known as CDK inhibitors or CKIs. p21 was the first molecule identified to act as a member of the CKI family and its function in cell cycle control has been well established. It has been reported that over-expression of p21 leads to G1-, G2- (Niculescu et al. 1998), or S-phase arrest (Ogryzko et al. 1997; Radhakrishnan et al. 2004).
In response to radiation, p21 level commonly changes and regulates the cell cycle progress. We have tabulated relevant studies in Table 2. Which may help to understand p21 function in cell cycle after radiation. Most of the studies show that radiation-induced p21 caused cell arrest in normal and carcinoma cells. Nevertheless, only one study suggests that repression of p21 expression after irradiation resulted in a decrease of G1 and G2 arrest (Liu et al. 2006). Although p21 was down-regulated by radiation in this research, p21 still acted as a negative regulatory factor of cell cycle. Based on the results of these studies, radiation-induced p21 usually gives rise to G1/S or G2/M arrest. Concretely, p21 can suppress CDKs activities via directly binding with their N-terminal region or indirectly blocking the phosphorylation of CDK1 and CDK2 (Abbas and Dutta 2009). Besides, p21 can also interact with the cyclin subunit by binding to cyclin binding motif 1 (Cy1) and cyclin binding motif 2 (Cy2), which exist in the N-terminal and C-terminal domains, respectively. These binding elements are obbligato for p21-modulated repression of cyclin-CDK complexes. p21 hinders cell cycle progression during G1 and S phases through inhibiting the activity of cyclin-CDK2, cyclin-CDK1, and cyclin-CDK4, 6 complexes (Bertoli et al. 2013; Georgakilas et al. 2017). It has also been observed that p21 induced by p53 following DNA damage causes cell growth arrest at the G2 phase via inhibiting CDK1 (Bunz et al. 1998; Niculescu et al. 1998).
Table 2.
Influence of p21 on cell cycle after irradiation
| Experimental material | Treatment | p21 influence on cell cycle after irradiation | Study |
|---|---|---|---|
| Cardiac microvascular endothelial cells | X-ray | G1/S cell cycle arrest increased | Zeng et al. (2020) |
| HeLa cells | X-ray | Resulted in G2/M arrest | Furusawa et al. (2012) |
| Human oral cancer cell lines | X-ray | Arrested the cell cycle in the G2/M phase | (Ho et al. (2019) |
| Nasopharyngeal carcinoma cell line | X-ray | Repression of p21 expression resulted in G1 and G2 arrest decrease | Liu et al. 2006) |
| Non-small cell lung cancer cell lines | X-ray | Enhancing G2/M arrest | Wang et al. (2017) |
| Breast cancer lines | X-ray | Resulted in G2/M arrest | Yang et al. (2012) |
| Human uveal melanoma 92–1 cells | X-ray and Fe ions | Resulted long-term G2 arrest | Zhang et al. (2016) |
| Normal human foreskin AG1523 diploid fibroblasts | UV irradiation | p21 induced by irradiation bound to CDK2 and CDK4 inhibiting their activity | Poon et al. (1996) |
| N-TERT keratinocytes | UVB irradiation | Caused G1 arrest | Collier et al. (2018) |
| C57BL/6 and BALB/c mice, murine colorectal carcinoma cell line CT26 and IEC6 | γ-Irradiation from a 137Cs source | Activation of p53/p21 mediated reversible cell cycle arrest | Nag et al. (2019) |
| Human colon carcinoma cells (HCT116/p21+/+) | γ-Irradiation from a 137Cs source | Inhibition of p21 induced by irradiation resulted in disappearance of G1 phase arrest | Tian et al. (2000) |
| HCT116 cell line | γ-Irradiation | Wild-type cells were permanently arrested in the G2-M phase, while p21-deficient cells did not occur cycle arrest | Sohn et al. (2006) |
| MCF-7 and HCT116 cell lines | γ-Irradiation | Irradiation -induced p21 and G2/M arrest | Wendt et al. (2006) |
| Brain cell PC12 | 60Co and UVA | Cells stagnated in G1 phase | Liu et al. (2020) |
| Human epidermoid carcinoma cells A431 | Photodynamic therapy | Caused G1/S cell cycle arrest | Ahmad et al. (1998) |
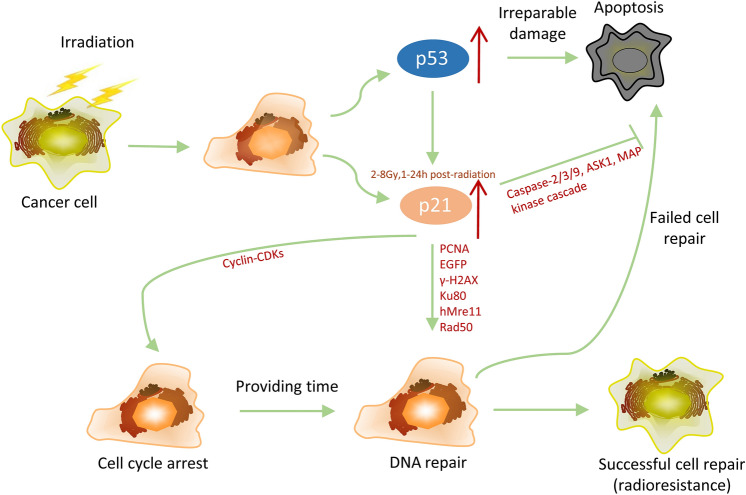
Cell cycle arrest caused by p21 augment provides more time for DNA repair. In normal cells, this phenomenon is conducive to ensure genomic stability. However, these mechanisms offer opportunities for cancer cells to live and protect the malignant cells from apoptosis. Therefore, p21-induced cell cycle arrest for cancer cells is unfavorable for patient treatment during radiotherapy.
p21 and DNA damage repair
DNA damage caused by ionizing radiation is the key theoretical basis of radiotherapy for cancer treatment. Ionizing radiation results in several types of DNA damage, including base modifications, crosslinks, single-strand breaks (SSBs) and double-strand breaks (DSBs) (Vens and Begg 2010). Cells evolve multiple mechanisms to repair particular types of DNA damage via DNA damage response (DDR) pathways, which provide chance for precise DNA repair aiming at definite damage sites. There are five major DNA repair pathways activated throughout different stages of the cell cycle, including DNA damage base excision repair (BER), mismatch repair (MMR), nucleotide excision repair (NER), homologous recombination (HR), and nonhomologous end joining (NHEJ). Unlike UV radiation, cells exposed to ionizing radiation undergo different extents of DNA damage directly by secondary electrons and/or indirectly by reactive oxygen species (ROS), which can lead to SSBs or DSBs. However, most studies on p21 involved in DNA repair concentrate on the UV radiation and we demonstrate these studies in Table 3.
Table 3.
Influence of p21 on DNA repair after irradiation
| Experimental material | Treatment | P21 influence on DNA repair after irradiation | Study |
|---|---|---|---|
| U2OS cell line, HCT116 and HCT116 p21–/– cell line | UV irradiation | p21 inhibited NER via binding with PCNA and preventing its interaction with TLS and the assembly of pol η foci after UV | Soria et al. (2008) |
| HeLa S3 cell line, mouse C2C12 myoblasts, human embryonic lung fibroblasts | UV irradiation | p21 is recruited to DNA-damage sites and colocalized with PCNA and PCNA-interacting proteins involved in nucleotide excision repair (NER), such as DNA polymerase δ, XPG and CAF-1 | Perucca et al. 2006) |
| Normal human fibroblasts | UV irradiation | p21 recruited to DNA excision repair foci in the UV-induced DNA damage response | Stivala and Prosperi 2004) |
| N-TERT keratinocytes | UVB irradiation | p21 facilitates DNA repair | Zhang et al. (2016) |
| DLD1 colorectal carcinoma cells | UV irradiation | p21 may modulate the nucleotide excision repair process to facilitate the repair of UV-induced DNA damage even in the absence of wild-type p53 | Sheikh et al. (1997) |
| H1299 human lung epithelial carcinoma cells, HCT116 and RKO human colorectal cancer cell lines and WI38 human fibroblasts | UV irradiation | Downregulation of p21 after UV radiation was required for efficient PCNA ubiquitination, which was conducive to PCNA-dependent DNA repair | Soria et al. (2006) |
| Normal human lung embryonic fibroblasts | UV irradiation | Loss of p21 was found to induce an increase in UV sensitivity, together with a reduction in NER capacity | Stivala et al. (2001) |
| Murine lung epithelial cell line, human cervical carcinoma cell line, human diploid lung fibroblast cell line, Chinese hamster ovary cell line | Laser irradiation | EGFP-p21 accumulated rapidly at irradiated sites, and colocalized with the DSB marker γ-H2AX and with the DSB sensor protein Ku80 | Koike et al. (2011) |
| 8–10‑week‑old male albino Wistar rats | X-ray | Increasing expression of p21 in rat peripheral blood assists in DNA double‑strand breaks repair | Rezaeejam et al. (2018) |
| Normal human foreskin fibroblasts | Heavy ion bombardment | p21 bound to DNA damage sites and colocalized with hMre11 and Rad50 | Jakob et al. (2002) |
| HeLa cell cline | Kr ions | p21 is recruited to DNA-damage sites and colocalized with γ-H2AX | Tobias et al. (2010) |
For UV radiation, there is a result showing that p21 is involved in NER via recruiting to DNA-damage sites and colocalized with PCNA and PCNA-interacting proteins such as DNA polymerase δ, XPG and CAF-1. Besides, p21 does not inhibit PCNA-dependent DNA-repair and DNA synthesis (Perucca et al. 2006). However, Soria et al. demonstrate that p21 inhibited NER via binding with PCNA and preventing its interaction with TLS (translation DNA synthesis) and the recruitment of pol η foci after UV irradiation (Soria et al. 2008). These apparent contradictory results might be explained by the differences in genetic background of cell lines used in the experiments. Additionally, other studies found that p21 facilitates DNA repair after UV irradiation. Down-regulation of p21 after UV irradiation was required for efficient PCNA ubiquitination, which was conducive to the PCNA-dependent DNA repair (Soria et al. 2006). Depletion of p21 was found to cause lung embryonic fibroblasts more susceptible to UV, together with a decrease in NER capacity (Stivala et al. 2001). Furthermore, p21 modulates the nucleotide excision repair progress to promote the repair of UV-induced DNA damage even in the deficiency of wild-type p53 (Sheikh et al. 1997). There are also several studies which claim that p21 recruits to DNA-damage sites and colocalizes with DNA repair proteins. Assembly of p21 to DNA excision repair foci is initiated in the UV-induced DNA damage response (Stivala and Prosperi 2004). EGFP-p21 was recruited rapidly at laser-irradiated sites, and colocalized with the DSB marker γ-H2AX and with the DSB sensor protein Ku80 (Koike et al. 2011). p21 is bound to DNA damage sites caused by heavy ion bombardment and colocalized with hMre11, Rad50 (Jakob et al. 2002) and γ-H2AX (Tobias et al. 2010). Based on the results of the current studies, it is certain that at least p21 participates in DNA repair; however, we have no definite answers about p21: what functions does it fulfil in DNA repair after irradiation?
However, the roles of p21 in DNA repair caused by other factors can be used as a ready reference. Evidence from several studies indicates that the roles of p21 in DNA repair are paradoxical: p21 is considered to be depressant (Pan et al. 1995; Podust et al. 1995), useless (Adimoolam et al. 2001; Wani et al. 2002) or even necessary (Mauro et al. 2012; McDonald et al. 1996), mostly depending on the type of damage/repair mechanism, the experimental treatment, genetic background of cell line or/and the degree of DNA damage. p21 contributes to DNA repair and synthesis via interfering with PCNA-DNMT1 binding (Parveen et al. 2016). However, p21 also exhibits inhibitory effect on the PCNA-dependent base excision repair and mismatch repair (Abbas and Dutta 2009). Moreover, p21 plays a part in DSB repair, including homologous recombination (HR) and nonhomologous DNA-end-joining (NHEJ). Lessened replication-coupled HR, enhanced MRE11 nuclear foci and CDK-mediated BRCA2 phosphorylation are shown in p21 deficient cell line (Mauro et al. 2012). Furthermore, it has been reported that the Her2/NeuT oncogene reduces the level of major DNA repair factor histone H2AX via the p21-CDK-Rb pathway, giving rise to repression of DNA repair and promotion genome instability in Her2-positive cancer (Yaglom et al. 2014).
To sum up, the above-mentioned studies indicate the different functions of p21 in distinct types of DNA repair course involving in varying degrees of DNA damage. Therefore, the extent of genotoxic stress and cell type may determine the function of p21 in genome stability. However, which role dose p21 play in DNA repair upon ionizing radiation is still unclear, thus further studies on this topic may help us clarify the detailed mechanisms and then better understand cancer radiotherapy.
p21 and apoptosis
Apoptosis, a type of programmed cell death, is induced by the damage to DNA or stress conditions, such as ionizing radiation, cytotoxic drugs, hypoxia, oxidative stress and high temperature (Elmore 2007; Mendez-Armenta et al. 2014; Moeller et al. 2007). Ionizing radiation induces various degrees of DNA damage, which results in an increase of the ratio of Bax to Bcl-2, thereby inducing the intrusion of Bax into the inner layer of mitochondria, which creates liberation of cytochrome C and the formation of the apoptosome complex (Elumalai et al. 2012; Fulda and Debatin 2006). Cancerous cells exposed to radiation often undergo cell cycle arrest to repair DNA lesions, and then apoptosis is activated when the cellular damages are irreparable. Numerous studies threw light upon the dual role of p21 in regulating radiation-induced apoptosis (listed in Table 4). Although p21 is well known for its proliferation-inhibitory functions, p21 also suppresses apoptosis in many studies.
Table 4.
Influence of p21 on radiation-induced apoptosis
| Experimental material | Treatment | p21 influence on irradiation-induced apoptosis | Study |
|---|---|---|---|
| Human epidermoid carcinoma cells A431 | Photodynamic therapy | Upregulation of p21 is accompanied by apoptosis | Ahmad et al. (1998) |
| Normal volunteers | UV irradiation | Upregulation of p21 is accompanied by apoptosis | (Murphy et al. (2002) |
| Human colon carcinoma cells (HCT116/p21+/+) | γ-Irradiation from a 137Cs source | Downregulation of p21 is accompanied by apoptosis | Tian et al. (2000) |
| Large intestine cancer HT29 cells | X-ray | Downregulation of p21 is accompanied by apoptosis | (Zuo et al. (2020) |
| GBM cell lines | X-ray | Promoting apoptosis | Shu et al. (1998) |
| Primary normal human skin fibroblast HFSN1 cells | UV irradiation | Suppressing apoptosis | Al-Khalaf et al. (2012) |
| Human glioma cell lines (T98G, U251MG, and U87MG) | γ-Irradiation | Suppressing apoptosis | Kokunai et al. (2001) |
| HCT-116 cell line | γ-Irradiation | Suppressing apoptosis | Waldman and B. 1996) |
| HCT116 human colon carcinoma cells | γ-Irradiation from a 137Cs source | Suppressing apoptosis | (Wouters et al. (1999) |
| HCT116 cell line | γ-Irradiation | Suppressing apoptosis | Sohn et al. (2006) |
| MCF-7 and HCT116 cell lines | γ-Irradiation | Suppressing apoptosis | Wendt et al. (2006) |
| DLD-1 human colorectal carcinoma cells | γ-Irradiation | Suppressing apoptosis | Lu et al. (1998) |
| HeLa cells | X-ray | Suppressing apoptosis | Furusawa et al. (2012) |
| Invasive cervical cancer clinical samples | Patients were treated with a combination of external whole pelvis and intracavitary irradiation | Suppressing apoptosis | Niibe et al. (1999) |
Although fluctuation of p21 triggered by radiation is accompanied by augment of apoptosis, there is no evidence to support the regulatory function of p21 on radiation-induced apoptosis in some studies (Ahmad et al. 1998; Murphy et al. 2002; Tian et al. 2000). However, modulation of p21 level dose have an effect on apoptosis caused by radiation. Using antisense oligonucleotide to inhibit the expression of p21 is able to enhance the radiation-induced cytotoxicity and apoptosis in glioma cells (Kokunai et al. 2001). Moreover, HCT-116/p21−/− cells have been found to be more prone to apoptosis after irradiation with γ-rays than their p21+/+ counterparts in both in vitro (Waldman et al. 1996) and in vivo (Wouters et al. 1999) studies. Concretely, caspase-9 and caspase-3 are activated and subsequently apoptosis occurs only in p21-deficient HCT-116/p21−/− after irradiation, while p21 wild-type cells are perennially blocked at G2/M phase, accompanying with the occurrence of cellular senescence (Sohn et al. 2006). Mutational p21 lacking any cdk-inhibitory activity also is unable to prevent DLD-1 human colorectal carcinoma cells from apoptosis induced by γ-Irradiation (Lu et al. 1998). Additionally, radiation-induced p21 expression and G2/M arrest inhibit apoptosis caused by γ- (Wendt et al. 2006) or X-rays (Furusawa et al. 2012). There are also clinical data in support of this point. Study on cervical patients receiving combined therapy of external whole pelvis and intracavitary irradiation found that apoptosis and expression of p21 were induced during radiotherapy, but p21 played a inhibitory role in apoptosis (Niibe et al. 1999). Study in another way reported that ATR inhibited degradation of p21 and decreased apoptosis after irradiation (Al-Khalaf et al. 2012), which also sustains the above conclusions. Only a few studies considered that the increase of p21 level promoted apoptosis after irradiation (Shu et al. 1998). These studies revealed that p21 plays a regulatory role in radiation-induced apoptosis, but the specific molecular mechanisms were not clarified in detail. A thorough dissection of the relevant literature summarizes that p21 influences radiation-induced apoptosis mainly from two aspects. On the one hand, p21 triggered by radiation sustains cell cycle arrest allowing DNA repair to protect cells from apoptosis. On the other hand, p21 itself participates in the regulation of the apoptotic process. Several studies have expounded the apoptosis inhibitory function of p21. p21 could directly interact with and suppress the effect of some apoptosis-related proteins, such as protease precursors and kinases. Concretely, p21 binds with procaspase 3 to stem its transformation from inactivated into active form, thereby blocking Fas-mediated apoptosis (Suzuki et al. 1999). Besides, caspase 2 also is inhibited by p21 in a p53-dependent manner (Baptiste-Okoh et al. 2008). There are also several studies suggesting that p21 plays an inhibitory role of apoptosis via repressing apoptosis signal-regulating kinase 1 (ASK1) and stress-activated MAP kinase cascade (Asada et al. 1999). Moreover, p21 also promotes the expression of some factors which play anti-apoptotic or pro-mitogenic roles (Chang et al. 2000). It is noteworthy that p21 plays an inconsistent role in facilitating apoptosis. This conclusion is drawn from the study revealing that up-regulation of p21 promotes apoptosis caused by cisplatin in glioma and ovarian carcinoma cells in vitro (Gartel 2005). In addition, p21 increases MAPK-dependent apoptosis triggered by bile acid in hepatocytes (Qiao et al. 2002). p21 also enhances ceramide-stimulated apoptosis by up-regulating the level of the proapoptotic protein Bax (Kang et al. 1999). Collectively, p21 acts as pro- or anti-apoptotic factor via various molecular mechanisms, showing its regulatory function of apoptosis after irradiation. Dual effects of p21 on radiation-induced apoptosis depend on cell type, cellular context and irradiation mode. Additionally, p21 functions as a key determinant to decide the cell fate of survival or death after irradiation. However, future studies are needed to elucidate the detailed molecular mechanisms of p21 regulatory function on radiation-induced apoptosis, because the current studies are still limited to the effects of p21 on apoptosis phenomenon, lacking in the detailed description about the underlying mechanisms, and the studies under the other treatment conditions can only be considered as a reference. The present conclusions are still trivial and it would be challenging to gain a global picture concerning the influence of p21 on apoptosis induced by radiation.
p21 and other cellular processes
Cellular senescence, an irreversible cell cycle arrest condition obtained during cell division (Pazolli and Stewart 2008), has a beneficial or harmful effect on normal tissue via an inflammatory response and senescence-associated secretion phenotype (SASP) (Nguyen et al. 2018). p21 is firstly confirmed as a biomarker of senescence (Noda et al. 1994) and able to promote premature senescence in both normal and tumour cells in a p53-independent manner (Fang et al. 1999; McConnell et al. 1998; Wang et al. 1999b). Ionizing radiation-induced senescence is closely related to p21. According to the current studies, radiation-induced p21 leads to cell growth arrest and at the same time the occurrence of senescence in normal (Cao et al. 2014; Crochemore et al. 2019; Liu et al. 2020; Mirzayans et al. 2008) and carcinoma cell (Liu et al. 2018; Sohn et al. 2006; Wang et al. 1999b; Zhang et al. 2016). Down-regulation of p21 expression at the transcriptional level drove irradiated carcinoma cell lines from different entities into cell death rather than into senescence (Radine et al. 2020). The function of senescence in cancer therapy gets more attention, because it has been proved that senescence preferentially occurs rather than apoptosis after chemotherapy or radiotherapy in lung cancer and glioblastoma (Hotta et al. 2007; Jeon et al. 2016; Joyner et al. 2006). Moreover, some components of the SASP are conducive to reduce or prevent tumour growth. Maspin, one of the SASP elements, inhibits angiogenesis, which is needed for tumour to expand (Nickoloff et al. 2004). Another component, interleukins, contributes to enhance the extent of senescence in damaged cells (Kuilman et al. 2010). Senescence induced by radiation was believed to be capable of repressing tumour progression via inhibiting tumour cell proliferation; however, some evidence supported that senescence should be considered as a tumour promoter (Milanovic et al. 2018). Senescent cells excrete multiple dissolvable factors facilitating tissue repair, chronic inflammation, invasiveness of surrounding cells and tumour development (Rodier and Campisi 2011), and conduce to the escape of therapy-induced apoptosis (Gartel 2009). Most of the current studies demonstrated that radiation-induced p21 could contribute to cellular senescence, but very few studies reported the exact impact of p21 on radiotherapy efficacy.
Apart from several cellular processes mentioned above, autophagy is another response manner to ionizing radiation. Autophagy is a catabolic course by which cells realize the digestion and recycle of their own cytoplasmic ingredients (Tam et al. 2017). It has been reported that ionizing radiation is one of the stimuli that could activate autophagy in both normal and cancer cells (Kim et al. 2015). An in vitro study reported that glioblastoma cells were killed by radiation via autophagy rather than apoptosis (Daido et al. 2005). So far there have been rare studies investigating the relationship between p21 and autophagy after irradiation. However, there is also evidence to show the association between them. The existing study indicated cordycepin and X-ray combined treatment triggered increase of p21 level in an autophagy cascade-dependent manner. In addition, the regulatory role of p21 in autophagy in response to metabolic stress suggested that p21 might be involved in the autophagy pathway (Manu et al. 2019).
Except for the occurrence of DNA damages in directly irradiated cells, signal molecules released from the irradiated cells would influence the neighboring non-irradiated cells. This is defined as the radiation-induced bystander effect (RIBE). There have been few studies on the involvement of p21 in RIBE, but available evidence suggests that p21 plays a critical role in RIBE. Azzam et al. observed that the signaling molecules secreted from irradiated cells enhanced p21 level in the non-irradiated bystander cells, and the increase of p21 level in the neighboring cells was related to radiation-induced ROS (Azzam et al. 2001, 2002). In addition, p21 deficient mouse embryo fibroblasts were incapable of producing bystander signals (Zhao et al. 2015). Without doubt, it is highly interesting to investigate the function of p21 in RIBE in further detail, because radiation tolerance of the normal tissues is also a limitation of radiotherapy. As the RIBE mechanisms continue to be elucidated, the efforts will not only make a contribution to guide radiation treatment planning, but also play a more relevant role in radiation protection of normal tissues during radiotherapy.
p21 and tumour radioresistance
Radioresistance, considered as the main reason of radiotherapy failure, leads to tumour recurrence and metastases after treatment and brings poor prognosis to cancer patients. Due to the complexity and incompleteness of the corresponding mechanisms, radioresistance is still a significant challenge in radiotherapy. Tumour radiosensitivity is strongly dependent upon its genetic background, which determines the response mechanisms of tumour to radiation and the characteristics of tumor microenvironment. p21, an important regulator involved in almost all aspects of the response to radiation, plays a pivotal role in tumour radiosensitivity. Studies on the effect of p21 on radioresistance are summarized in Table 5, possibly helping us sort out their relationship. Probably, p21 may play different roles in different cancers. According to the literature, up-regulation of p21 in non-small cell lung cancer decreases the tumour radioresistance, while p21 over-expression enhances radioresistance in colorectal cancer in the most studies (Table 5). Paradoxically, a few studies have drawn opposite conclusions in non-small cell lung cancer (Niu et al. 2020) and colorectal cancer (Fu et al. 1998; Zheng et al. 2015). It is noted that in the literature, p21 deficiency had no effect on the radiosensitivity (Fan et al. 1997) and thermal radiosensitization (Larsson and Ng 2003) in colorectal cancer. Different types of cell lines used in the experiments might be the most likely cause of the inconsistent conclusions, because p21 itself possesses the dual effect characteristic. p21 exhibits a promoting effect on radioresistance in other tumors, including brain, prostate, cervical, esophageal, large intestine cancer and nasopharyngeal carcinoma (Table 5). Interestingly, p21 in peripheral blood from post-irradiated breast cancer patients was capable of predicting early effects of radiotherapy, and patients with low level of p21, who underwent severe reactions to radiotherapy, had an inherent radiosensitivity (Badie et al. 2008). In addition, Zhou et al. reported that the radiation sensitivity of breast cancer cell line with knock-down p21 was significantly enhanced and bioinformatics analysis of clinical data in breast cancer showed that the prognosis of patients with low p21 expression is significantly better than that of the patient with high p21 expression. Multivariate cox analysis manifested that p21 expression level is an independent prognostic factor in breast cancer patient (Zhou et al. 2020). It is noteworthy that there is also evidence from the literature suggesting the promotive functions of p21 on radioresistance in normal cells both in vitro (Soysa et al. 2019; Stivala et al. 2001) and in vivo (Wang et al. 1997).
Table 5.
Influence of p21 on radioresistance
| Experimental material | Treatment | p21 influence on radioresistance | Study |
|---|---|---|---|
| Lung cancer cell lines A549, H1299, and H460 | Ionizing radiation | Decreasing radioresistance | Biswas et al. (2017) |
| Non-small cell lung cancer A549 cells | γ-Irradiation | Decreasing radioresistance | Kim et al. (2010) |
| Human lung cancer cell lines A549 and H1299 | X-ray | Decreasing radioresistance | Zhang et al. (2018) |
| Non-small cell and lung cancer cell lines | X-ray | Decreasing radioresistance | Wang et al. (2017) |
| Lung adenocarcinoma cell lines (A549, H1299, and H1975) | X-ray | Increasing radioresistance | Niu et al. (2020) |
| Patients with rectal carcinomas | Preoperative radiation | Decreasing radioresistance | Fu et al. (1998) |
| HCT116 p21–/– and HCT116 p21+/+ cell lines | γ-Irradiation | Increasing radioresistance | Waldman et al. (1997) |
| HCT-116 cell line | γ-Irradiation | Increasing radioresistance | (Waldman and B. 1996) |
| HCT116 human colon carcinoma cells | γ-Irradiation from a 137Cs source | Increasing radioresistance | Wouters et al. (1999) |
| Colon carcinoma HCT-116 cells | γ-Irradiation from a 137Cs source | p21 deficiency had no effect on radiosensitivity | Fan et al. (1997) |
| C57BL/6 and BALB/c mice, murine colorectal carcinoma cell line CT26 and IEC6 | γ-Irradiation from a 137Cs source | Increasing radioresistance | Nag et al. (2019) |
| HT-29 (HTB-38) and HCT-116 (CCL-247) colorectal cancer cells | X-ray | Increasing radioresistance | Huerta et al. (2013) |
| HCT116 human colorectal carcinoma cells | High-dose-rate X radiation, hyperthermia or a combination of the two treatments | p21 does not play an important role in the expression of thermal radiosensitization | Larsson and Ng 2003) |
| Human colonic carcinoma cell lines | X-ray | Decreasing radioresistance | Zheng et al. (2015) |
| Large intestine cancer HT29 cells | X-ray | Increasing radioresistance | Zuo et al. (2020) |
| Breast cancer lines | X-ray | Decreasing radioresistance | Yang et al. (2012) |
| Human triple-negative breast cancer cell line MDA-MB-231 | X-ray | Increasing radioresistance | Zhou et al. (2020) |
| Lymphocyte samples of sporadic breast cancer patients | X-ray | Increasing radioresistance | Badie et al. (2008) |
| Brain tumour cell lines | γ-Irradiation | Increasing radioresistance | Kokunai and Tamaki 1999) |
| Human glioma cell lines (T98G, U251MG, and U87MG) | γ-Irradiation | Increasing radioresistance | (Kokunai et al. (2001) |
| Glioblastoma cell lines | γ-Irradiation from a 137Cs source | Increasing radioresistance | Wang et al. (2006) |
| Sprague Dawley rats injected with RT-2 rat glioma cells | γ-Irradiation | Descreasing radioresistance | Hsiao et al. (1997) |
| Human esophageal squamous cancer cell line | X-ray | Increasing radioresistance | Zheng et al. (2017) |
| Esophageal squamous cell carcinomas cell line KYSE510 | X-ray | Increasing radioresistance | (Kuo et al. (2019) |
| Esophageal Adenocarcinoma cell line | Ionizing radiation | Increasing radioresistance | (Hotte et al. (2012) |
| Prostate cancer cell lines C4-2, RWPE-1 and CWR22Rv1 | γ-Irradiation | Increasing radioresistance | Xie et al. (2016) |
| Cervical cancer cell lines | X-ray | Increasing radioresistance | Pedroza-Torres et al. (2018) |
| Nasopharyngeal carcinoma cell lines HNE‑1 and CNE‑2 | Ionizing radiation | Increasing radioresistance | Du et al. (2017) |
| The oral carcinoma 3 (OC3) cell line | γ-Irradiation from a 60Co source | Decreasing radioresistance | Wu et al. (2013) |
| Rhabdomyosarcoma cell lines | X-ray | Decreasing radioresistance | Petragnano et al. (2020a) |
| All cells were derived from appropriate matings of mice carrying p21 and atm null alleles | γ-Irradiation from a 137Cs source | Increasing radioresistance | Wang et al. (1997) |
| Normal human lung embryonic fibroblasts | UV irradiation | Increasing radioresistance | Stivala et al. (2001) |
| Liver Kupffer cells | Irradiation | Increasing radioresistance | Soysa et al. (2019) |
Endogenous p21 expression status usually determines the intrinsic radiosensitivity of tumour cells. In non-small cell lung cancer, high level of p21 was significantly correlated with poor prognosis and positively associated with the resistance to X-rays (Niu et al. 2020). However, another study considered that patients with lower p21 expression represented the resistance to preoperative radiation and had poor prognosis (Fu et al. 1998). The results of the studies on colorectal cancer are relatively consistent in vitro and in vivo. HCT-116/p21−/− cells have been found to be more readily killed by γ-Irradiation than their p21+/+ counterparts (Huerta et al. 2013; Waldman et al. 1996). The p21+/+ tumour kept growing, while the p21−/− tumour disappeared after radiotherapy (Waldman et al. 1997). In addition, there was higher p21 expression in radioresistant astrocytic tumours than in more radiosensitive brain tumours (Kokunai and Tamaki 1999), but p21 expression increased the radiosensitivity of RT-2 rat glioma cells (Hsiao et al. 1997). In another interesting study, Petragnano et al. obtained clinically relevant radioresistant (RR) rhabdomyosarcoma (RMS) cell lines by hypo-fractionated schedule. Ionizing radiation (IR) up-regulated p21 expression in parental (PR) RMS cells, while RR cells significantly counteracted IR-induced p21 over-expression. IR increased the percentage of cells at G2/M phase more efficiently in PR cells compared to RR cells, and RR cells escaped from G2/M growth arrest more quickly than PR cells (Petragnano et al. 2020a), suggesting that p21-related molecular mechanism is highly correlated with tumour radioresistance. Besides, miRNAs maintain their effects on the radiosensitity via modulating the expression of target gene p21. High level of miR-125a sensitized cervical cancer cells to radiation therapy by down-regulating the expression of p21 (Pedroza-Torres et al. 2018) and mi-R373 has a similar effect in glioblastoma (Peng et al. 2020), while miRNA-17-5p (Wu et al. 2013), miRNA-106b (Zheng et al. 2015) and miR-208a (Tang et al. 2016) increased the radioresistance of cancer cells by targeting p21. Therefore, in tumours with different genetic backgrounds, p21 shows significant differences in expression and biological function on radioresistance. Participation of p21 in multiple intricate cellular processes mentioned above may explain the observed differences.
Some drugs and inhibitors are utilized as radiosensitizer via regulating p21 level to play their roles. Bromodomain and extra-terminal (BET) bromodomain inhibitors JQ1 and Nedd8 activating enzyme inhibitor MLN42924 increased radiation sensitivity of non-small cell lung cancer by up-regulating p21 and enhancing G2/M arrest, respectively, in non-small cell lung cancer (Wang et al. 2017) and breast cancer (Yang et al. 2012). In the radiosensitizing studies of colon tumor, auraptene was found to increase the radiosensitivity of colon adenocarcinoma cells by up-regulating p21 (Moussavi et al. 2017). Another drug auranofin not only prevented normal intestinal tissue from radiation toxicity and improved survival with the activation of p53/p21–mediated reversible cell cycle arrest but also inhibited malignant tissue growth (Nag et al. 2019). Conversely, repression of p21 via antisense oligonucleotide enhanced the γ-irradiation-induced apoptosis and cytotoxicity in radioresistant glioma cells (Kokunai et al. 2001). Farnesyltransferase inhibitors R115777 increased radiation sensitivity of glioblastoma cells via inhibiting p21 (Wang et al. 2006). Zoledronic acid augmented the radiosensitivity of cancer cells via depleting p21 (Du et al. 2017). Palbociclib could be a novel radiosensitizer agent and it increases the radiosensitivity of cancer cells via blocking the transcriptional activity of p53 and p21 (Fernandez-Aroca et al. 2019). Although there are the contradictory results in the studies, they still provide a theoretical basis for considering p21 as a radiotherapy target.
Another interesting point is that prostate cancer cells increased radiotherapy resistance via recruiting mast cell to up-regulate p21 (Xie et al. 2016), implying that p21 is associated with tumor microenvironment-mediated radioresistance. Additionally, our unpublished (data not shown) results showed that p21 elevated radiation resistance of GBM via promoting the glycolysis under hypoxia. Western blot and Luciferase reporter analyses showed that p21 was transcriptionally activated by HIF-1α in GBM cells under hypoxic conditions. Interestingly, we also observed that augment of p21 is necessary for promoting HIF-1α transcription. Under oxygen deficiency, the presence of mutual positive feedback between HIF-1α and p21 induced the glycolysis via up-regulation of the expression of Glut1 and LDHA and enhanced the radioresistance of GBM cells. The positive feedback loop between HIF-1α and p21 might play a crucial role in enhancing the radioresistance of GBM under hypoxia.
Targeting p21 in cancer radiotherapy
According to the functions of p21 in radiotherapy mentioned above, p21 definitely plays a dual role in tumour cells. In the main cellular processes induced by ionizing radiation, including cell cycle arrest, DNA repair, apoptosis and senescence, although the up- or down-regulatory role of p21 strongly depends on the cell type, there is no doubt that p21 has an important function in radiotherapy. In line with this, understanding the reasons for the dual role of p21 will help to define the relationship between p21 and radiosensitivity. More importantly, p21 should serve as a biomarker for specific therapies or prognosis, because it is related not only to the radiosensitivity but also to the chemoresistance (Liu et al. 2003) of tumour.
Based on the results of the retrospective studies summarized in this paper, nearly 70% of the studies considered that p21 could improve tumor resistance to radiotherapy, while a small portion of the studies showed contrary observations. A relatively complete chain of evidence about increasing the radioresistance of cancer by p21 has been formulated according to the findings from the existing literature (shown in Fig. 2). A study on extrahepatic cholangiocarcinoma has drawn the conclusion that both low and high p21 levels are effective biomarkers of negative prognosis, whereas moderate levels of p21 afford a more advantageous indication (Li et al. 2000). As mentioned above, the expression disorder of p21 is significantly associated with poor prognosis in several tumours. Accordingly, simply modulating p21 as a target of radiotherapy would increase risks and unanticipated side effects. Given the “antagonistic duality” of p21 (Gartel and Tyner 2002), cancer radiotherapy targeting p21 should manipulate the level of p21 on the basis of its function in specific tumour type.
Fig. 2.
p21 determines the fate of cells exposed to radiation. The p53 pathway is activated after DNA damage caused by radiation and leads to either apoptosis or anti-apoptosis. The subsequent increase in p21 allows cells to execute the DNA repair process and develop radiatoresistance
First, up-regulation of p21 should be considered in malignant cells with low level of p21, which maintains the radioresistance of tumour. Histone deacetylase inhibitors (Ocker and Schneider-Stock 2007), agonist of p53 (Martinez 2010; Shen and Maki 2011) or stagnating the degradation of p21 by proteasome inhibitors (Rastogi and Mishra 2012) could be utilized to facilitate the function of p21 as tumor suppressor and inhibit the tumor development. On the other hand, the high expression of p21 in normal tissues might play a role of radioprotection. There is a study indicating that increased p21 expression is conducive to reinstate intestinal integrity after radiation (Pant et al. 2019). Enhancing p21 activity could be a viable prophylactic strategy for alleviating gastrointestinal syndrome in patients undergoing radiotherapy.
Even more importantly, down-regulation of p21 should be implemented in tumours with highly expressed p21, because over-expression of p21 not only increases the radioresistance of tumours (Table 5) but also plays an anti-drug role in the studies, where doxorubicin (Martinez et al. 2002), camptothecin (Han et al. 2002) and taxol (Li et al. 2002) were used to treat tumors. Various methods reducing p21 expression such as antisense oligonucleotides (ASOs) (Kokunai et al. 2001), small interfering RNA (siRNA), synthetic miRNA mimics (Ivanovska et al. 2008; van Beijnum et al. 2017) and a promising targeted therapy utilizing the clustered regularly interspaced short palindromic repeats (CRISPR) technique (Ghosh et al. 2019) were applied. Down-regulating p21 by ASOs has been employed to induce proliferation block and/or apoptosis in breast cancer (Weiss et al. 2003), colorectal cancer (Tian et al. 2000), glioma (Kokunai et al. 2001), myeloid leukemia (Freemerman et al. 1997) and renal cell carcinoma cell lines (Park et al. 2008a). The technique of transient transfection is usually limited by its short duration and difficulty of transport to tumour tissue. So there are some difficulties to put ASOs, siRNA and miRNA into clinical use (Burnett et al. 2011; Liu et al. 2013). Moreover, small molecule inhibitors of p21 have been discovered and used in some studies. Butyrolactone I (BL) (Liu et al. 2013), butyrolactone (Sax et al. 2002) and LLW10 (Park et al. 2008b) act as effective inhibitors of p21 via promoting p21 proteasomal degradation to reduce its expression. Given the limitation of their specificity on targeting p21, these inhibitors might not be a preferred choice for clinical use. It is a potential and selective intervening strategy to hinder the oncogenic functions of p21 by inhibiting its combination with PCNA using small molecules like T2AA (Punchihewa et al. 2012).
So many researchers believe that cytoplasmic p21 and nuclear p21 have opposite functions, tumorigenic and tumor-suppressive roles, respectively. Phosphorylated p21 is normally localized in the cytoplasm. Cytoplasmic localization of p21 is involved in cell proliferation and survival, by facilitating the combination of the cyclinD with CDK4/6 and preventing tumours from apoptosis, respectively (Abbas and Dutta 2009). In addition, cytoplasmic p21 may also function as a chaperone for cyclin E to sponsor G1/S progression (Georgakilas et al. 2017). On the contrary, nuclear p21 supports its anticancer effects by blocking cell division and growth and represses some DNA repair pathways in the nucleus leading to senescence (Abbas and Dutta 2009; Gawriluk et al. 2016; Georgakilas et al. 2017). Yang et al. found that IFITM1 knockdown could result in p21 up-regulation and p-p21 down-regulation, which increased the effectiveness of radiotherapy (Yang et al. 2018). In line with this, targeting cytoplasmic p21 or obstructing nuclear-cytoplasmic shuttling of p21 might be a desirable strategy. UC2288, a hopeful small molecule inhibitor, reduces tumour growth by targeting cytoplasmic p21 without disturbing nuclear p21 levels and p53 (Liu et al. 2013). The phosphorylation by Akt kinase of p21 at different sites may lead to its cytoplasmic localization (Kreis et al. 2015). Akt inhibitors, therefore, would be a decent choice for contributing to the nuclear relocation of p21 and sensitizing cells to anticancer therapy (Koster et al. 2010). However, there are few investigations on the functions of cytoplasmic p21 and nuclear p21 in radiotherapy and only one study reported that radiation-induced p21 expression was restricted to the nucleus (Sohn et al. 2006). Hence the use of these drugs may need further investigation in this regard.
What’s more, a therapeutic strategy specially aiming at radiotherapy is based on the expression of p21. Endogenous p21, an excellent molecular biomarker in vivo, could be induced by external beam radiation or intracellular concentrated radionuclides (McCarthy et al. 2007) and its expression has a good linear relationship with radiation dose (Nenoi et al. 2006). McMahon et al. set up a new p21 reporter mouse model, which was able to reflects the extent of ionizing radiation-induced DNA damage clearly by means of p21 levels (McMahon et al. 2016). More importantly, it has been reported that a clinically relevant strategy of p21-driven inducible nitric oxide synthase (iNOS) gene therapy significantly sensitized tumour cells to fractionated radiotherapy in vitro and in vivo (McCarthy et al. 2007). iNOS could generate the potent radiosensitizer, nitric oxide. Besides, p21 gene promoter in combination with recombinant adeno-associated virus (rAAV) vector is potentially usable in developing a radiation-inducible vector for cancer gene therapy (Nenoi et al. 2006). These studies highlight the application of p21-driven chimeric promoter or gene therapy in radiotherapy.
In summary, it is vital to implement selective interference to tumorigenic function of p21, meanwhile, maintaining its role in cell cycle regulation and genome stability. There is a clear need for further studies to solve the mystery of p21’s duality and to assure that p21 is a safe target for radiotherapy in certain tumour types.
Conclusions and future directions
Studies on p21 lasted over 25 years and found that p21 is a complex and multidimensional protein. p21 controls multiple cellular processes of tumour in response to radiation exposure, including cell cycle, DNA repair, apoptosis, senescence, autophagy and tumor microenvironment. Even more confusing is the fact that it plays dual role in these processes. Therefore, thorough comprehension of p21 is still a huge challenge. Powerful regulation function forces researchers to attach importance to p21 and consider it as a promising radiotherapeutic target. In follow-up studies, focus should be concentrated on the mechanisms of p21 playing opposite roles and how to selectively control its oncogenicity. p21 with different post-translational modification sites may play diverse roles in biological systems. An equilibrium between the different functions of p21 might be the key to successful radiotherapy.
Author contributions
YK had the idea for the review and performed the writing of the full text. JK, HL, BL, XZ and LL participated in the collection of literature. XJ and QL critically revised the work. All authors read and approved the final manuscript.
Funding
This work was jointly supported by the National Key Research Program of China (Grant nos. 2018YFC0115700, 2018YFC0115702), the National Natural Science Foundation of China (Grant nos. 11875299, U1532264), the Key Deployment Project of Chinese Academy of Sciences (Grant No. KFZD-SW-222) and the National Natural Science Foundation of Chinese Academy of Engineering Physics (Grant no. U1730133).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Consent for publication
All authors agree with its publication.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaodong Jin, Email: jinxd@impcas.ac.cn.
Qiang Li, Email: liqiang@impcas.ac.cn.
References
- Abbas T, Dutta A (2009) p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9:400–414. 10.1038/nrc2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adimoolam S, Lin CX, Ford JM (2001) The p53-regulated cyclin-dependent kinase inhibitor, p21 (cip1, waf1, sdi1), is not required for global genomic and transcription-coupled nucleotide excision repair of UV-induced DNA photoproducts. J Biol Chem 276:25813–25822. 10.1074/jbc.M102240200 [DOI] [PubMed] [Google Scholar]
- Ahmad N, Feyes DK, Agarwal R, Mukhtar H (1998) Photodynamic therapy results in induction of WAF1/CIP1/P21 leading to cell cycle arrest and apoptosis. Proc Natl Acad Sci USA 95:6977–6982. 10.1073/pnas.95.12.6977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khalaf HH, Hendrayani SF, Aboussekhra A (2012) ATR controls the p21(WAF1/Cip1) protein up-regulation and apoptosis in response to low UV fluences. Mol Carcinog 51:930–938. 10.1002/mc.20864 [DOI] [PubMed] [Google Scholar]
- Asada M, Yamada T, Ichijo H, Delia D, Miyazono K, Fukumuro K, Mizutani S (1999) Apoptosis inhibitory activity of cytoplasmic p21(Cip1/WAF1) in monocytic differentiation. EMBO J 18:1223–1234. 10.1093/emboj/18.5.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari M, Sobti RC, Nikbakht M, Sharma SC (2013) Aberrant promoter hypermethylation of p21 (WAF1/CIP1) gene and its impact on expression and role of polymorphism in the risk of breast cancer. Mol Cell Biochem 382:19–26. 10.1007/s11010-013-1696-5 [DOI] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB (2001) Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 98:473–478. 10.1073/pnas.011417098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, De Toledo SM, Spitz DR, Little JB (2002) Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62:5436–5442 [PubMed] [Google Scholar]
- Badie C et al (2008) Aberrant CDKN1A transcriptional response associates with abnormal sensitivity to radiation treatment. Br J Cancer 98:1845–1851. 10.1038/sj.bjc.6604381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptiste-Okoh N, Barsotti AM, Prives C (2008) Caspase 2 is both required for p53-mediated apoptosis and downregulated by p53 in a p21-dependent manner. Cell Cycle 7:1133–1138. 10.4161/cc.7.9.5805 [DOI] [PubMed] [Google Scholar]
- Bertoli C, Skotheim JM, de Bruin RA (2013) Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14:518–528. 10.1038/nrm3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Sarkar S, Du K, Brautigan DL, Abbas T, Larner JM (2017) The E3 ligase CHIP mediates p21 degradation to maintain radioresistance. Mol Cancer Res 15:651–659. 10.1158/1541-7786.MCR-16-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott SR, Arya M, Kirby RS, Williamson M (2005) p21WAF1/CIP1 gene is inactivated in metastatic prostatic cancer cell lines by promoter methylation. Prostate Cancer Prostatic Dis 8:321–326. 10.1038/sj.pcan.4500822 [DOI] [PubMed] [Google Scholar]
- Bunz F et al (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497–1501. 10.1126/science.282.5393.1497 [DOI] [PubMed] [Google Scholar]
- Burnett JC, Rossi JJ, Tiemann K (2011) Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J 6:1130–1146. 10.1002/biot.201100054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylicky MA, Mueller GP, Day RM (2019) Radiation resistance of normal human astrocytes: the role of non-homologous end joining DNA repair activity. J Radiat Res 60:37–50. 10.1093/jrr/rry084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L et al (2014) A novel ATM/TP53/p21-mediated checkpoint only activated by chronic gamma-irradiation. PLoS ONE 9:e104279. 10.1371/journal.pone.0104279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB (2000) Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA 97:4291–4296. 10.1073/pnas.97.8.4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ (1999) The p21(Cip1) and p27(Kip1) CDK “inhibitors” are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 18:1571–1583. 10.1093/emboj/18.6.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AE, Spandau DF, Wek RC (2018) Translational control of a human CDKN1A mRNA splice variant regulates the fate of UVB-irradiated human keratinocytes. Mol Biol Cell 29:29–41. 10.1091/mbc.E17-06-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochemore C, Fernandez-Molina C, Montagne B, Salles A, Ricchetti M (2019) CSB promoter downregulation via histone H3 hypoacetylation is an early determinant of replicative senescence. Nat Commun 10:5576. 10.1038/s41467-019-13314-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daido S, Yamamoto A, Fujiwara K, Sawaya R, Kondo S, Kondo Y (2005) Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy. Cancer Res 65:4368–4375. 10.1158/0008-5472.CAN-04-4202 [DOI] [PubMed] [Google Scholar]
- De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM, Hegi-Johnson F (2019) Radiotherapy toxicity. Nat Rev Dis Primers 5:13. 10.1038/s41572-019-0064-5 [DOI] [PubMed] [Google Scholar]
- Du C, Wang Y, Li H, Huang Y, Jiang O, You Y, Luo F (2017) Zoledronic acid augments the radiosensitivity of cancer cells through perturbing S- and M-phase cyclins and p21(CIP1) expression. Oncol Lett 14:4237–4242. 10.3892/ol.2017.6710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry WS et al (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825. 10.1016/0092-8674(93)90500-p [DOI] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elumalai P et al (2012) Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett 215:131–142. 10.1016/j.toxlet.2012.10.008 [DOI] [PubMed] [Google Scholar]
- Fan S, Chang JK, Smith ML, Duba D, Fornace AJ Jr, O’Connor PM (1997) Cells lacking CIP1/WAF1 genes exhibit preferential sensitivity to cisplatin and nitrogen mustard. Oncogene 14:2127–2136. 10.1038/sj.onc.1201052 [DOI] [PubMed] [Google Scholar]
- Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA (1999) p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 18:2789–2797. 10.1038/sj.onc.1202615 [DOI] [PubMed] [Google Scholar]
- Fernandez-Aroca DM et al (2019) P53 pathway is a major determinant in the radiosensitizing effect of Palbociclib: implication in cancer therapy. Cancer Lett 451:23–33. 10.1016/j.canlet.2019.02.049 [DOI] [PubMed] [Google Scholar]
- Fournier C, Wiese C, Taucher-Scholz G (2004) Accumulation of the cell cycle regulators TP53 and CDKN1A (p21) in human fibroblasts after exposure to low- and high-LET radiation. Radiat Res 161:675–684. 10.1667/rr3182 [DOI] [PubMed] [Google Scholar]
- Freemerman AJ, Vrana JA, Tombes RM, Jiang H, Chellappan SP, Fisher PB, Grant S (1997) Effects of antisense p21 (WAF1/CIP1/MDA6) expression on the induction of differentiation and drug-mediated apoptosis in human myeloid leukemia cells (HL-60). Leukemia 11:504–513. 10.1038/sj.leu.2400625 [DOI] [PubMed] [Google Scholar]
- Fu CG et al (1998) Role of p53 and p21/WAF1 detection in patient selection for preoperative radiotherapy in rectal cancer patients. Dis Colon Rectum 41:68–74. 10.1007/BF02236898 [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM (2006) Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 25:4798–4811. 10.1038/sj.onc.1209608 [DOI] [PubMed] [Google Scholar]
- Furusawa Y et al (2012) TGF-beta-activated kinase 1 promotes cell cycle arrest and cell survival of X-ray irradiated HeLa cells dependent on p21 induction but independent of NF-kappaB, p38 MAPK and ERK phosphorylations. Radiat Res 177:766–774. 10.1667/rr2792.1 [DOI] [PubMed] [Google Scholar]
- Gartel AL (2005) The conflicting roles of the cdk inhibitor p21(CIP1/WAF1) in apoptosis. Leuk Res 29:1237–1238. 10.1016/j.leukres.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Gartel AL (2009) p21(WAF1/CIP1) and cancer: a shifting paradigm? BioFactors 35:161–164. 10.1002/biof.26 [DOI] [PubMed] [Google Scholar]
- Gartel AL, Tyner AL (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1:639–649 [PubMed] [Google Scholar]
- Gawriluk TR et al (2016) Comparative analysis of ear-hole closure identifies epimorphic regeneration as a discrete trait in mammals. Nat Commun 7:11164. 10.1038/ncomms11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas AG, Martin OA, Bonner WM (2017) p21: a two-faced genome guardian trends. Mol Med 23:310–319. 10.1016/j.molmed.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Ghosh D, Venkataramani P, Nandi S, Bhattacharjee S (2019) CRISPR-Cas9 a boon or bane: the bumpy road ahead to cancer therapeutics. Cancer Cell Int 19:12. 10.1186/s12935-019-0726-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham K, Moran-Jones K, Sansom OJ, Brunton VG, Frame MC (2011) FAK deletion promotes p53-mediated induction of p21, DNA-damage responses and radio-resistance in advanced squamous cancer cells. PLoS ONE 6:e27806. 10.1371/journal.pone.0027806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z et al (2002) Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J Biol Chem 277:17154–17160. 10.1074/jbc.M112401200 [DOI] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805–816. 10.1016/0092-8674(93)90499-g [DOI] [PubMed] [Google Scholar]
- Ho SY et al (2019) Cordycepin enhances radiosensitivity in oral squamous carcinoma cells by inducing autophagy and apoptosis through cell cycle arrest. Int J Mol Sci. 10.3390/ijms20215366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K et al (2007) Gefitinib induces premature senescence in non-small cell lung cancer cells with or without EGFR gene mutation. Oncol Rep 17:313–317 [PubMed] [Google Scholar]
- Hotte GJ, Linam-Lennon N, Reynolds JV, Maher SG (2012) Radiation sensitivity of esophageal adenocarcinoma: the contribution of the RNA-binding protein RNPC1 and p21-mediated cell cycle arrest to radioresistance. Radiat Res 177:272–279. 10.1667/rr2776.1 [DOI] [PubMed] [Google Scholar]
- Hsiao M, Tse V, Carmel J, Costanzi E, Strauss B, Haas M, Silverberg GD (1997) Functional expression of human p21(WAF1/CIP1) gene in rat glioma cells suppresses tumor growth in vivo and induces radiosensitivity. Biochem Biophys Res Commun 233:329–335. 10.1006/bbrc.1997.6450 [DOI] [PubMed] [Google Scholar]
- Huerta S, Gao X, Dineen S, Kapur P, Saha D, Meyer J (2013) Role of p53, Bax, p21, and DNA-PKcs in radiation sensitivity of HCT-116 cells and xenografts. Surgery 154:143–151. 10.1016/j.surg.2013.03.012 [DOI] [PubMed] [Google Scholar]
- Ivanovska I et al (2008) MicroRNAs in the miR-106b family regulate p21/CDKN1A and promote cell cycle progression. Mol Cell Biol 28:2167–2174. 10.1128/MCB.01977-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob B, Scholz M, Taucher-Scholz G (2002) Characterization of CDKN1A (p21) binding to sites of heavy-ion-induced damage: colocalization with proteins involved in DNA repair. Int J Radiat Biol 78:75–88. 10.1080/09553000110090007 [DOI] [PubMed] [Google Scholar]
- Jeon HY et al (2016) Irradiation induces glioblastoma cell senescence and senescence-associated secretory phenotype. Tumour Biol 37:5857–5867. 10.1007/s13277-015-4439-2 [DOI] [PubMed] [Google Scholar]
- Jin Q, Lin C, Zhu X, Cao Y, Guo C, Wang L (2020) (125)I seeds irradiation inhibits tumor growth and induces apoptosis by Ki-67, P21, survivin, livin and caspase-9 expression in lung carcinoma xenografts. Radiat Oncol 15:238. 10.1186/s13014-020-01682-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner DE, Bastar JD, Randall RL (2006) Doxorubicin induces cell senescence preferentially over apoptosis in the FU-SY-1 synovial sarcoma cell line. J Orthop Res 24:1163–1169. 10.1002/jor.20169 [DOI] [PubMed] [Google Scholar]
- Jung YS, Qian Y, Chen X (2010) Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal 22:1003–1012. 10.1016/j.cellsig.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KH, Kim WH, Choi KH (1999) p21 promotes ceramide-induced apoptosis and antagonizes the antideath effect of Bcl-2 in human hepatocarcinoma cells. Exp Cell Res 253:403–412. 10.1006/excr.1999.4644 [DOI] [PubMed] [Google Scholar]
- Keam SP et al (2018) The transcriptional landscape of radiation-treated human prostate cancer: analysis of a prospective tissue cohort. Int J Radiat Oncol Biol Phys 100:188–198. 10.1016/j.ijrobp.2017.09.037 [DOI] [PubMed] [Google Scholar]
- Kim EJ, Lee SY, Kim TR, Choi SI, Cho EW, Kim KC, Kim IG (2010) TSPYL5 is involved in cell growth and the resistance to radiation in A549 cells via the regulation of p21(WAF1/Cip1) and PTEN/AKT pathway. Biochem Biophys Res Commun 392:448–453. 10.1016/j.bbrc.2010.01.045 [DOI] [PubMed] [Google Scholar]
- Kim BM et al (2015) Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci 16:26880–26913. 10.3390/ijms161125991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Yutoku Y, Koike A (2011) Accumulation of p21 proteins at DNA damage sites independent of p53 and core NHEJ factors following irradiation. Biochem Biophys Res Commun 412:39–43. 10.1016/j.bbrc.2011.07.032 [DOI] [PubMed] [Google Scholar]
- Kokunai T, Tamaki N (1999) Relationship between expression of p21WAF1/CIP1 and radioresistance in human gliomas. Jpn J Cancer Res 90:638–646. 10.1111/j.1349-7006.1999.tb00795.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokunai T, Urui S, Tomita H, Tamaki N (2001) Overcoming of radioresistance in human gliomas by p21WAF1/CIP1 antisense oligonucleotide. J Neurooncol 51:111–119. 10.1023/a:1010645205169 [DOI] [PubMed] [Google Scholar]
- Koster R et al (2010) Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J Clin Invest 120:3594–3605. 10.1172/JCI41939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A, Gross MW, Knuechel R, Munkel K, Neff F, Schlegel J (2000) Aberrant p21 regulation in radioresistant primary glioblastoma multiforme cells bearing wild-type p53. J Neurosurg 93:863–872. 10.3171/jns.2000.93.5.0863 [DOI] [PubMed] [Google Scholar]
- Kreis NN, Louwen F, Yuan J (2015) Less understood issues: p21(Cip1) in mitosis and its therapeutic potential. Oncogene 34:1758–1767. 10.1038/onc.2014.133 [DOI] [PubMed] [Google Scholar]
- Kreis NN, Louwen F, Yuan J (2019) The multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation migration and cancer therapy. Cancers (Basel). 10.3390/cancers11091220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24:2463–2479. 10.1101/gad.1971610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo IY, Huang YL, Lin CY, Lin CH, Chang WL, Lai WW, Wang YC (2019) SOX17 overexpression sensitizes chemoradiation response in esophageal cancer by transcriptional down-regulation of DNA repair and damage response genes. J Biomed Sci 26:20. 10.1186/s12929-019-0510-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBaer J et al (1997) New functional activities for the p21 family of CDK inhibitors. Genes Dev 11:847–862. 10.1101/gad.11.7.847 [DOI] [PubMed] [Google Scholar]
- Larsson C, Ng CE (2003) p21+/+ (CDKN1A+/+) and p21-/- (CDKN1A-/-) human colorectal carcinoma cells display equivalent amounts of thermal radiosensitization. Radiat Res 160:205–209. 10.1667/3031 [DOI] [PubMed] [Google Scholar]
- Lee EW et al (2009) Differential regulation of p53 and p21 by MKRN1 E3 ligase controls cell cycle arrest and apoptosis. EMBO J 28:2100–2113. 10.1038/emboj.2009.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Lee KS, Nam KS (2019) The association of changes in RAD51 and survivin expression levels with the proton beam sensitivity of Capan1 and Panc1 human pancreatic cancer cells. Int J Oncol 54:744–752. 10.3892/ijo.2018.4642 [DOI] [PubMed] [Google Scholar]
- Li X, Hui A, Takayama T, Cui X, Shi Y, Makuuchi M (2000) Altered p21(WAF1/CIP1) expression is associated with poor prognosis in extrahepatic bile duct carcinoma. Cancer Lett 154:85–91. 10.1016/s0304-3835(00)00383-9 [DOI] [PubMed] [Google Scholar]
- Li Y, Dowbenko D, Lasky LA (2002) AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem 277:11352–11361. 10.1074/jbc.M109062200 [DOI] [PubMed] [Google Scholar]
- Liu S, Bishop WR, Liu M (2003) Differential effects of cell cycle regulatory protein p21(WAF1/Cip1) on apoptosis and sensitivity to cancer chemotherapy. Drug Resist Updat 6:183–195. 10.1016/s1368-7646(03)00044-x [DOI] [PubMed] [Google Scholar]
- Liu XF et al (2006) The effect of p21 antisense oligodeoxynucleotides on the radiosensitivity of nasopharyngeal carcinoma cells with normal p53 function. Cell Biol Int 30:283–287. 10.1016/j.cellbi.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Liu R, Wettersten HI, Park SH, Weiss RH (2013) Small-molecule inhibitors of p21 as novel therapeutics for chemotherapy-resistant kidney cancer. Future Med Chem 5:991–994. 10.4155/fmc.13.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X et al (2018) Inhibition of survivin enhances radiosensitivity of esophageal cancer cells by switching radiation-induced senescence to apoptosis. Oncol Targets Ther 11:3087–3100. 10.2147/OTT.S166798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X et al (2020) Astragaloside IV ameliorates radiation-induced senescence via antioxidative mechanism. J Pharm Pharmacol 72:1110–1118. 10.1111/jphp.13284 [DOI] [PubMed] [Google Scholar]
- Lu Y, Yamagishi N, Yagi T, Takebe H (1998) Mutated p21(WAF1/CIP1/SDI1) lacking CDK-inhibitory activity fails to prevent apoptosis in human colorectal carcinoma cells. Oncogene 16:705–712. 10.1038/sj.onc.1201585 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M (2001) To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 1:222–231. 10.1038/35106065 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30:630–641. 10.1016/j.tibs.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Manu KA, Cao PHA, Chai TF, Casey PJ, Wang M (2019) p21cip1/waf1 coordinate autophagy, proliferation and apoptosis in response to metabolic stress. Cancers (Basel). 10.3390/cancers11081112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JD (2010) Restoring p53 tumor suppressor activity as an anticancer therapeutic strategy. Future Oncol 6:1857–1862. 10.2217/fon.10.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LA et al (2002) p21 modulates threshold of apoptosis induced by DNA-damage and growth factor withdrawal in prostate cancer cells. Carcinogenesis 23:1289–1296. 10.1093/carcin/23.8.1289 [DOI] [PubMed] [Google Scholar]
- Mauro M, Rego MA, Boisvert RA, Esashi F, Cavallo F, Jasin M, Howlett NG (2012) p21 promotes error-free replication-coupled DNA double-strand break repair. Nucleic Acids Res 40:8348–8360. 10.1093/nar/gks612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy HO et al (2007) p21((WAF1))-mediated transcriptional targeting of inducible nitric oxide synthase gene therapy sensitizes tumours to fractionated radiotherapy. Gene Ther 14:246–255. 10.1038/sj.gt.3302871 [DOI] [PubMed] [Google Scholar]
- McConnell BB, Starborg M, Brookes S, Peters G (1998) Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol 8:351–354. 10.1016/s0960-9822(98)70137-x [DOI] [PubMed] [Google Scholar]
- McDonald ER 3rd, Wu GS, Waldman T, El-Deiry WS (1996) Repair defect in p21 WAF1/CIP1-/- human cancer cells. Cancer Res 56:2250–2255 [PubMed] [Google Scholar]
- McMahon M, Frangova TG, Henderson CJ, Wolf CR (2016) Olaparib, monotherapy or with ionizing radiation, exacerbates DNA damage in normal tissues: insights from a new p21 reporter mouse. Mol Cancer Res 14:1195–1203. 10.1158/1541-7786.MCR-16-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Armenta M, Nava-Ruiz C, Juarez-Rebollar D, Rodriguez-Martinez E, Gomez PY (2014) Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxid Med Cell Longev 2014:293689. 10.1155/2014/293689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic M, Yu Y, Schmitt CA (2018) The senescence-stemness alliance—a cancer-hijacked regeneration principle. Trends Cell Biol 28:1049–1061. 10.1016/j.tcb.2018.09.001 [DOI] [PubMed] [Google Scholar]
- Mirzayans R, Scott A, Andrais B, Pollock S, Murray D (2008) Ultraviolet light exposure triggers nuclear accumulation of p21(WAF1) and accelerated senescence in human normal and nucleotide excision repair-deficient fibroblast strains. J Cell Physiol 215:55–67. 10.1002/jcp.21284 [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Richardson RA, Dewhirst MW (2007) Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev 26:241–248. 10.1007/s10555-007-9056-0 [DOI] [PubMed] [Google Scholar]
- Moussavi M, Haddad F, Rassouli FB, Iranshahi M, Soleymanifard S (2017) Synergy between Auraptene, Ionizing radiation, and anticancer drugs in colon adenocarcinoma cells. Phytother Res 31:1369–1375. 10.1002/ptr.5863 [DOI] [PubMed] [Google Scholar]
- Murphy M et al (2002) The expression of p53, p21, Bax and induction of apoptosis in normal volunteers in response to different doses of ultraviolet radiation. Br J Dermatol 147:110–117. 10.1046/j.1365-2133.2002.04749.x [DOI] [PubMed] [Google Scholar]
- Nag D et al (2019) Auranofin protects intestine against radiation injury by modulating p53/p21 pathway and radiosensitizes human colon tumor. Clin Cancer Res 25:4791–4807. 10.1158/1078-0432.CCR-18-2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenoi M, Daino K, Ichimura S, Takahash S, Akuta T (2006) Low-dose radiation response of the p21WAF1/CIP1 gene promoter transduced by adeno-associated virus vector. Exp Mol Med 38:553–564. 10.1038/emm.2006.65 [DOI] [PubMed] [Google Scholar]
- Nenoi M, Daino K, Nakajima T, Wang B, Taki K, Kakimoto A (2009) Involvement of Oct-1 in the regulation of CDKN1A in response to clinically relevant doses of ionizing radiation. Biochim Biophys Acta 1789:225–231. 10.1016/j.bbagrm.2008.12.002 [DOI] [PubMed] [Google Scholar]
- Nguyen HQ, To NH, Zadigue P, Kerbrat S, De La Taille A, Le Gouvello S, Belkacemi Y (2018) Ionizing radiation-induced cellular senescence promotes tissue fibrosis after radiotherapy. A review. Crit Rev Oncol Hematol 129:13–26. 10.1016/j.critrevonc.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Nguyen HQ et al (2020) Human CCR6+ Th17 lymphocytes are highly sensitive to radiation-induced senescence and are a potential target for prevention of radiation-induced toxicity. Int J Radiat Oncol Biol Phys 108:314–325. 10.1016/j.ijrobp.2019.10.045 [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ et al (2004) Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res 64:2956–2961. 10.1158/0008-5472.can-03-2388 [DOI] [PubMed] [Google Scholar]
- Niculescu AB 3rd, Chen X, Smeets M, Hengst L, Prives C, Reed SI (1998) Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol 18:629–643. 10.1128/mcb.18.1.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niibe Y, Nakano T, Ohno T, Tsujii H, Oka K (1999) Relationship between p21/WAF-1/CIP-1 and apoptosis in cervical cancer during radiation therapy. Int J Radiat Oncol Biol Phys 44:297–303. 10.1016/s0360-3016(99)00026-7 [DOI] [PubMed] [Google Scholar]
- Niu H et al (2020) Knockdown of SMAD3 inhibits the growth and enhances the radiosensitivity of lung adenocarcinoma via p21 in vitro and in vivo. Int J Biol Sci 16:1010–1022. 10.7150/ijbs.40173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A, Ning Y, Venable SF, Pereira-Smith OM, Smith JR (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res 211:90–98. 10.1006/excr.1994.1063 [DOI] [PubMed] [Google Scholar]
- Ocker M, Schneider-Stock R (2007) Histone deacetylase inhibitors: signalling towards p21cip1/waf1. Int J Biochem Cell Biol 39:1367–1374. 10.1016/j.biocel.2007.03.001 [DOI] [PubMed] [Google Scholar]
- Ogryzko VV, Wong P, Howard BH (1997) WAF1 retards S-phase progression primarily by inhibition of cyclin-dependent kinases. Mol Cell Biol 17:4877–4882. 10.1128/mcb.17.8.4877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ZQ, Reardon JT, Li L, Flores-Rozas H, Legerski R, Sancar A, Hurwitz J (1995) Inhibition of nucleotide excision repair by the cyclin-dependent kinase inhibitor p21. J Biol Chem 270:22008–22016. 10.1074/jbc.270.37.22008 [DOI] [PubMed] [Google Scholar]
- Pant V et al (2019) Transient enhancement of p53 activity protects from radiation-induced gastrointestinal toxicity. Proc Natl Acad Sci USA 116:17429–17437. 10.1073/pnas.1909550116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Park JY, Weiss RH (2008a) Antisense attenuation of p21 sensitizes kidney cancer to apoptosis in response to conventional DNA damaging chemotherapy associated with enhancement of phospho-p53. J Urol 180:352–360. 10.1016/j.juro.2008.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Wang X, Liu R, Lam KS, Weiss RH (2008b) High throughput screening of a small molecule one-bead-one-compound combinatorial library to identify attenuators of p21 as chemotherapy sensitizers. Cancer Biol Ther 7:2015–2022. 10.4161/cbt.7.12.7069 [DOI] [PubMed] [Google Scholar]
- Parveen A, Akash MS, Rehman K, Kyunn WW (2016) Dual role of p21 in the progression of cancer and its treatment. Crit Rev Eukaryot Gene Expr 26:49–62. 10.1615/CritRevEukaryotGeneExpr.v26.i1.60 [DOI] [PubMed] [Google Scholar]
- Pavlides SC et al (2016) TGF-beta activates APC through Cdh1 binding for Cks1 and Skp2 proteasomal destruction stabilizing p27kip1 for normal endometrial growth. Cell Cycle 15:931–947. 10.1080/15384101.2016.1150393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazolli E, Stewart SA (2008) Senescence: the good the bad and the dysfunctional. Curr Opin Genet Dev 18:42–47. 10.1016/j.gde.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Pedroza-Torres A et al (2018) MicroRNA-125 modulates radioresistance through targeting p21 in cervical cancer. Oncol Rep 39:1532–1540. 10.3892/or.2018.6219 [DOI] [PubMed] [Google Scholar]
- Peng T, Wang T, Liu G, Zhou L (2020) Effects of miR-373 inhibition on glioblastoma growth by reducing Limk1 in vitro. J Immunol Res 2020:7671502. 10.1155/2020/7671502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca P et al (2006) Spatiotemporal dynamics of p21CDKN1A protein recruitment to DNA-damage sites and interaction with proliferating cell nuclear antigen. J Cell Sci 119:1517–1527. 10.1242/jcs.02868 [DOI] [PubMed] [Google Scholar]
- Petragnano F et al (2020) Clinically relevant radioresistant rhabdomyosarcoma cell lines: functional, molecular and immune-related characterization. J Biomed Sci 27:90. 10.1186/s12929-020-00683-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petragnano F et al (2020) Modulating the dose-rate differently affects the responsiveness of human epithelial prostate- and mesenchymal rhabdomyosarcoma-cancer cell line to radiation. Int J Radiat Biol 96:823–835. 10.1080/09553002.2020.1739774 [DOI] [PubMed] [Google Scholar]
- Podust VN, Podust LM, Goubin F, Ducommun B, Hubscher U (1995) Mechanism of inhibition of proliferating cell nuclear antigen-dependent DNA synthesis by the cyclin-dependent kinase inhibitor p21. Biochemistry 34:8869–8875. 10.1021/bi00027a039 [DOI] [PubMed] [Google Scholar]
- Poon RY, Jiang W, Toyoshima H, Hunter T (1996) Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage. J Biol Chem 271:13283–13291. 10.1074/jbc.271.22.13283 [DOI] [PubMed] [Google Scholar]
- Punchihewa C et al (2012) Identification of small molecule proliferating cell nuclear antigen (PCNA) inhibitor that disrupts interactions with PIP-box proteins and inhibits DNA replication. J Biol Chem 287:14289–14300. 10.1074/jbc.M112.353201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L et al (2002) Cyclin kinase inhibitor p21 potentiates bile acid-induced apoptosis in hepatocytes that is dependent on p53. Hepatology 36:39–48. 10.1053/jhep.2002.33899 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan SK, Feliciano CS, Najmabadi F, Haegebarth A, Kandel ES, Tyner AL, Gartel AL (2004) Constitutive expression of E2F–1 leads to p21-dependent cell cycle arrest in S phase of the cell cycle. Oncogene 23:4173–4176. 10.1038/sj.onc.1207571 [DOI] [PubMed] [Google Scholar]
- Radine C, Peters D, Reese A, Neuwahl J, Budach W, Janicke RU, Sohn D (2020) The RNA-binding protein RBM47 is a novel regulator of cell fate decisions by transcriptionally controlling the p53–p21-axis. Cell Death Differ 27:1274–1285. 10.1038/s41418-019-0414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N, Mishra DP (2012) Therapeutic targeting of cancer cell cycle using proteasome inhibitors. Cell Div 7:26. 10.1186/1747-1028-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaeejam H, Shirazi A, Izadi P, Bazzaz JT, Ghazi-Khansari M, Valizadeh M, Tabesh GA (2018) Radioprotective effect of melatonin on expression of Cdkn1a and Rad50 genes in rat peripheral blood. J Cancer Res Ther 14:S1070–S1075. 10.4103/0973-1482.196758 [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192:547–556. 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Gomez J et al (2002) 5’ CpG island hypermethylation is associated with transcriptional silencing of the p21(CIP1/WAF1/SDI1) gene and confers poor prognosis in acute lymphoblastic leukemia. Blood 99:2291–2296. 10.1182/blood.v99.7.2291 [DOI] [PubMed] [Google Scholar]
- Sax JK, Dash BC, Hong R, Dicker DT, El-Deiry WS (2002) The cyclin-dependent kinase inhibitor butyrolactone is a potent inhibitor of p21 (WAF1/CIP1 expression). Cell Cycle 1:90–96 [PubMed] [Google Scholar]
- Sheikh MS, Chen YQ, Smith ML, Fornace AJ Jr (1997) Role of p21Waf1/Cip1/Sdi1 in cell death and DNA repair as studied using a tetracycline-inducible system in p53-deficient cells. Oncogene 14:1875–1882. 10.1038/sj.onc.1201004 [DOI] [PubMed] [Google Scholar]
- Shen H, Maki CG (2011) Pharmacologic activation of p53 by small-molecule MDM2 antagonists. Curr Pharm Des 17:560–568. 10.2174/138161211795222603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu HK, Kim MM, Chen P, Furman F, Julin CM, Israel MA (1998) The intrinsic radioresistance of glioblastoma-derived cell lines is associated with a failure of p53 to induce p21(BAX) expression. Proc Natl Acad Sci USA 95:14453–14458. 10.1073/pnas.95.24.14453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn D, Essmann F, Schulze-Osthoff K, Janicke RU (2006) p21 blocks irradiation-induced apoptosis downstream of mitochondria by inhibition of cyclin-dependent kinase-mediated caspase-9 activation. Cancer Res 66:11254–11262. 10.1158/0008-5472.CAN-06-1569 [DOI] [PubMed] [Google Scholar]
- Soria G, Podhajcer O, Prives C, Gottifredi V (2006) P21Cip1/WAF1 downregulation is required for efficient PCNA ubiquitination after UV irradiation. Oncogene 25:2829–2838. 10.1038/sj.onc.1209315 [DOI] [PubMed] [Google Scholar]
- Soria G, Speroni J, Podhajcer OL, Prives C, Gottifredi V (2008) p21 differentially regulates DNA replication and DNA-repair-associated processes after UV irradiation. J Cell Sci 121:3271–3282. 10.1242/jcs.027730 [DOI] [PubMed] [Google Scholar]
- Soysa R, Lampert S, Yuen S, Douglass AN, Li W, Pfeffer K, Crispe IN (2019) Fetal origin confers radioresistance on liver macrophages via p21(cip1/WAF1). J Hepatol 71:553–562. 10.1016/j.jhep.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stivala LA, Prosperi E (2004) Analysis of p21CDKN1A recruitment to DNA excision repair foci in the UV-induced DNA damage response. Methods Mol Biol 281:73–89. 10.1385/1-59259-811-0:073 [DOI] [PubMed] [Google Scholar]
- Stivala LA, Riva F, Cazzalini O, Savio M, Prosperi E (2001) p21(waf1/cip1)-null human fibroblasts are deficient in nucleotide excision repair downstream the recruitment of PCNA to DNA repair sites. Oncogene 20:563–570. 10.1038/sj.onc.1204132 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Tsutomi Y, Miura M, Akahane K (1999) Caspase 3 inactivation to suppress Fas-mediated apoptosis: identification of binding domain with p21 and ILP and inactivation machinery by p21. Oncogene 18:1239–1244. 10.1038/sj.onc.1202409 [DOI] [PubMed] [Google Scholar]
- Szoltysek K et al (2018) RRAD, IL4I1, CDKN1A, and SERPINE1 genes are potentially co-regulated by NF-kappaB and p53 transcription factors in cells exposed to high doses of ionizing radiation. BMC Genom 19:813. 10.1186/s12864-018-5211-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam SY, Wu VW, Law HK (2017) Influence of autophagy on the efficacy of radiotherapy. Radiat Oncol 12:57. 10.1186/s13014-017-0795-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y et al (2016) Radiation-induced miR-208a increases the proliferation and radioresistance by targeting p21 in human lung cancer cells. J Exp Clin Cancer Res 35:7. 10.1186/s13046-016-0285-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramen H et al (2011) Aberrant methylation of p21 gene in lung cancer and malignant pleural mesothelioma. Acta Med Okayama 65:179–184. 10.18926/AMO/46629 [DOI] [PubMed] [Google Scholar]
- Tian H, Wittmack EK, Jorgensen TJ (2000) p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res 60:679–684 [PubMed] [Google Scholar]
- Tobias F, Durante M, Taucher-Scholz G, Jakob B (2010) Spatiotemporal analysis of DNA repair using charged particle radiation. Mutat Res 704:54–60. 10.1016/j.mrrev.2009.11.004 [DOI] [PubMed] [Google Scholar]
- van Beijnum JR, Giovannetti E, Poel D, Nowak-Sliwinska P, Griffioen AW (2017) miRNAs: micro-managers of anticancer combination therapies. Angiogenesis 20:269–285. 10.1007/s10456-017-9545-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vens C, Begg AC (2010) Targeting base excision repair as a sensitization strategy in radiotherapy. Semin Radiat Oncol 20:241–249. 10.1016/j.semradonc.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Waldman T, Lengauer C, Kinzler KW, Vogelstein B (1996) Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381:713–716 [DOI] [PubMed] [Google Scholar]
- Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J (1997) Cell-cycle arrest versus cell death in cancer therapy. Nat Med 3:1034–1036. 10.1038/nm0997-1034 [DOI] [PubMed] [Google Scholar]
- Wang YA, Elson A, Leder P (1997) Loss of p21 increases sensitivity to ionizing radiation and delays the onset of lymphoma in atm-deficient mice. Proc Natl Acad Sci USA 94:14590–14595. 10.1073/pnas.94.26.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Blandino G, Givol D (1999b) Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene 18:2643–2649. 10.1038/sj.onc.1202632 [DOI] [PubMed] [Google Scholar]
- Wang JA, Fan S, Yuan RQ, Ma YX, Meng Q, Goldberg ID, Rosen EM (1999a) Ultraviolet radiation down-regulates expression of the cell-cycle inhibitor p21WAF1/CIP1 in human cancer cells independently of p53. Int J Radiat Biol 75:301–316. 10.1080/095530099140483 [DOI] [PubMed] [Google Scholar]
- Wang CC, Liao YP, Mischel PS, Iwamoto KS, Cacalano NA, McBride WH (2006) HDJ-2 as a target for radiosensitization of glioblastoma multiforme cells by the farnesyltransferase inhibitor R115777 and the role of the p53/p21 pathway. Cancer Res 66:6756–6762. 10.1158/0008-5472.CAN-06-0185 [DOI] [PubMed] [Google Scholar]
- Wang J et al (2017) The BET bromodomain inhibitor JQ1 radiosensitizes non-small cell lung cancer cells by upregulating p21. Cancer Lett 391:141–151. 10.1016/j.canlet.2017.01.031 [DOI] [PubMed] [Google Scholar]
- Wani MA, Wani G, Yao J, Zhu Q, Wani AA (2002) Human cells deficient in p53 regulated p21(waf1/cip1) expression exhibit normal nucleotide excision repair of UV-induced DNA damage. Carcinogenesis 23:403–410. 10.1093/carcin/23.3.403 [DOI] [PubMed] [Google Scholar]
- Weiss RH, Marshall D, Howard L, Corbacho AM, Cheung AT, Sawai ET (2003) Suppression of breast cancer growth and angiogenesis by an antisense oligodeoxynucleotide to p21(Waf1/Cip1). Cancer Lett 189:39–48. 10.1016/s0304-3835(02)00495-0 [DOI] [PubMed] [Google Scholar]
- Wendt J et al (2006) Induction of p21CIP/WAF-1 and G2 arrest by ionizing irradiation impedes caspase-3-mediated apoptosis in human carcinoma cells. Oncogene 25:972–980. 10.1038/sj.onc.1209031 [DOI] [PubMed] [Google Scholar]
- Wouters BG, Denko NC, Giaccia AJ, Brown JM (1999) A p53 and apoptotic independent role for p21waf1 in tumour response to radiation therapy. Oncogene 18:6540–6545. 10.1038/sj.onc.1203053 [DOI] [PubMed] [Google Scholar]
- Wu L, Levine AJ (1997) Differential regulation of the p21/WAF-1 and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol Med 3:441–451 [PMC free article] [PubMed] [Google Scholar]
- Wu SY et al (2013) MicroRNA-17–5p post-transcriptionally regulates p21 expression in irradiated betel quid chewing-related oral squamous cell carcinoma cells. Strahlenther Onkol 189:675–683. 10.1007/s00066-013-0347-9 [DOI] [PubMed] [Google Scholar]
- Wu C et al (2020) Radiation-induced DNMT3B promotes radioresistance in nasopharyngeal carcinoma through methylation of p53 and p21. Mol Ther Oncolytics 17:306–319. 10.1016/j.omto.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Li C, Dang Q, Chang LS, Li L (2016) Infiltrating mast cells increase prostate cancer chemotherapy and radiotherapy resistances via modulation of p38/p53/p21 and ATM signals. Oncotarget 7:1341–1353. 10.18632/oncotarget.6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaglom JA, McFarland C, Mirny L, Sherman MY (2014) Oncogene-triggered suppression of DNA repair leads to DNA instability in cancer. Oncotarget 5:8367–8378. 10.18632/oncotarget.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Tan M, Wang G, Sun Y (2012) The p21-dependent radiosensitization of human breast cancer cells by MLN4924, an investigational inhibitor of NEDD8 activating enzyme. PLoS ONE 7:e34079. 10.1371/journal.pone.0034079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J et al (2018) Combination of IFITM1 knockdown and radiotherapy inhibits the growth of oral cancer. Cancer Sci 109:3115–3128. 10.1111/cas.13640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng ZM et al (2020) BRCA1 protects cardiac microvascular endothelial cells against irradiation by regulating p21-mediated cell cycle arrest. Life Sci 244:117342. 10.1016/j.lfs.2020.117342 [DOI] [PubMed] [Google Scholar]
- Zhang XR, Liu YA, Sun F, Li H, Lei SW, Wang JF (2016) p21 is responsible for ionizing radiation-induced bypass of mitosis. Biomed Environ Sci 29:484–493. 10.3967/bes2016.064 [DOI] [PubMed] [Google Scholar]
- Zhang M et al (2018) Long noncoding RNA CRNDE/PRC2 participated in the radiotherapy resistance of human lung adenocarcinoma through targeting p21 expression. Oncol Res 26:1245–1255. 10.3727/096504017X14944585873668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y et al (2015) The Roles of p21(Waf1/CIP1) and Hus1 in generation and transmission of damage signals stimulated by low-dose alpha-particle irradiation. Radiat Res 184:578–585. 10.1667/RR4165.1 [DOI] [PubMed] [Google Scholar]
- Zheng L et al (2015) MiR-106b induces cell radioresistance via the PTEN/PI3K/AKT pathways and p21 in colorectal cancer. J Transl Med 13:252. 10.1186/s12967-015-0592-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Liu Y, Zhang X, Zhao P, Deng Q (2017) miRNA-200c enhances radiosensitivity of esophageal cancer by cell cycle arrest and targeting P21. Biomed Pharmacother 90:517–523. 10.1016/j.biopha.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Zhou ZR et al (2020) Building radiation-resistant model in triple-negative breast cancer to screen radioresistance-related molecular markers. Ann Transl Med 8:108. 10.21037/atm.2019.12.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Ji S, He L, Zhang Y, Peng Z, Han J (2020) LncRNA TTN-AS1/miR-134–5p/PAK3 axis regulates the radiosensitivity of human large intestine cancer cells through the P21 pathway and AKT/GSK-3beta/beta-catenin pathway. Cell Biol Int 44:2284–2292. 10.1002/cbin.11436 [DOI] [PubMed] [Google Scholar]