Abstract
We develop a mechanistic mathematical model of the G-protein coupled signaling pathway responsible for generating current responses in frog olfactory receptor neurons. The model incorporates descriptions of ligand-receptor interaction, intracellular transduction events involving the second messenger cAMP, effector ion-channel activity, and calcium-mediated feedback steps. We parameterized the model with respect to suction pipette current recordings from single cells stimulated with multiple odor concentrations. The proposed model accurately predicts the receptor-current response of the neuron to brief and prolonged odorant exposure and is able to produce the adaptation observed under repeated or sustained stimulation.
Keywords: mathematical model, receptor neuron, olfaction, signal transduction, cilia
Sensory transduction of odors occurs in olfactory receptor neurons (ORNs) located in the olfactory epithelium of vertebrates and the antennal structures of invertebrates (1). The first step in transduction is the binding of an odorant molecule to a seven-transmembrane-domain receptor protein in the cilia of an ORN. This interaction triggers a G-protein-coupled cascade that activates the enzyme adenylyl cyclase, resulting in an increase in intraciliary adenosine-3′,5′-cyclic monophosphate (cAMP). When cAMP increases, it opens cyclic nucleotide-gated (CNG) channels, allowing calcium and other extracellular cations into the cilia and generating an inward current. Elevated Ca2+ levels in the cilia activate Ca2+-gated chloride [Cl(Ca)] channels, creating an outward flow of Cl- ions and producing amplification of the inward current (2–4) that results in membrane depolarization and the generation of action potentials in the cell soma (5). This increase in intracellular calcium initiates termination and adaptation of the cell's response by means of deactivation and feedback mechanisms, including interactions mediated by Ca2+-calmodulin (CaCaM) and Ca2+/calmodulin-kinase II (CaMK) (6–10). The Cl(Ca) channels remain open until enough calcium is extruded from the cilia via the Na/Ca exchanger (NCX) (11).
After stimulation, an ORN's response to subsequent stimuli becomes attenuated. Short-term adaptation is observed as a decrease in responsiveness to odor presentations that occur within a few seconds after a brief stimulus (7); it may be mediated in part by CaCaM inhibition of the CNG channel (12). Desensitization occurs when an odor is experienced for a sustained interval of at least several seconds: the ORN's response declines while the stimulus continues to be present (13, 14). In this case, several aspects of the response to a subsequent stimulus are altered, including a decrease in the slope of the rising phase, as well as an increase in the speed of decay, of the stimulus-induced inward current (7, 13). Experiments (15) have demonstrated that the molecular mechanisms responsible for desensitization are likely to act upstream of cAMP production; inhibition of adenylyl cyclase activity by CaMK is a possible mechanism (13). Longer-lasting adaptation effects with recovery time constants of minutes also have been reported (7). In addition, oscillations in the receptor current have been observed when ORNs are exposed to prolonged stimulation (16, 17).
It is not yet clear to what extent each of these mechanisms contributes to the shaping of odor responses and modulation of cell sensitivity across different timescales. Mathematical modeling of olfactory transduction can help to elucidate how these various cellular processes participate in the coding of odor stimuli. Previous models have focused on the input and output stages of chemoelectrical transduction, i.e., receptor binding (18–20) and generation of an electrical response, from the triggering of conductance changes in effector channels to the production of a receptor potential and action potentials (21–24). Few models have investigated the intracellular processes of transduction (25, 26), and, of those, most concentrate on specific aspects of the cascade (25, 27). Here, we construct a mechanistic model of the intraciliary signaling pathway, which simulates the receptor current during odor stimulation and is capable of both adapting to a stimulus and oscillating in response to prolonged odor exposure. We compare our model output with data from vertebrate ORNs recorded during odor stimulation and identify potential cellular mechanisms involved in sensory transduction and odor adaptation.
Model Development
First, we constructed a parsimonious model consisting of four differential equations that describe ligand-receptor binding, G-protein activation, and concentrations of the messengers cAMP and Ca2+. This simple model was expanded by adding equations for biologically motivated feedback mechanisms and the evolution of membrane potential under non-voltage-clamped conditions. By using parameter estimation methods that we developed, we tested the model's capacity to fit odorant-induced current responses from salamander (28, 29) and frog ORNs (16, 30). The minimal model that reproduces adaptation consists of the following components.
Ligand-Receptor Binding and G-Protein Activation. Equations for the proportion of receptors that are ligand-bound (bLR) and proportion of G-proteins that are in the active Gα-GTP state (aG) are as follows:
 |
 |
We modeled the presentation of odorant (Ostim) in different experiments by taking
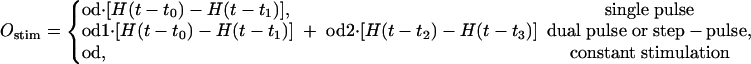 |
where H(·) is a Heaviside step function, and od, od1, and od2 represent odorant concentrations.
Intracellular Signaling. Equations that describe the biochemical reactions of the transduction cascade, involving concentrations of cAMP (cAMP), cytosolic free Ca2+ (Ca), Ca2+-calmodulin (CaCaM), and active CaMK (aCaMK), are as follows:
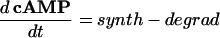 |
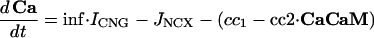 |
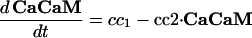 |
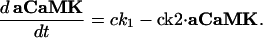 |
To start with, we used the simple formulas
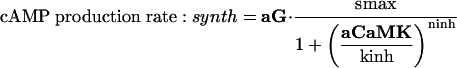 |
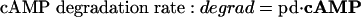 |
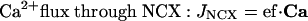 |
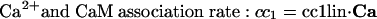 |
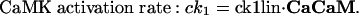 |
Effector Channel Activity/Receptor Currents. Equations for the CNG current (ICNG), Cl(Ca) current (ICl(Ca)), current arising from the electrogenic NCX (INCX), the combination of all other currents (Iother), and membrane potential (V) are as follows:
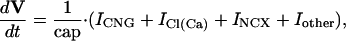 |
where
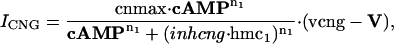 |
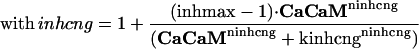 |
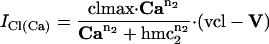 |
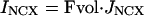 |
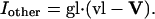 |
The predicted receptor current (our output measure for the model) is defined as
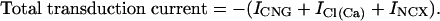 |
In the formulas, lightface roman symbols represent constants; boldface symbols denote dynamical state variables of the system, each of which has a differential equation associated with it; and italicized symbols denote functions and auxiliary variables. The CNG channel inhibition factor inhcng represents the fold increase in K1/2 of the CNG channel as a function of CaCaM concentration (31). The parameter inhmax (≥1) is the greatest attainable factor of increase in the cAMP concentration needed for achieving half-maximal channel activation; the minimal factor (when no CaCaM is present) is 1. Other parameters are explained in Supporting Text and Tables 1 and 2, which are published as supporting information on the PNAS web site.
During the model-fitting process, we found that the system of equations stated above was sufficient for simulating adaptation and oscillatory behavior. However, to adequately match the termination phase of responses to brief stimuli at high concentrations, we needed to postulate additional mechanisms that could result in intracellular Ca2+ concentration remaining elevated for some time after stimulus presentation. We considered the possibilities that G-protein lifetime or cAMP presence could be extended through, respectively, Ca2+-dependent slowing of receptor or G-protein deactivation (similar to the phototransduction case as modeled in ref. 32) or inhibition of the rate of cAMP hydrolysis by phosphodiesterases. A simple way of modeling these scenarios is to modify the G-protein deactivation rate to r2·aG/{1 + (IX/kI)nI}, or change the expression for degrad to pd·cAMP/{1 + (IX/kI)nI}, where IX is an intermediate Ca2+-stimulated substance governed by the equation dIX/dt = cx1lin·Ca - cx2·IX. In addition, we considered the hypothesis that the rate of Ca2+ extrusion through the NCX may become reduced when intracellular Ca2+ concentration is high. This scenario can be modeled phenomenologically by taking JNCX = ef·Ca/{1 + (IX/kI)nI}. We also experimented with more complicated forms of JNCX along the lines of refs. 25 and 33, with nonlinear calcium, voltage, and sodium dependence. An example is JNCX = ef·fallosteric·felectrochemical, where
 |
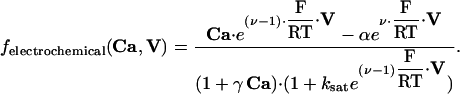 |
Equations incorporating nonlinear voltage-dependence and gating dynamics of the CNG and Ca(Cl) channels were considered as well (see Supporting Text), and we compared their simulation results with those obtained from the simpler equations shown earlier.
We investigated how accurately the model could capture the responses of frog ORNs to single pulses of odorant (see figure 2A of ref. 30), a step–pulse stimulation protocol (see figure 5 A–D of ref. 30), and to prolonged stimulation (see figure 7A of ref. 16). In these experiments, ORN responses were measured as receptor currents collected by suction-pipette; voltage was unclamped. Parameter values were estimated by using a genetic algorithm modified by an active-set procedure, as well as a roughness penalty minimization approach for the oscillatory data (see Supporting Text). Parameter sensitivity analysis was used to determine which aspects of the model exerted the greatest influence on particular response properties.
Response to Single-Pulse Stimulation
The response of an ORN to an odorant pulse of 1-s duration (30) is characterized by a latency period, a delay from the onset of stimulation to any noticeable generation of receptor current, followed by a rise in inward current to reach a peak, after which the current declines toward baseline. With increasing odorant concentration, the latency and time-to-peak decrease (i.e., the rate of rise of the current increases); the peak itself also becomes narrower. Simulations showed that negative feedback to the CNG channel alone was insufficient for generating a sharp peak, whereas a model that included inhibition of cAMP synthesis but not the CNG channel was able to produce this phenomenon.
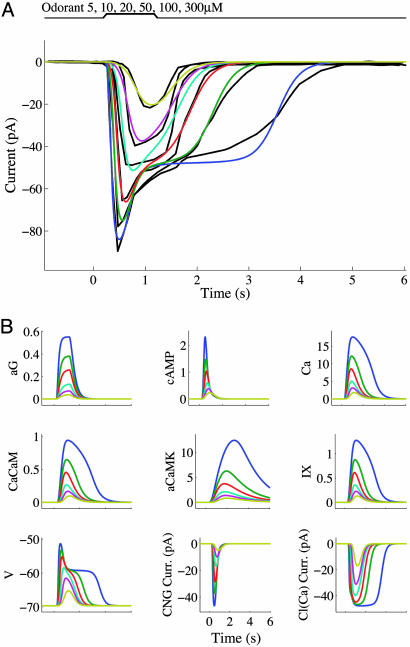
Another prominent aspect of the current response at high stimulus concentrations is the rapid relaxation to a plateau phase immediately after the initial peak (see Fig. 1); the plateau can outlast the end of stimulation by several seconds, and its length increases with concentration. By comparing likelihood measures computed from fitting different model versions to the data, we found that the best fit for the response termination phase was associated with having a reduced sodium/calcium exchange rate at high calcium concentrations. This decrease in exchanger efficacy may be a direct consequence of nonlinear calcium-dependence in the JNCX function, or it could result from indirect modulation by a Ca2+-activated substance or by the depolarization that accompanies a rise in ciliary calcium. We present in Fig. 1 the output obtained from taking JNCX = ef·Ca/{1 + (IX/kI)nI}. In the remainder of this work, “the model” will refer to this version of the equations, comprising a total of 8 variables and 32 kinetic parameters. This system generates the plateau current most robustly and contains fewer parameters than if JNCX were endowed with intrinsic nonlinear Ca and V dependence by means of allosteric and electrochemical components. Estimated parameter values and comparisons with other model versions are presented in Supporting Text, Tables 3–5, and Figs. 4–9, which are published as supporting information on the PNAS web site.
Fig. 1.
Model predictions for stimulation of an ORN with a 1-s odorant pulse of various concentrations as in figure 2A of ref. 30. (A) Simulated receptor currents associated with exposure to 5 (yellow), 10 (purple), 20 (cyan), 50 (red), 100 (green), or 300 (blue) μM cineole, compared with current traces obtained from the experimental data of Reisert and Matthews (30) (black curves). [Black current traces in the image are reprinted with permission from ref. 30 (Copyright 1999, Blackwell Publishing).] (B) Time courses for various quantities in the model as follows: aG, active G-proteins; Ca, intracellular free Ca2+; CaCaM, Ca2+-associated calmodulin; aCaMK, active CaMK; IX, intermediate Ca2+-stimulated substance; V, membrane potential; CNG current; and Cl(Ca) current. The variable bLR (proportion of ligand-bound receptors) is not depicted because its time course closely resembles that of aG. Color coding is the same as in A.
Adaptation Experiments
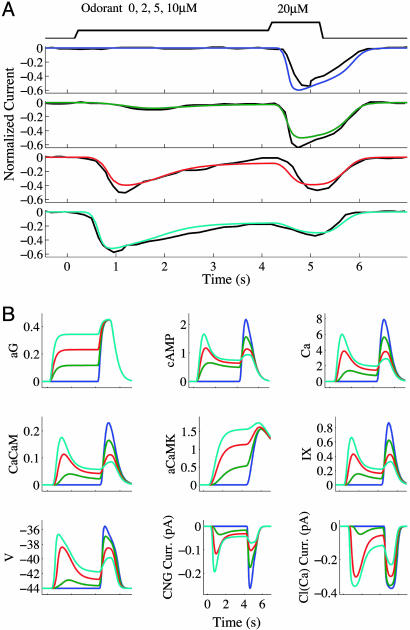
To examine the model's ability to predict the current response of an ORN during adaptation, we fit the model to the data in figure 5 of ref. 30. In this experiment, an ORN was exposed to different odor concentrations delivered over a 4-s conditioning step, immediately followed by a 20 μM test pulse of 1-s duration. The degree of modulation in response sensitivity increased with concentration of the conditioning stimulus and resulted in progressively diminished test-pulse responses. The model fitted to these data are shown in Fig. 2. Down-regulation of cAMP production by aCaMK was responsible for the desensitization seen during the 4-s conditioning step. Although parameter estimates showed that CaCaM can decrease CNG channel sensitivity by up to 5-fold in the model, we observed from parameter sensitivity analyses that this mechanism had much less influence on the accuracy of fit than did CaMK feedback to adenylyl cyclase. In fact, models that do not explicitly include a CaCaM equation, so that activation of CaMK and inhibition of the CNG channel are approximated by direct actions of Ca2+ without the intermediate calmodulin association step, are also capable of simulating responses in the step-pulse adaptation experiments. CaCaM does, however, play an essential role in capturing the correct time course of recovery from short-term adaptation as shown in ref. 12. Our model was able to reproduce the qualitative behavior demonstrated in the dual brief-pulse experiments of Kurahashi and Menini (12) (see Supporting Text and Fig. 10, which is published as supporting information on the PNAS web site), whereas models without an explicit dynamical equation for CaCaM failed to do so.
Fig. 2.
Model predictions for the step-pulse adaptation experiment in figure 5 A–D of ref. 30. (A) Simulated receptor currents associated with a 4-s exposure to 0 (blue), 2 (green), 5 (red), or 10 (cyan) μM odorant, followed by a 1-s 20 μM test-pulse; black traces were obtained from the experimental data of Reisert and Matthews (30). [Black current traces in the image are reprinted with permission from ref. 30 (Copyright 1999, Blackwell Publishing).] All currents have been normalized to the peak amplitude evoked by a 300 μM stimulus. (B) Time courses for various quantities in the model (same as in Fig. 1).
Response to Prolonged Stimulation
A single frog ORN exposed to odor stimulation sustained for 30–60 s exhibited oscillatory current responses (16). Although the experimentally observed oscillations deviated from strict periodicity, our model was able to match the qualitative nature of these data. For the model output shown in Fig. 3, we calculated the amplitude of oscillatory peaks to be 10.14 ± 1.46 pA (upon ignoring the initial peak, which is considerably larger than subsequent ones), whereas the oscillation period was 6.34 ± 0.31 s. For the experimental data, from figure 7A of ref. 16, the oscillations had a peak amplitude of 12.41 ± 3.12 pA and period of 6.57 ± 0.45 s. Oscillations in the model were found to be highly sensitive to the parameters smax, kinh, pd, and ef that control Ca2+ and cAMP dynamics. CaCaM, through its influence on CaMK activation and on the CNG channel, appeared to exert strong control over the shape and width of the oscillatory peaks. Further investigation of parameter space and model variations suggested that CaCaM's desensitizing effect on the CNG channel was not as salient as the delay to CaMK activation induced by the Ca2+ and calmodulin association step; modulation of peak width would be difficult to achieve in a model that does not incorporate explicit CaCaM dynamics.
Fig. 3.
Model predictions for the exposure of a frog ORN to 100 μM odorant for 60 s as in figure 7A of ref. 16. (A) Simulated receptor current (blue) compared with filtered data from the experiments of Reisert and Matthews (16) (black trace). [Black current trace in the image is reprinted with permission from ref. 16 (Copyright 2001, Blackwell Publishing).] (B) Time courses for various quantities in the model (same as in Fig. 1).
Discussion
We present a computational model that simulates the receptor current of frog ORNs and takes into account the dynamics of intracellular signaling, including feedback processes. Our model has the ability to capture odor-induced responses and adaptation phenomena over different timescales and under various stimulation protocols. When the number of variables in the system is reduced by eliminating intermediate equations, the model is no longer capable of supporting the full range of responses discussed here.
The model of Suzuki et al. (26) also considers the vertebrate olfactory transduction pathway in detail, but the context of that study differs from ours in several significant ways. Suzuki et al. (26) measured whole-cell currents generated by rainbow trout ORNs in response to stimulation with amino acid mixtures; in particular, they were interested in modeling the damped oscillations elicited by single pulses of stimuli (ranging from 25 ms to a few seconds duration). We are not aware of observations of similar short-term oscillatory behavior in the ORNs of frog or other vertebrate species. In addition, these experiments were conducted under voltage-clamp conditions, whereas to fit the suction pipette recordings of refs. 16 and 30, it is necessary to have membrane potential as a variable in our model. The model in ref. 26, despite incorporating multistep feedback processes, shows no desensitization and limited short-term adaptation. It also contains considerable redundancy for producing the oscillatory responses it was designed to model. Our model, although simpler, has the capacity not only to reproduce receptor currents evoked by short-term single-pulse odor presentations but also to generate adaptation and oscillatory responses under repeated or prolonged stimulation.
There are many similarities between our ORN model and previously reported models of phototransduction (32, 34–36). However, these models are able to reproduce various light adaptation phenomena even when the negative feedback steps, e.g., to the cyclase or to rhodopsin kinase (receptor deactivation), are expressed as functions of calcium itself. In this case, intermediate processes and calcium-binding proteins do not need to be modeled explicitly to simulate adaptation. In contrast, we found that models that simplify intermediate steps by approximating them with direct, nondelayed actions of Ca2+ could not provide accurate fits to experimental data from ORNs. This property is due to the presence of an amplifying Ca2+-activated Cl- current, which photoreceptors lack.
We observed that even when initial parameter estimates were set so that the CNG current was dominant in the response, the optimization always drifted toward a parameter regime in which the Cl(Ca) current dominated. Thus, the amplification of a small inward current through the CNG cation channel by a large outward chloride ion flux is a strongly identifiable aspect of this model. Contribution of NCX electrogenicity to the receptor current was negligible. Instantaneous current–voltage relations for CNG channels are in fact nonlinear (9), and complicated effects such as channel block by divalent ions are present. Computational experimentation has shown that assuming linear voltage-dependence for both the CNG and Cl(Ca) channels does not significantly reduce the quality of fit between our model and the data sets considered here; but more detailed analyses are needed to understand how physiologically realistic ways of implementing voltage sensitivity play a role in shaping the responses.
Rising Phase of the Receptor Current. Action potentials generated by an ORN occur predominantly during the rising phase of the receptor current. Reisert and Matthews (30) observed that although the latency of response shortened with increasing odorant concentration, a minimal latency of 170 ms existed beyond which no further decrease was possible. The observation of a lower limit to response latency indicates the presence of a rate-limiting step in the transduction cascade. In view of the experiments in refs. 12 and 15, which compare responses evoked by odorants with those induced by flash photolysis of caged cAMP, this rate-limiting step should occur early in the pathway. Our simulations suggest that it is likely to be activation of G-proteins rather than adenylyl cyclase activation or cAMP synthesis, because response latency in the model is primarily dependent on the rate k2. The slope of rise of the receptor current is very sensitive to the ligand-receptor binding constant k1, and the dynamic range of the concentration–response relation is determined principally by the binding affinity k1/r1 (or equivalently, the dissociation constant r1/k1). We observed that the rising phase and peak amplitudes of current responses can be fitted well by using a two-variable model that describes only the interactions between odor molecules, receptors, and G-proteins, coupled to a function representing the total current flowing through effector channels. Thus, explicit equations involving cAMP, Ca2+, etc. are not necessary for adequate simulation of the early-stage receptor current.
Plateau Currents. Certain spiking properties of ORNs, such as latency, frequency, and number of spikes fired, can be characterized by mathematical models that consider simply the early steps in the signal transduction cascade (21, 24), without including feedback processes. However, later phases of the response do have important implications for sensory adaptation. Therefore, it is essential to elucidate the mechanisms underlying later-stage phenomena if we wish to obtain a full understanding of the information processing capabilities of ORNs.
Several putative mechanisms, all involving an elevated ciliary calcium signal extending beyond the time of odor exposure, could account for the pronounced plateau in current observed during the response-termination phase at higher stimulus concentrations (J. Reisert, personal communication). One hypothesis is that a large amount of Ca2+ enters the cilia through CNG channels at the beginning of the response; the CNG conductance then rapidly deactivates, so that virtually no additional calcium enters thereafter. The calcium that had entered the cell earlier on maintains the Cl(Ca) current until the NCX eventually extrudes all of it. If enough Ca2+ enters during the peak of the response to saturate the chloride channels, the current will stay nearly constant for a while after the peak. This plateau lasts until intraciliary Ca2+ concentration falls below the level needed for chloride channel saturation; the response then ends with an abrupt reduction in current. By using the basic model in which NCX was assumed to work at a steady specific rate (JNCX = ef·Ca), we were unable to obtain good fits to the single-pulse data based on this hypothesis. However, predictions from versions of the model that describe JNCX as a nonlinear function were found to be consistent with this mechanism.
A different scenario is that CNG channels stay open throughout the plateau phase, serving as a continuous calcium source to maintain the Cl(Ca) conductance. During the plateau, the rates of Ca2+ influx via CNG channels and efflux via the NCX are balanced. Eventually, the CNG channels close, the NCX reduces Ca2+ to prestimulus levels so that Cl(Ca) channel activity falls, and the current response terminates. This mechanism is predicated on elevated cAMP levels throughout the plateau phase, which may be due to (i) extended G-protein lifetime; (ii) prolonged adenylyl cyclase activity, i.e., cAMP production; or (iii) prolonged presence of the cAMP that has already been produced. From numerical explorations, we observed that the single-pulse dose-response traces can be adequately simulated by using (i) or (iii). In the case of (iii), we needed to assume that phosphodiesterase activity (cAMP degradation) becomes reduced at higher calcium concentrations. However, mechanisms of phosphodiesterase inhibition are unknown in this system; in fact, olfactory phosphodiesterase appears to be stimulated by CaCaM (37).
A third possibility is that the rate of Ca2+ removal is decreased during the plateau. There is experimental evidence that Ca2+ flux through cardiac NCX can be reduced either by an unknown inhibitor that increases in response to mitochondrial Ca2+ concentration (38) or by Ca2+ buffering (39). By using our model, we tested a hypothesis whereby the specific rate of Ca2+ extrusion through the NCX is lowered by an inhibitor IX. This mechanism indeed led to significant prolongation of elevated intraciliary Ca and produced sharp transitions into and out of the plateau phase. Better fits to the single-pulse data were obtained from this model version than from those discussed in the preceding paragraph. Unfortunately, no evidence currently exists for substances that down-regulate the NCX in ORNs. We therefore explored the possibility that “inhibition” of the sodium/calcium-exchange rate can be attributed to the exchanger's intrinsic nonlinear dependence on membrane potential and on intraciliary concentrations of calcium and sodium. By using functional forms for JNCX based on the cardiac myocyte model in ref. 33, we were able to obtain simulated currents that agree well with the data. The plateau phase was verified to be coincident with a drop in the specific calcium extrusion rate occurring at elevated levels of intracellular Ca2+ and/or voltage.
Desensitization and Adaptation. Takeuchi and Kurahashi (15) found that in ORNs, currents evoked in response to sustained odor stimulation decayed faster than those induced by the photolysis of caged cAMP. This finding demonstrated that desensitization occurs as a result of negative feedback onto some component upstream of cAMP production (e.g., receptor, G-protein, or adenylyl cyclase). Our model incorporates a down-regulating effect of CaMK on adenylyl cyclase activity, embodied as a noncompetitive inhibition factor in the definition of synth (cAMP synthesis rate). Simulation results showed that this mechanism was the predominant one underlying the adaptation demonstrated in figure 5 of ref. 30. Thus, although CaCaM inhibits Ca2+ influx via the CNG channel, it appears that the role of this feedback in shaping responses to the step-pulse stimulation protocol is secondary to the action of CaMK on adenylyl cyclase. We found that when CaCaM was eliminated from the model, and CaMK activation was described as being directly dependent on Ca2+, the simpler model still exhibited adaptation. However, it was less able to accurately match the decay phases of the current responses. Indeed, the delaying effect of CaCaM on CaMK activation and CNG channel modulation seems to be important in setting the sharpness, shape of decline, and other properties of current peaks.
Oscillations. The origin of the oscillatory receptor current is thought to be a Ca2+-mediated negative feedback that leads to cyclic suppression of the CNG conductance (16, 29). This feedback mechanism could be the direct inhibitory action of CaCaM on the CNG channel or a more indirect effect of calcium on levels of the channel-gating molecule cAMP. In simulations of the prolonged-stimulus experiment, oscillations occur in the variables cAMP, Ca, CaCaM, aCaMK, and V, whereas proportions of active G-protein and bound receptors are constant. The parameters that play a primary role in producing oscillations are found in the equations for Ca2+, CaMK, and cAMP, which supports the finding of Reisert and Matthews (16) that a coupled oscillation between calcium and cyclic nucleotide concentrations is responsible for oscillatory current responses. We found that simplified models that include CaMK but eliminate intermediate CaCaM dynamics remain capable of displaying oscillations. Our simulations also suggest that during oscillations, the attenuation in amplitude of subsequent peaks relative to the first few is the result of accumulation of active CaMK. However, to adequately fit the shape of receptor current traces, CaCaM was required; CaCaM contributed to determining the width of the current peak (hence the duration of rising and decay phases) within each cycle of the oscillatory response. We find in our model that the frequency of oscillation depends on the sodium/calcium exchange rate (as well as other parameters related to Ca2+). This outcome is in accordance with the experimental observations of ref. 29 and is as one would expect if, after each oscillatory peak, the Ca2+ concentration must return to near baseline levels to relieve suppression of the CNG conductance and allow the next rise in current.
Unlike the remarkably uniform populations of rod and cone photoreceptors in the vertebrate visual system, which exhibit highly reproducible properties, there is great variability in the biochemical kinetics and response characteristics of ORNs. This diversity may partly explain the inconsistencies between parameter estimates obtained from fitting the model to data sets originating from different ORNs. It would be interesting to investigate which components of the transduction machinery are primarily responsible for the heterogeneity that underlies an ORN population's vast coding capacity. Some analysis of this issue (along with quantitative interpretation of the variability in experimental data) has been done by Rospars et al. (40), and further work addressing such questions could be undertaken. Other areas needing further research involve incorporating more realistic dynamics for the channel and enzyme inhibition processes, extending the model to include dendrite and soma (spike-generating) compartments, and developing similar models for invertebrate olfactory signaling.
Supplementary Material
Acknowledgments
We thank S. Kleene, J. Reisert, and J. Sneyd for enlightening discussions and advice; J. Reisert for providing the filtered experimental data in Fig. 3; and J. Reisert and J.-P. Rospars for their constructive comments that greatly helped to improve the manuscript. This work is based on research supported by National Science Foundation Agreement 0112050. G.A.W. was partially supported by National Institutes of Health–National Center for Research Resources Grant 9 R01 RR1466 (awarded to B. H. Smith).
Abbreviations: CaCaM, Ca2+-calmodulin; CaMK, Ca2+/calmodulin-kinase II; Cl(Ca), Ca2+-activated chloride; CNG, cyclic nucleotide-gated; NCX, Na/Ca exchanger; ORN, olfactory receptor neuron.
References
- 1.Hildebrand, J. G. & Shepherd, G. M. (1997) Annu. Rev. Neurosci. 20, 595-631. [DOI] [PubMed] [Google Scholar]
- 2.Hallani, M., Lynch, J. W. & Barry, P. H. (1998) J. Membr. Biol. 161, 163-171. [DOI] [PubMed] [Google Scholar]
- 3.Kleene, S. J. (1993) Neuron 11, 123-132. [DOI] [PubMed] [Google Scholar]
- 4.Kleene, S. J. (1997) Biophys. J. 73, 1110-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold, G. H. (1999) Annu. Rev. Physiol. 61, 857-871. [DOI] [PubMed] [Google Scholar]
- 6.Menini, A. (1999) Curr. Opin. Neurobiol. 9, 419-426. [DOI] [PubMed] [Google Scholar]
- 7.Zufall, F. & Leinders-Zufall, T. (2000) Chem. Senses 25, 473-481. [DOI] [PubMed] [Google Scholar]
- 8.Matthews, H. R. & Reisert, J. (2003) Curr. Opin. Neurobiol. 13, 469-475. [DOI] [PubMed] [Google Scholar]
- 9.Kaupp, U. B. & Seifert, R. (2002) Physiological Reviews 82, 769-824. [DOI] [PubMed] [Google Scholar]
- 10.Trudeau, M. C. & Zagotta, W. N. (2003) J. Biol. Chem. 278, 18705-18708. [DOI] [PubMed] [Google Scholar]
- 11.Reisert, J. & Matthews, H. R. (1998) J. Gen. Physiol. 112, 529-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurahashi, T. & Menini, A. (1997) Nature 385, 725-729. [DOI] [PubMed] [Google Scholar]
- 13.Leinders-Zufall, T., Ma, M. & Zufall, F. (1999) J. Neurosci. 19, 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagostena, L. & Menini, A. (2003) Chem. Senses 28, 705-716. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi, H. & Kurahashi, T. (2002) J. Physiol. (London) 541, 825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reisert, J. & Matthews, H. R. (2001) J. Physiol. (London) 534, 179-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisert, J. & Matthews, H. R. (2001) J. Physiol. (London) 530, 113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lansky, P. & Rospars, J.-P. (1998) BioSystems 48, 131-138. [DOI] [PubMed] [Google Scholar]
- 19.Rospars, J.-P., Krivan, V. & Lansky, P. (2000) Chem. Senses 25, 293-311. [DOI] [PubMed] [Google Scholar]
- 20.Rospars, J.-P., Lansky, P. & Krivan, V. (2003) Chem. Senses 28, 509-522. [DOI] [PubMed] [Google Scholar]
- 21.Rospars, J.-P., Lansky, P., Tuckwell, H. C. & Vermeulen, A. (1996) J. Comput. Neurosci. 3, 51-72. [DOI] [PubMed] [Google Scholar]
- 22.Getz, W. M. (1999) Chem. Senses 24, 497-508. [DOI] [PubMed] [Google Scholar]
- 23.Vidybida, A. K. (2000) BioSystems 58, 125-132. [DOI] [PubMed] [Google Scholar]
- 24.Rospars, J.-P., Lansky, P., Duchamp-Viret, P. & Duchamp, A. (2001) Neurocomputing 38–40, 319-325. [Google Scholar]
- 25.Lindemann, B. (2001) Biophys. J. 80, 1712-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki, N., Takahata, M. & Sato, K. (2002) Chem. Senses 27, 789-801. [DOI] [PubMed] [Google Scholar]
- 27.Larsson, H. P., Kleene, S. J. & Lecar, H. (1997) Biophys. J. 72, 1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firestein, S., Picco, C. & Menini, A. (1993) J. Physiol. (London) 468, 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisert, J. & Matthews, H. R. (2001) J. Physiol. (London) 535, 637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisert, J. & Matthews, H. R. (1999) J. Physiol. (London) 519, 801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen, T.-Y. & Yau, K.-W. (1994) Nature 368, 545-548. [DOI] [PubMed] [Google Scholar]
- 32.Hamer, R. D. (2000) Mol. Vision 6, 265-286. [PMC free article] [PubMed] [Google Scholar]
- 33.Weber, C. R., Ginsburg, K. S., Philipson, K. D., Shannon, T. R. & Bers, D. M. (2001) J. Gen. Physiol. 117, 119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sneyd, J. & Tranchina, D. (1989) Bull. Math. Biol. 51, 749-784. [DOI] [PubMed] [Google Scholar]
- 35.Forti, S., Menini, A., Rispoli, G. & Torre, V. (1989) J. Physiol. (London) 419, 265-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koutalos, Y. & Yau, K.-W. (1996) Trends Neurosci. 19, 73-81. [DOI] [PubMed] [Google Scholar]
- 37.Borisy, F. F., Ronnett, G. V., Cunningham, A., Juilfs, D., Beavo, J. & Snyder, S. H. (1991) J. Neurosci. 12, 915-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opuni, K. & Reeves, J. P. (2000) J. Biol. Chem. 275, 21549-21554. [DOI] [PubMed] [Google Scholar]
- 39.Reeves, J. P. & Condrescu, M. (2003) J. Gen. Physiol. 122, 621-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rospars, J.-P., Lansky, P., Duchamp, A. & Duchamp-Viret, P. (2003) Eur. J. Neurosci. 18, 1135-1154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.