Abstract
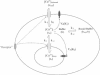
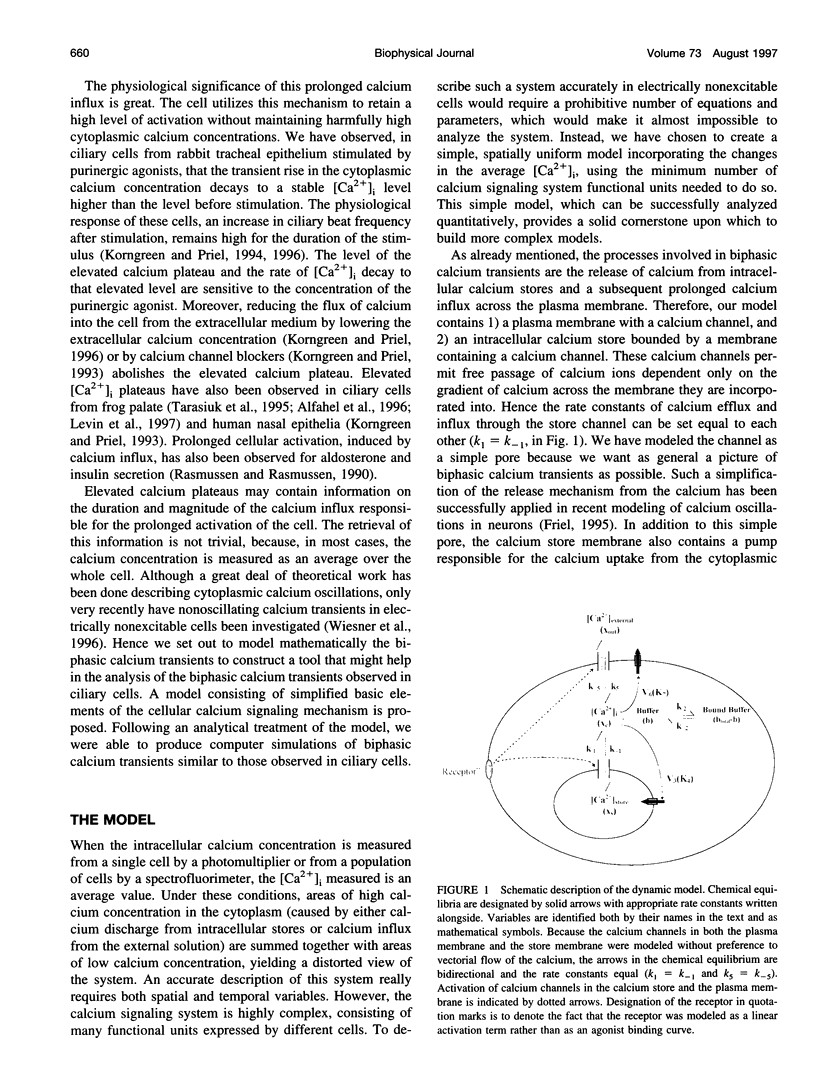
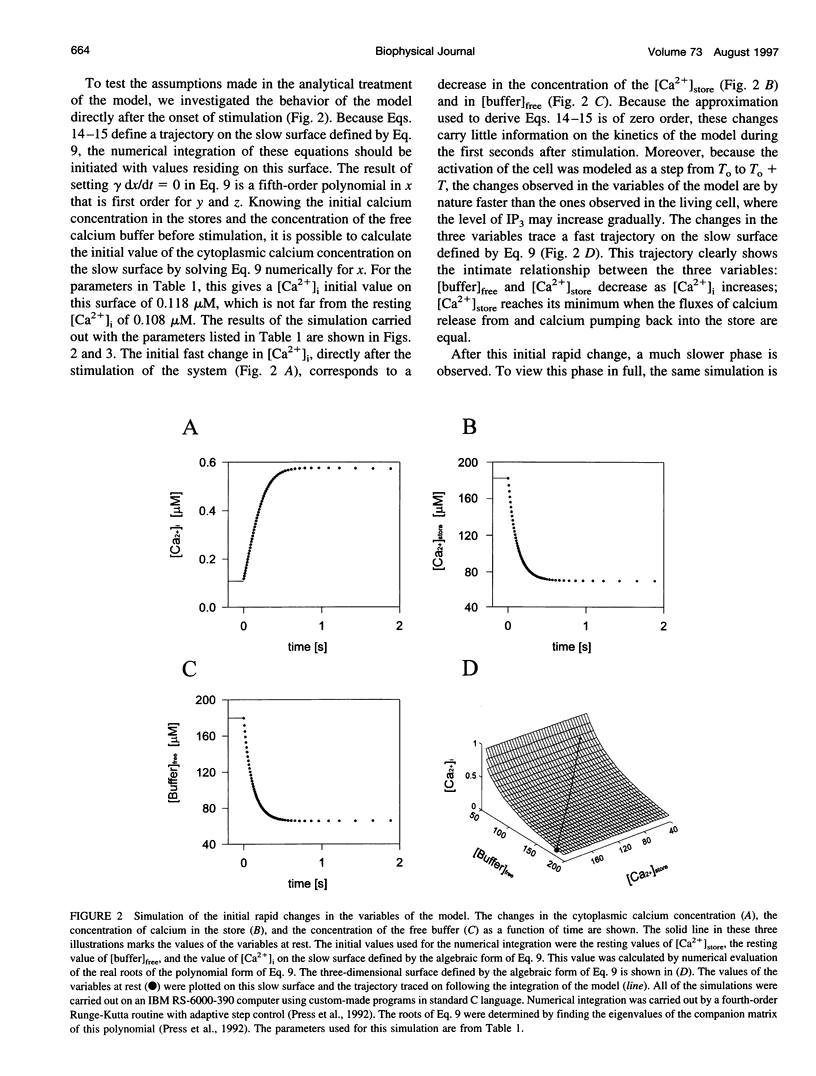
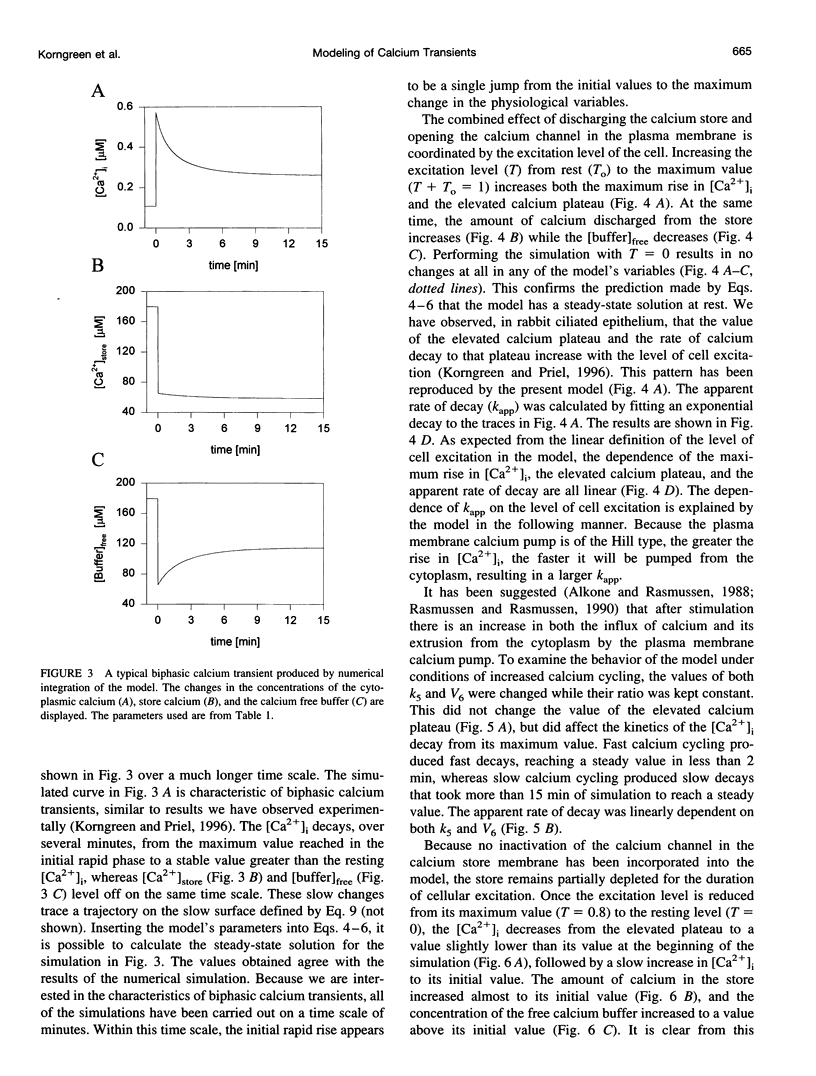
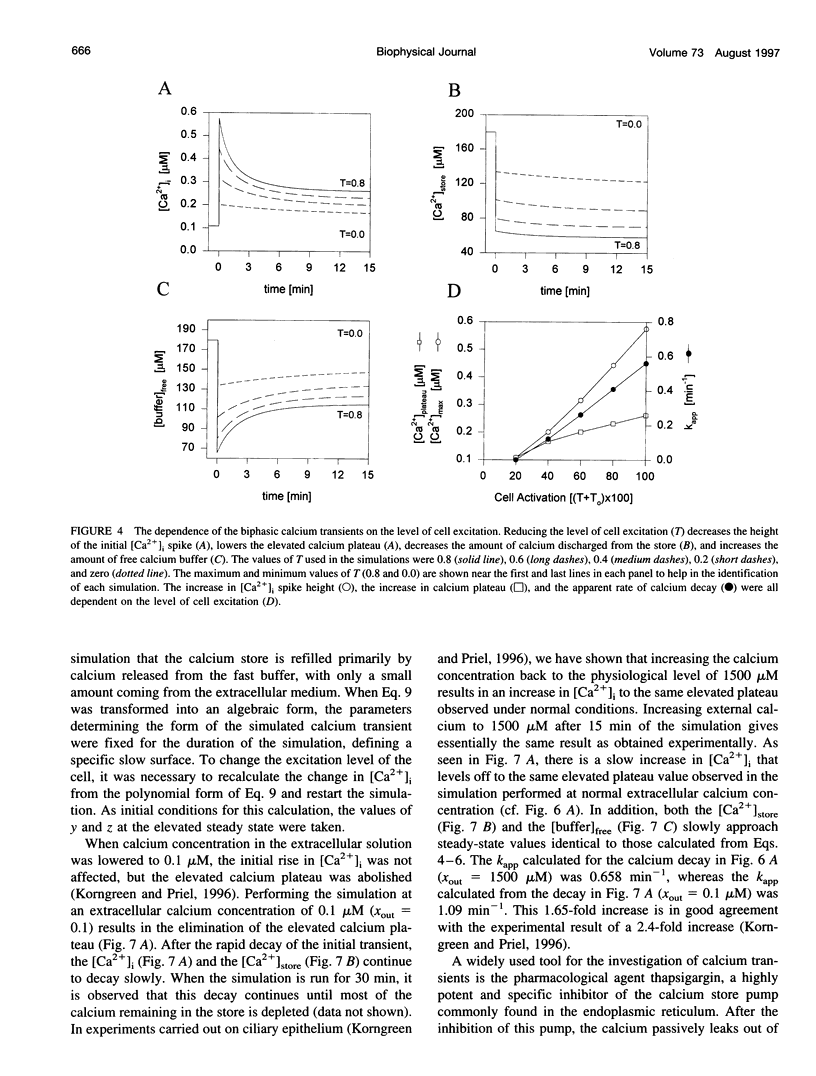
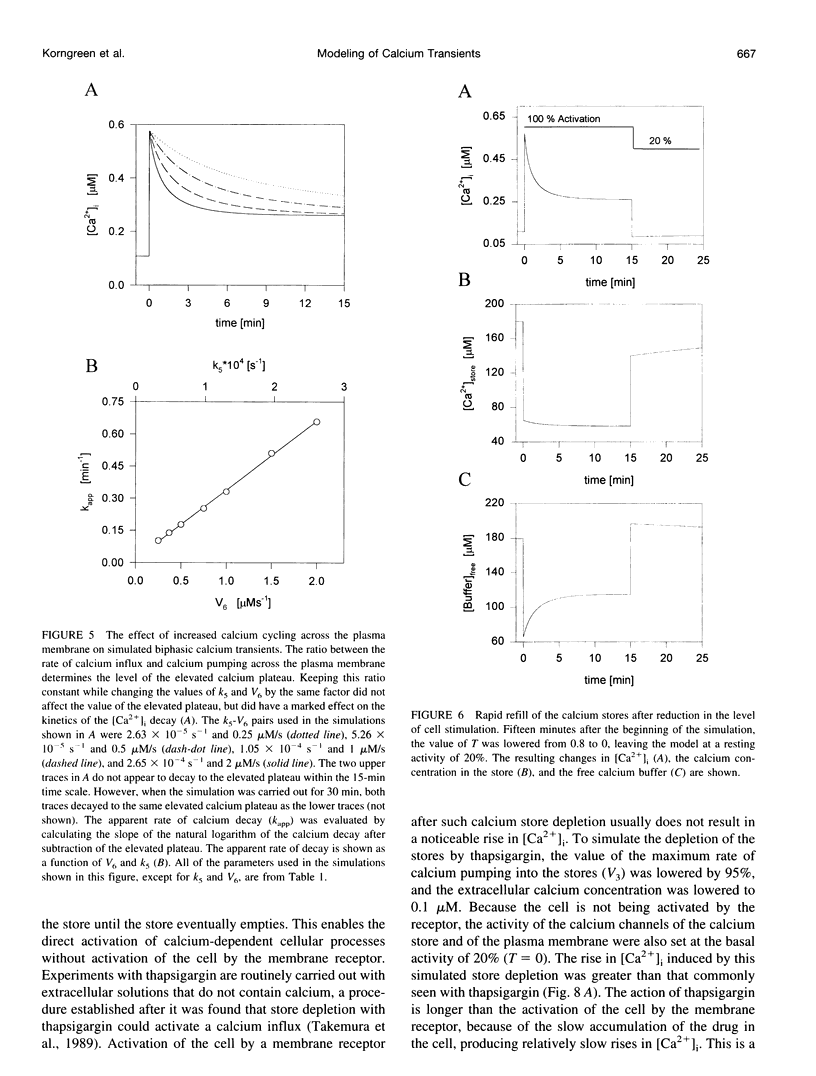
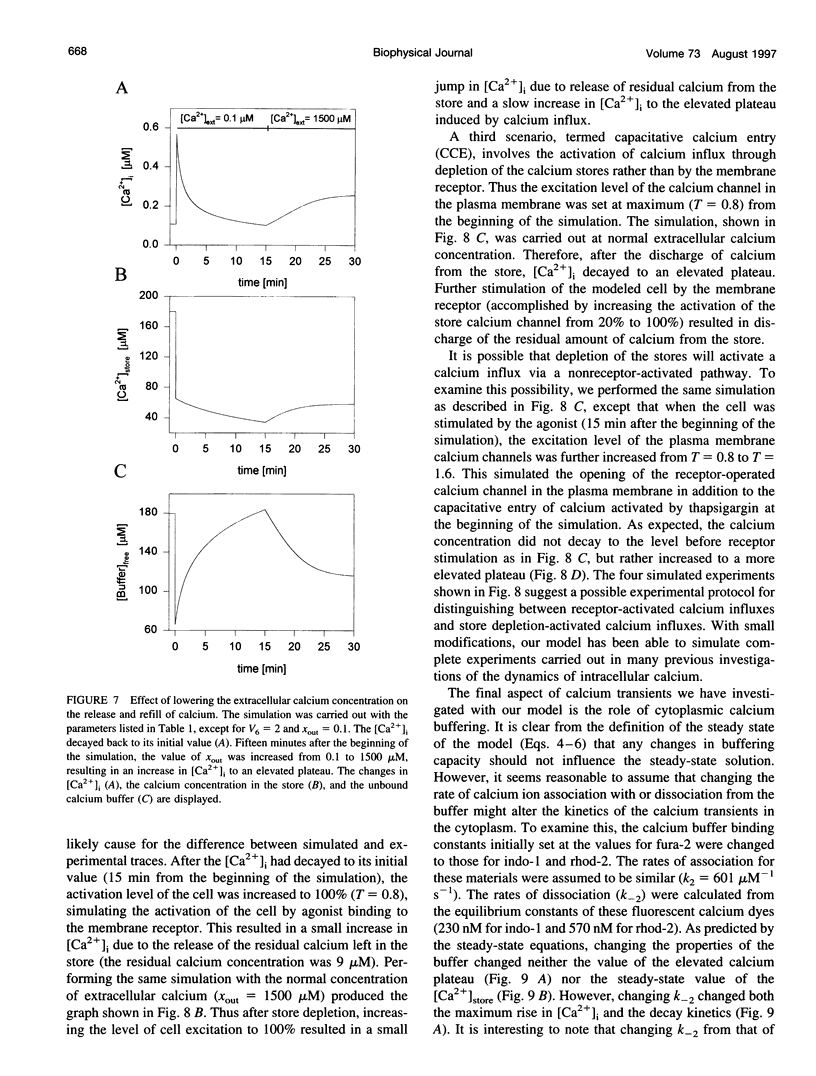
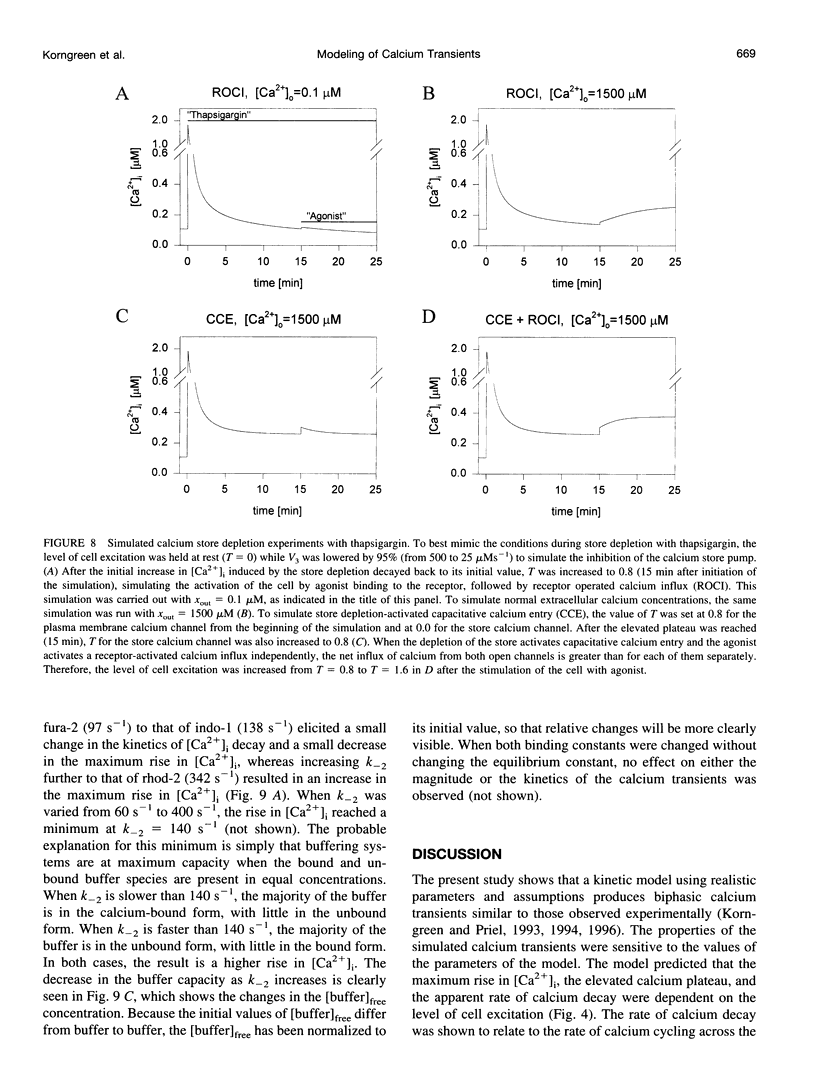
In many electrically nonexcitable cells, the release of calcium from internal stores is followed by a much slower phase in which the intracellular calcium concentration decreases gradually to a sustained value higher than the concentration before stimulation. This elevated calcium plateau has been shown to be the result of calcium influx. The model presented in this work describes a system consisting of a cytoplasmic calcium store and a plasma membrane calcium channel, both excitable by a membrane receptor; a fast cytoplasmic calcium buffer; and calcium pumps in both the calcium store and cellular membranes. Inherent difficulties in the numerical evaluation of the model, caused by very large calcium fluxes across the store membrane, were overcome by analytically separating the fast processes of calcium release from the slower processes of calcium cycling across the plasma membrane. This enabled the simulation of realistic biphasic calcium transients similar to those observed experimentally. The model predicted 1) a strong correlation between the rate of calcium cycling across the plasma membrane and the rate of calcium decay; and 2) a dependence on the level of cell excitation of the maximum rise in cytoplasmic calcium concentration, the level of the elevated calcium plateau, and the rate of calcium decay. Using the model, we simulated the washout of agonist from the bathing solution and the depletion of the calcium store by a pharmacological agent (such as thapsigargin) under several experimental conditions.
Full text
PDF



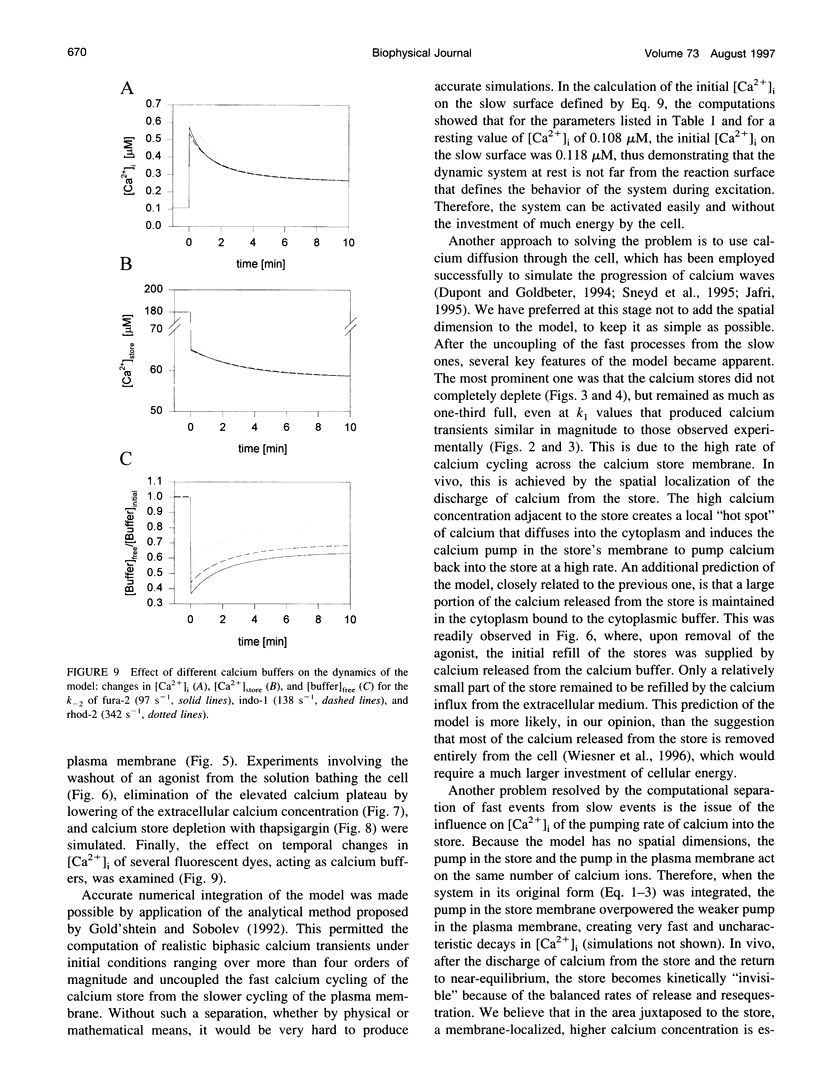



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfahel E., Korngreen A., Parola A. H., Priel Z. Purinergically induced membrane fluidization in ciliary cells: characterization and control by calcium and membrane potential. Biophys J. 1996 Feb;70(2):1045–1053. doi: 10.1016/S0006-3495(96)79650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Berlin J. R., Bassani J. W., Bers D. M. Intrinsic cytosolic calcium buffering properties of single rat cardiac myocytes. Biophys J. 1994 Oct;67(4):1775–1787. doi: 10.1016/S0006-3495(94)80652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Boitano S., Dirksen E. R., Sanderson M. J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992 Oct 9;258(5080):292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Berridge M. J. The elemental principles of calcium signaling. Cell. 1995 Dec 1;83(5):675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Cuthbertson K. S., Chay T. R. Modelling receptor-controlled intracellular calcium oscillators. Cell Calcium. 1991 Feb-Mar;12(2-3):97–109. doi: 10.1016/0143-4160(91)90012-4. [DOI] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Properties of intracellular Ca2+ waves generated by a model based on Ca(2+)-induced Ca2+ release. Biophys J. 1994 Dec;67(6):2191–2204. doi: 10.1016/S0006-3495(94)80705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichwald C., Kaiser F. Model for receptor-controlled cytosolic calcium oscillations and for external influences on the signal pathway. Biophys J. 1993 Nov;65(5):2047–2058. doi: 10.1016/S0006-3495(93)81236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel D. D. [Ca2+]i oscillations in sympathetic neurons: an experimental test of a theoretical model. Biophys J. 1995 May;68(5):1752–1766. doi: 10.1016/S0006-3495(95)80352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M. S. A theoretical study of cytosolic calcium waves in Xenopus oocytes. J Theor Biol. 1995 Feb 7;172(3):209–216. doi: 10.1006/jtbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- Jafri M. S., Keizer J. On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys J. 1995 Nov;69(5):2139–2153. doi: 10.1016/S0006-3495(95)80088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P., Tsien R. Y. Ca2+ binding kinetics of fura-2 and azo-1 from temperature-jump relaxation measurements. Biophys J. 1988 Apr;53(4):635–639. doi: 10.1016/S0006-3495(88)83142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall J. M., Sala-Newby G., Ghalaut V., Dormer R. L., Campbell A. K. Engineering the CA(2+)-activated photoprotein aequorin with reduced affinity for calcium. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1091–1097. doi: 10.1016/0006-291x(92)91309-e. [DOI] [PubMed] [Google Scholar]
- Korngreen A., Priel Z. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J Physiol. 1996 Nov 15;497(Pt 1):53–66. doi: 10.1113/jphysiol.1996.sp021749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen A., Priel Z. Simultaneous measurement of ciliary beating and intracellular calcium. Biophys J. 1994 Jul;67(1):377–380. doi: 10.1016/S0006-3495(94)80492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M., Wolf B. Mathematical model for agonist-induced oscillatory calcium waves in non-excitable mammalian cells. Biol Signals. 1992 Mar-Apr;1(2):101–113. doi: 10.1159/000109316. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Peralta E., Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991 Apr 5;252(5002):123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Levin R., Braiman A., Priel Z. Protein kinase C induced calcium influx and sustained enhancement of ciliary beating by extracellular ATP. Cell Calcium. 1997 Feb;21(2):103–113. doi: 10.1016/s0143-4160(97)90034-8. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Meyer T., Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. J., Jacob R. Ionomycin enhances Ca2+ influx by stimulating store-regulated cation entry and not by a direct action at the plasma membrane. Biochem J. 1994 Jun 15;300(Pt 3):665–672. doi: 10.1042/bj3000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Tsien R. Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993 Aug 26;364(6440):809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Rasmussen J. E. Calcium as intracellular messenger: from simplicity to complexity. Curr Top Cell Regul. 1990;31:1–109. doi: 10.1016/b978-0-12-152831-7.50003-2. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Keizer J., Sanderson M. J. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J. 1995 Nov;9(14):1463–1472. doi: 10.1096/fasebj.9.14.7589988. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Tang Y., Othmer H. G. A model of calcium dynamics in cardiac myocytes based on the kinetics of ryanodine-sensitive calcium channels. Biophys J. 1994 Dec;67(6):2223–2235. doi: 10.1016/S0006-3495(94)80707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Othmer H. G. Frequency encoding in excitable systems with applications to calcium oscillations. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7869–7873. doi: 10.1073/pnas.92.17.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasiuk A., Bar-Shimon M., Gheber L., Korngreen A., Grossman Y., Priel Z. Extracellular ATP induces hyperpolarization and motility stimulation of ciliary cells. Biophys J. 1995 Mar;68(3):1163–1169. doi: 10.1016/S0006-3495(95)80292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribe R. M., Borin M. L., Blaustein M. P. Functionally and spatially distinct Ca2+ stores are revealed in cultured vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5908–5912. doi: 10.1073/pnas.91.13.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Wiesner T. F., Berk B. C., Nerem R. M. A mathematical model of cytosolic calcium dynamics in human umbilical vein endothelial cells. Am J Physiol. 1996 May;270(5 Pt 1):C1556–C1569. doi: 10.1152/ajpcell.1996.270.5.C1556. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]