Abstract
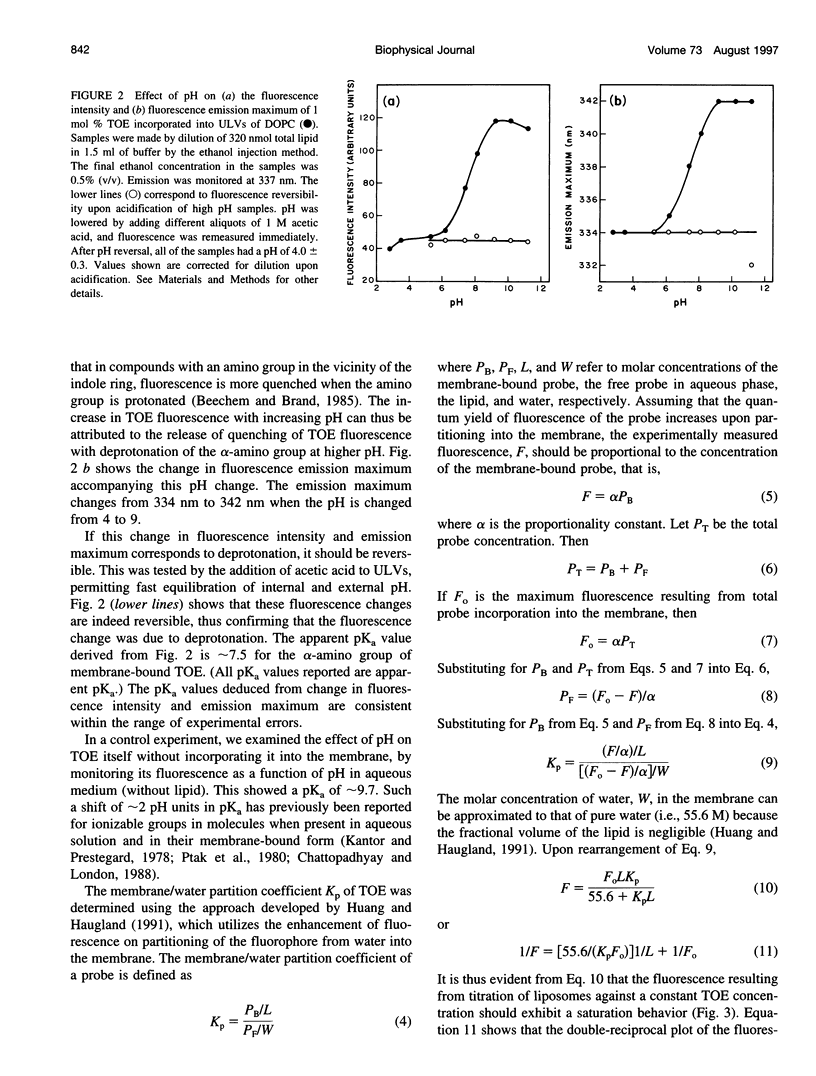
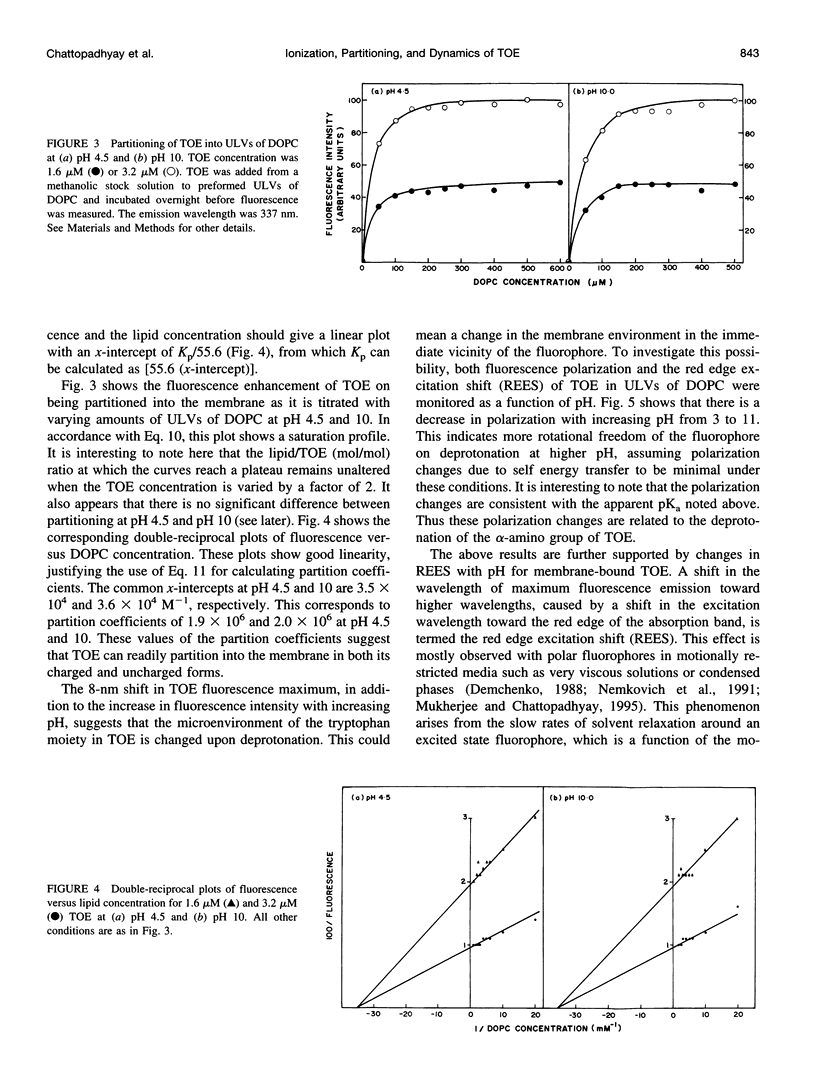
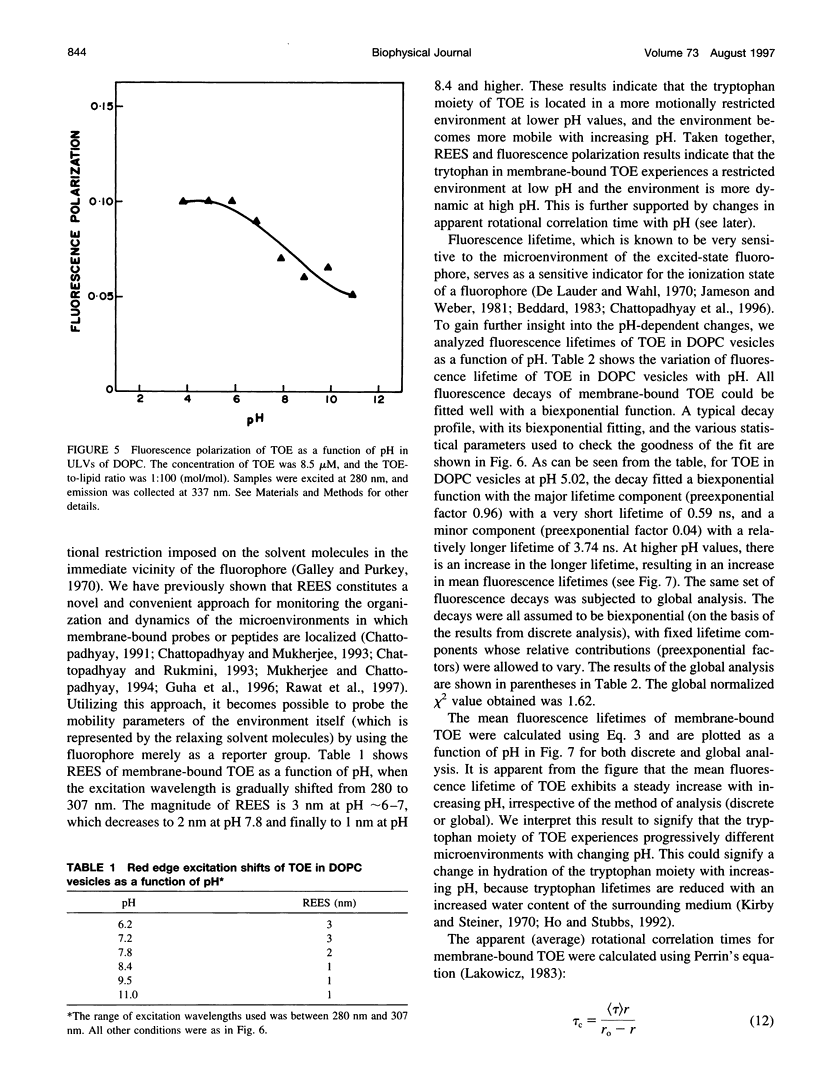
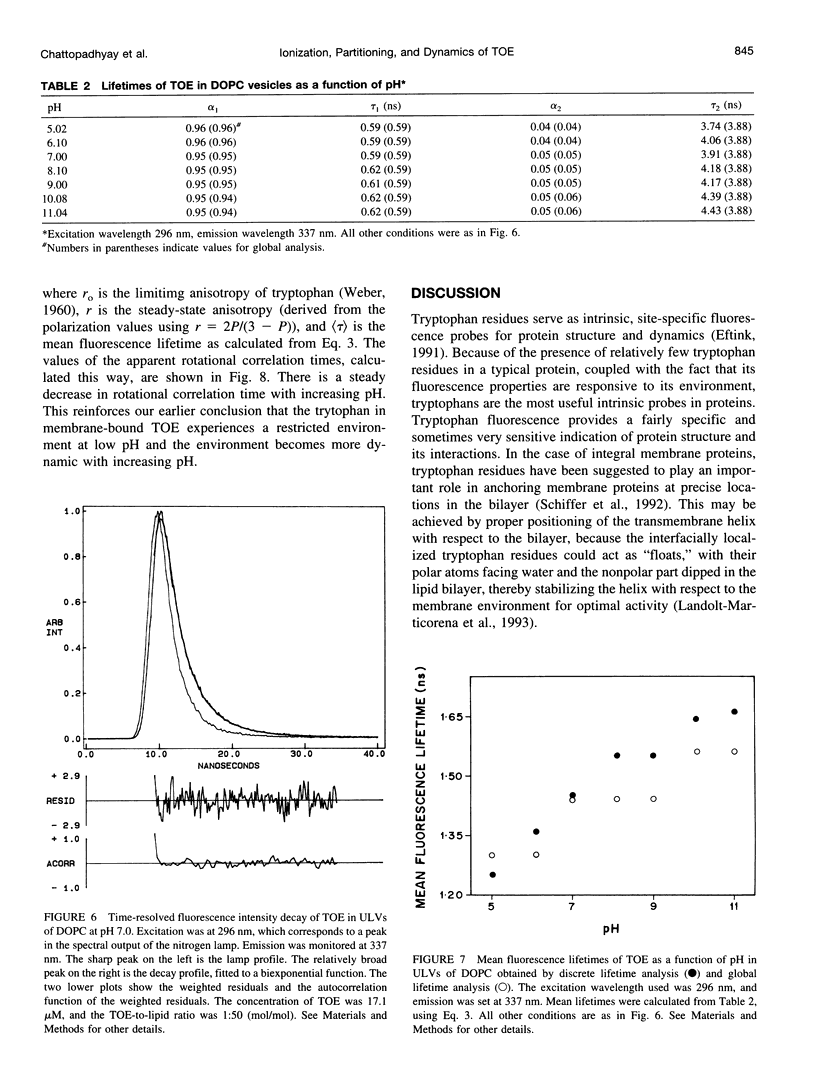
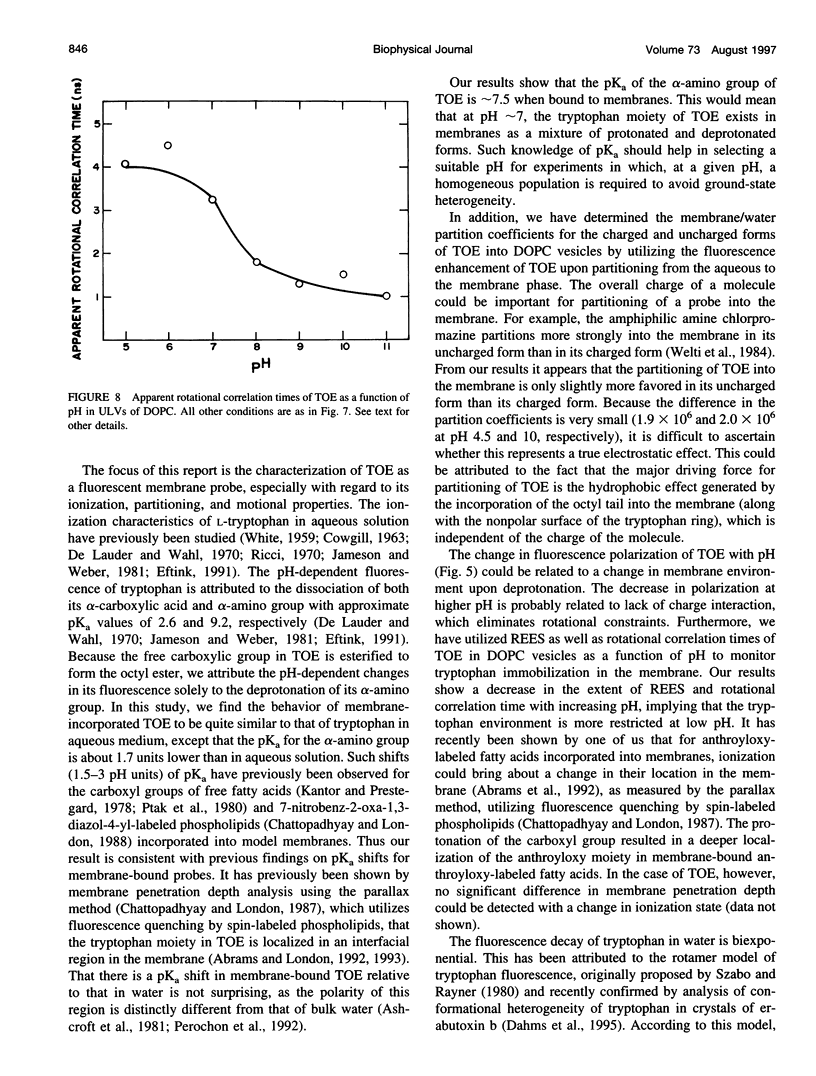
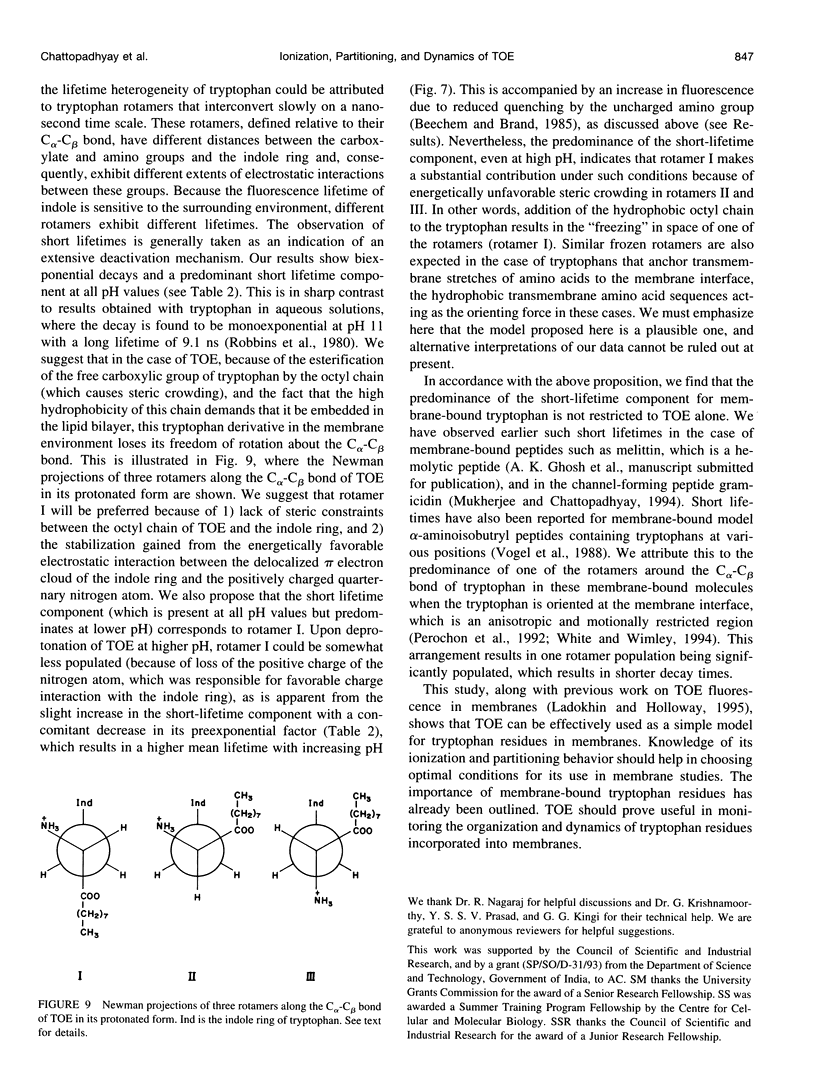
The presence of tryptophan residues as intrinsic fluorophores in most proteins makes them an obvious choice for fluorescence spectroscopic analyses of such proteins. Membrane proteins have been reported to have a significantly higher tryptophan content than soluble proteins. The role of tryptophan residues in the structure and function of membrane proteins has attracted a lot of attention. Tryptophan residues in membrane proteins and peptides are believed to be distributed asymmetrically toward the interfacial region. Tryptophan octyl ester (TOE) is an important model for membrane-bound tryptophan residues. We have characterized this molecule as a fluorescent membrane probe in terms of its ionization, partitioning, and motional characteristics in unilamellar vesicles of dioleoylphosphatidylcholine. The ionization property of this molecule in model membranes has been studied by utilizing its pH-dependent fluorescence characteristics. Analysis of pH-dependent fluorescence intensity and emission maximum shows that deprotonation of the alpha-amino group of TOE occurs with an apparent pKa of approximately 7.5 in the membrane. The fluorescence lifetime of membrane-bound TOE also shows pH dependence. The fluorescence lifetimes of TOE have been interpreted by using the rotamer model for the fluorescence decay of tryptophan. Membrane/water partition coefficients of TOE were measured in both its protonated and deprotonated forms. No appreciable difference was found in its partitioning behavior with ionization. Analysis of fluorescence polarization of TOE as a function of pH showed that there is a decrease in polarization with increasing pH, implying more rotational freedom on deprotonation. This is further supported by pH-dependent red edge excitation shift and the apparent rotational correlation time of membrane-bound TOE. TOE should prove useful in monitoring the organization and dynamics of tryptophan residues incorporated into membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams F. S., Chattopadhyay A., London E. Determination of the location of fluorescent probes attached to fatty acids using parallax analysis of fluorescence quenching: effect of carboxyl ionization state and environment on depth. Biochemistry. 1992 Jun 16;31(23):5322–5327. doi: 10.1021/bi00138a011. [DOI] [PubMed] [Google Scholar]
- Abrams F. S., London E. Calibration of the parallax fluorescence quenching method for determination of membrane penetration depth: refinement and comparison of quenching by spin-labeled and brominated lipids. Biochemistry. 1992 Jun 16;31(23):5312–5322. doi: 10.1021/bi00138a010. [DOI] [PubMed] [Google Scholar]
- Abrams F. S., London E. Extension of the parallax analysis of membrane penetration depth to the polar region of model membranes: use of fluorescence quenching by a spin-label attached to the phospholipid polar headgroup. Biochemistry. 1993 Oct 12;32(40):10826–10831. doi: 10.1021/bi00091a038. [DOI] [PubMed] [Google Scholar]
- Ashcroft R. G., Coster H. G., Smith J. R. The molecular organisation of bimolecular lipid membranes. The dielectric structure of the hydrophilic/hydrophobic interface. Biochim Biophys Acta. 1981 Apr 22;643(1):191–204. doi: 10.1016/0005-2736(81)90232-7. [DOI] [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Becker M. D., Greathouse D. V., Koeppe R. E., 2nd, Andersen O. S. Amino acid sequence modulation of gramicidin channel function: effects of tryptophan-to-phenylalanine substitutions on the single-channel conductance and duration. Biochemistry. 1991 Sep 10;30(36):8830–8839. doi: 10.1021/bi00100a015. [DOI] [PubMed] [Google Scholar]
- Beechem J. M. A second generation global analysis program for the recovery of complex inhomogeneous fluorescence decay kinetics. Chem Phys Lipids. 1989 Jun;50(3-4):237–251. doi: 10.1016/0009-3084(89)90052-2. [DOI] [PubMed] [Google Scholar]
- Beechem J. M., Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- Beechem J. M. Global analysis of biochemical and biophysical data. Methods Enzymol. 1992;210:37–54. doi: 10.1016/0076-6879(92)10004-w. [DOI] [PubMed] [Google Scholar]
- Befort K., Tabbara L., Kling D., Maigret B., Kieffer B. L. Role of aromatic transmembrane residues of the delta-opioid receptor in ligand recognition. J Biol Chem. 1996 Apr 26;271(17):10161–10168. doi: 10.1074/jbc.271.17.10161. [DOI] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science. 1985 Jul 5;229(4708):23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Weakly polar interactions in proteins. Adv Protein Chem. 1988;39:125–189. doi: 10.1016/s0065-3233(08)60376-9. [DOI] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- COWGILL R. W. Fluorescence and the structure of proteins. I. Effects of substituents on the fluorescence of indole and phenol compounds. Arch Biochem Biophys. 1963 Jan;100:36–44. doi: 10.1016/0003-9861(63)90031-6. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., London E. Parallax method for direct measurement of membrane penetration depth utilizing fluorescence quenching by spin-labeled phospholipids. Biochemistry. 1987 Jan 13;26(1):39–45. doi: 10.1021/bi00375a006. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., London E. Spectroscopic and ionization properties of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids in model membranes. Biochim Biophys Acta. 1988 Feb 8;938(1):24–34. doi: 10.1016/0005-2736(88)90118-6. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., McNamee M. G. Average membrane penetration depth of tryptophan residues of the nicotinic acetylcholine receptor by the parallax method. Biochemistry. 1991 Jul 23;30(29):7159–7164. doi: 10.1021/bi00243a017. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., Mukherjee S. Fluorophore environments in membrane-bound probes: a red edge excitation shift study. Biochemistry. 1993 Apr 13;32(14):3804–3811. doi: 10.1021/bi00065a037. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A., Rukmini R., Mukherjee S. Photophysics of a neurotransmitter: ionization and spectroscopic properties of serotonin. Biophys J. 1996 Oct;71(4):1952–1960. doi: 10.1016/S0006-3495(96)79393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay A., Rukmini R. Restricted mobility of the sole tryptophan in membrane-bound melittin. FEBS Lett. 1993 Dec 13;335(3):341–344. doi: 10.1016/0014-5793(93)80415-q. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- De Lauder W. B., Wahl P. pH dependence of the fluorescence decay of tryptophan. Biochemistry. 1970 Jun 23;9(13):2750–2754. doi: 10.1021/bi00815a025. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. The Photosynthetic Reaction Center from the Purple Bacterium Rhodopseudomonas viridis. Science. 1989 Sep 29;245(4925):1463–1473. doi: 10.1126/science.245.4925.1463. [DOI] [PubMed] [Google Scholar]
- Demchenko A. P. Site-selective excitation: a new dimension in protein and membrane spectroscopy. Trends Biochem Sci. 1988 Oct;13(10):374–377. doi: 10.1016/0968-0004(88)90173-9. [DOI] [PubMed] [Google Scholar]
- Eftink M. R. Fluorescence techniques for studying protein structure. Methods Biochem Anal. 1991;35:127–205. doi: 10.1002/9780470110560.ch3. [DOI] [PubMed] [Google Scholar]
- Ferrer-Montiel A. V., Sun W., Montal M. A single tryptophan on M2 of glutamate receptor channels confers high permeability to divalent cations. Biophys J. 1996 Aug;71(2):749–758. doi: 10.1016/S0006-3495(96)79274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca V., Daumas P., Ranjalahy-Rasoloarijao L., Heitz F., Lazaro R., Trudelle Y., Andersen O. S. Gramicidin channels that have no tryptophan residues. Biochemistry. 1992 Jun 16;31(23):5340–5350. doi: 10.1021/bi00138a014. [DOI] [PubMed] [Google Scholar]
- Galley W. C., Purkey R. M. Role of heterogeneity of the solvation site in electronic spectra in solution. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1116–1121. doi: 10.1073/pnas.67.3.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrisch K., Barry J. A., Holte L. L., Sinnwell T., Bergelson L. D., Ferretti J. A. Role of interactions at the lipid-water interface for domain formation. Mol Membr Biol. 1995 Jan-Mar;12(1):83–88. doi: 10.3109/09687689509038500. [DOI] [PubMed] [Google Scholar]
- Grinvald A., Steinberg I. Z. On the analysis of fluorescence decay kinetics by the method of least-squares. Anal Biochem. 1974 Jun;59(2):583–598. doi: 10.1016/0003-2697(74)90312-1. [DOI] [PubMed] [Google Scholar]
- Guha S., Rawat S. S., Chattopadhyay A., Bhattacharyya B. Tubulin conformation and dynamics: a red edge excitation shift study. Biochemistry. 1996 Oct 15;35(41):13426–13433. doi: 10.1021/bi961251g. [DOI] [PubMed] [Google Scholar]
- Ho C., Stubbs C. D. Hydration at the membrane protein-lipid interface. Biophys J. 1992 Oct;63(4):897–902. doi: 10.1016/S0006-3495(92)81671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Cross T. A. Tryptophan hydrogen bonding and electric dipole moments: functional roles in the gramicidin channel and implications for membrane proteins. Biochemistry. 1995 Oct 31;34(43):14147–14155. doi: 10.1021/bi00043a020. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Haugland R. P. Partition coefficients of fluorescent probes with phospholipid membranes. Biochem Biophys Res Commun. 1991 Nov 27;181(1):166–171. doi: 10.1016/s0006-291x(05)81396-8. [DOI] [PubMed] [Google Scholar]
- Ippolito J. A., Alexander R. S., Christianson D. W. Hydrogen bond stereochemistry in protein structure and function. J Mol Biol. 1990 Oct 5;215(3):457–471. doi: 10.1016/s0022-2836(05)80364-x. [DOI] [PubMed] [Google Scholar]
- Jacobs R. E., White S. H. The nature of the hydrophobic binding of small peptides at the bilayer interface: implications for the insertion of transbilayer helices. Biochemistry. 1989 Apr 18;28(8):3421–3437. doi: 10.1021/bi00434a042. [DOI] [PubMed] [Google Scholar]
- Jain M. K., Rogers J., Simpson L., Gierasch L. M. Effect of tryptophan derivatives on the phase properties of bilayers. Biochim Biophys Acta. 1985 Jun 11;816(1):153–162. doi: 10.1016/0005-2736(85)90403-1. [DOI] [PubMed] [Google Scholar]
- Kachel K., Asuncion-Punzalan E., London E. Anchoring of tryptophan and tyrosine analogs at the hydrocarbon-polar boundary in model membrane vesicles: parallax analysis of fluorescence quenching induced by nitroxide-labeled phospholipids. Biochemistry. 1995 Nov 28;34(47):15475–15479. doi: 10.1021/bi00047a012. [DOI] [PubMed] [Google Scholar]
- Kantor H. L., Prestegard J. H. Fusion of phosphatidylcholine bilayer vesicles: role of free fatty acid. Biochemistry. 1978 Aug 22;17(17):3592–3597. doi: 10.1021/bi00610a027. [DOI] [PubMed] [Google Scholar]
- Killian J. A., Salemink I., de Planque M. R., Lindblom G., Koeppe R. E., 2nd, Greathouse D. V. Induction of nonbilayer structures in diacylphosphatidylcholine model membranes by transmembrane alpha-helical peptides: importance of hydrophobic mismatch and proposed role of tryptophans. Biochemistry. 1996 Jan 23;35(3):1037–1045. doi: 10.1021/bi9519258. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Esker M. W., Pathmamanoharan C., Wiersema P. H. Vesicles of variable diameter prepared by a modified injection method. Biochemistry. 1977 Aug 23;16(17):3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- Ladokhin A. S., Holloway P. W. Fluorescence of membrane-bound tryptophan octyl ester: a model for studying intrinsic fluorescence of protein-membrane interactions. Biophys J. 1995 Aug;69(2):506–517. doi: 10.1016/S0006-3495(95)79924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt-Marticorena C., Williams K. A., Deber C. M., Reithmeier R. A. Non-random distribution of amino acids in the transmembrane segments of human type I single span membrane proteins. J Mol Biol. 1993 Feb 5;229(3):602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Pohle W., McElhaney R. N. The interfacial structure of phospholipid bilayers: differential scanning calorimetry and Fourier transform infrared spectroscopic studies of 1,2-dipalmitoyl-sn-glycero-3-phosphorylcholine and its dialkyl and acyl-alkyl analogs. Biophys J. 1996 Jun;70(6):2736–2746. doi: 10.1016/S0006-3495(96)79843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. A fluorescence-based detergent binding assay for protein hydrophobicity. Anal Biochem. 1986 Apr;154(1):57–63. doi: 10.1016/0003-2697(86)90495-1. [DOI] [PubMed] [Google Scholar]
- London E., Feigenson G. W. Fluorescence quenching in model membranes. 1. Characterization of quenching caused by a spin-labeled phospholipid. Biochemistry. 1981 Mar 31;20(7):1932–1938. doi: 10.1021/bi00510a032. [DOI] [PubMed] [Google Scholar]
- McClare C. W. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971 Feb;39(2):527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- Meers P. Location of tryptophans in membrane-bound annexins. Biochemistry. 1990 Apr 3;29(13):3325–3330. doi: 10.1021/bi00465a025. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Chattopadhyay A. Motionally restricted tryptophan environments at the peptide-lipid interface of gramicidin channels. Biochemistry. 1994 May 3;33(17):5089–5097. doi: 10.1021/bi00183a012. [DOI] [PubMed] [Google Scholar]
- Ptak M., Egret-Charlier M., Sanson A., Bouloussa O. A NMR study of the ionization of fatty acids, fatty amines and N-acylamino acids incorporated in phosphatidylcholine vesicles. Biochim Biophys Acta. 1980 Aug 4;600(2):387–397. doi: 10.1016/0005-2736(80)90442-3. [DOI] [PubMed] [Google Scholar]
- Pérochon E., Lopez A., Tocanne J. F. Polarity of lipid bilayers. A fluorescence investigation. Biochemistry. 1992 Aug 25;31(33):7672–7682. doi: 10.1021/bi00148a031. [DOI] [PubMed] [Google Scholar]
- Reithmeier R. A. Characterization and modeling of membrane proteins using sequence analysis. Curr Opin Struct Biol. 1995 Aug;5(4):491–500. doi: 10.1016/0959-440x(95)80034-4. [DOI] [PubMed] [Google Scholar]
- Ricci R. W. Deuterium-isotope effect on the fluorescence yields and lifetimes of indole derivatives--including tryptophan and tryptamine. Photochem Photobiol. 1970 Jul;12(1):67–75. doi: 10.1111/j.1751-1097.1970.tb06039.x. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Chang C. H., Stevens F. J. The functions of tryptophan residues in membrane proteins. Protein Eng. 1992 Apr;5(3):213–214. doi: 10.1093/protein/5.3.213. [DOI] [PubMed] [Google Scholar]
- Slater S. J., Ho C., Taddeo F. J., Kelly M. B., Stubbs C. D. Contribution of hydrogen bonding to lipid-lipid interactions in membranes and the role of lipid order: effects of cholesterol, increased phospholipid unsaturation, and ethanol. Biochemistry. 1993 Apr 13;32(14):3714–3721. doi: 10.1021/bi00065a025. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Meech S. R., Lee A. G., Phillips D. Solvent relaxation in lipid bilayers with dansyl probes. Biochim Biophys Acta. 1985 May 28;815(3):351–360. doi: 10.1016/0005-2736(85)90361-x. [DOI] [PubMed] [Google Scholar]
- Swaminathan R., Krishnamoorthy G., Periasamy N. Similarity of fluorescence lifetime distributions for single tryptophan proteins in the random coil state. Biophys J. 1994 Nov;67(5):2013–2023. doi: 10.1016/S0006-3495(94)80685-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable R. M., Zhang Y., Hardy B. J., Pastor R. W. Molecular dynamics simulations of a lipid bilayer and of hexadecane: an investigation of membrane fluidity. Science. 1993 Oct 8;262(5131):223–226. doi: 10.1126/science.8211140. [DOI] [PubMed] [Google Scholar]
- Vogel H., Nilsson L., Rigler R., Voges K. P., Jung G. Structural fluctuations of a helical polypeptide traversing a lipid bilayer. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5067–5071. doi: 10.1073/pnas.85.14.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in tyrosine, tryptophan and related compounds. Biochem J. 1960 May;75:335–345. doi: 10.1042/bj0750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE A. Effect of pH on fluorescence of tryosine, tryptophan and related compounds. Biochem J. 1959 Feb;71(2):217–220. doi: 10.1042/bj0710217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Abele U., Weckesser J., Welte W., Schiltz E., Schulz G. E. Molecular architecture and electrostatic properties of a bacterial porin. Science. 1991 Dec 13;254(5038):1627–1630. doi: 10.1126/science.1721242. [DOI] [PubMed] [Google Scholar]
- Welti R., Mullikin L. J., Yoshimura T., Helmkamp G. M., Jr Partition of amphiphilic molecules into phospholipid vesicles and human erythrocyte ghosts: measurements by ultraviolet difference spectroscopy. Biochemistry. 1984 Dec 4;23(25):6086–6091. doi: 10.1021/bi00320a028. [DOI] [PubMed] [Google Scholar]
- Wess J., Nanavati S., Vogel Z., Maggio R. Functional role of proline and tryptophan residues highly conserved among G protein-coupled receptors studied by mutational analysis of the m3 muscarinic receptor. EMBO J. 1993 Jan;12(1):331–338. doi: 10.1002/j.1460-2075.1993.tb05661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimley W. C., White S. H. Experimentally determined hydrophobicity scale for proteins at membrane interfaces. Nat Struct Biol. 1996 Oct;3(10):842–848. doi: 10.1038/nsb1096-842. [DOI] [PubMed] [Google Scholar]
- Wimley W. C., White S. H. Membrane partitioning: distinguishing bilayer effects from the hydrophobic effect. Biochemistry. 1993 Jun 29;32(25):6307–6312. doi: 10.1021/bi00076a001. [DOI] [PubMed] [Google Scholar]
- Wimley W. C., White S. H. Partitioning of tryptophan side-chain analogs between water and cyclohexane. Biochemistry. 1992 Dec 29;31(51):12813–12818. doi: 10.1021/bi00166a015. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. V., Cullis P. M., Southgate C. C. Water, protein folding, and the genetic code. Science. 1979 Nov 2;206(4418):575–577. doi: 10.1126/science.493962. [DOI] [PubMed] [Google Scholar]
- Woolf T. B., Roux B. Molecular dynamics simulation of the gramicidin channel in a phospholipid bilayer. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11631–11635. doi: 10.1073/pnas.91.24.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]


