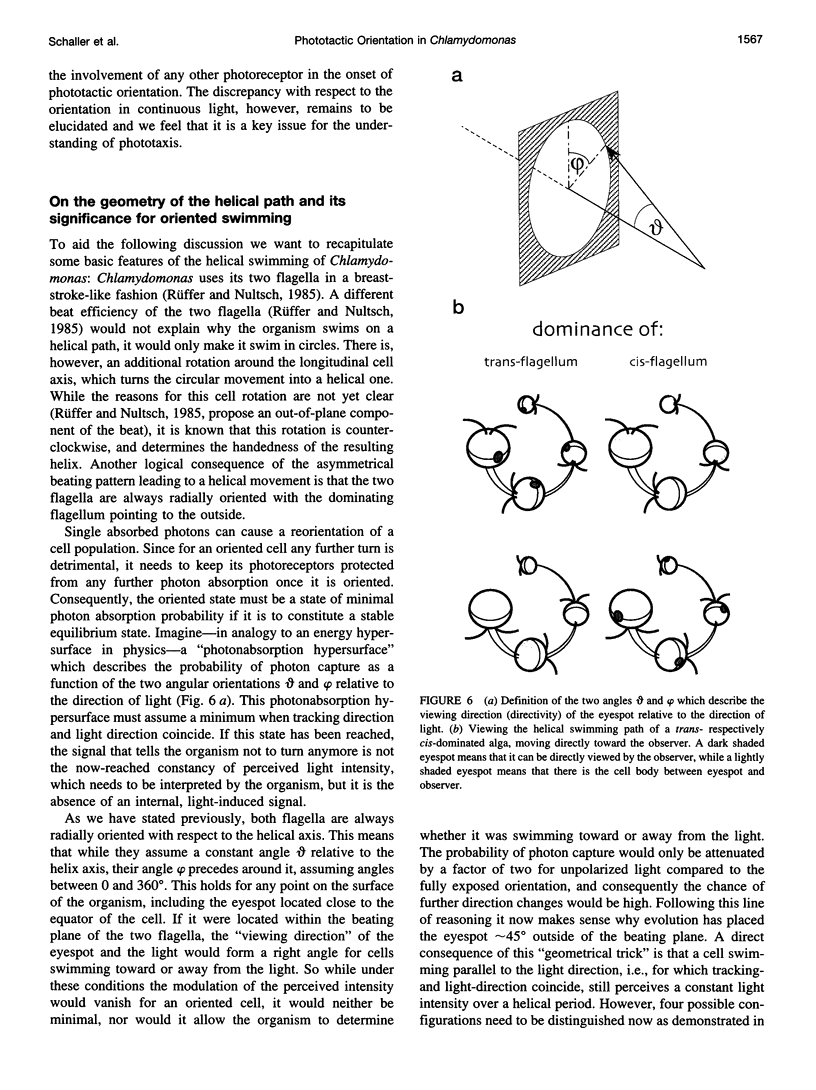
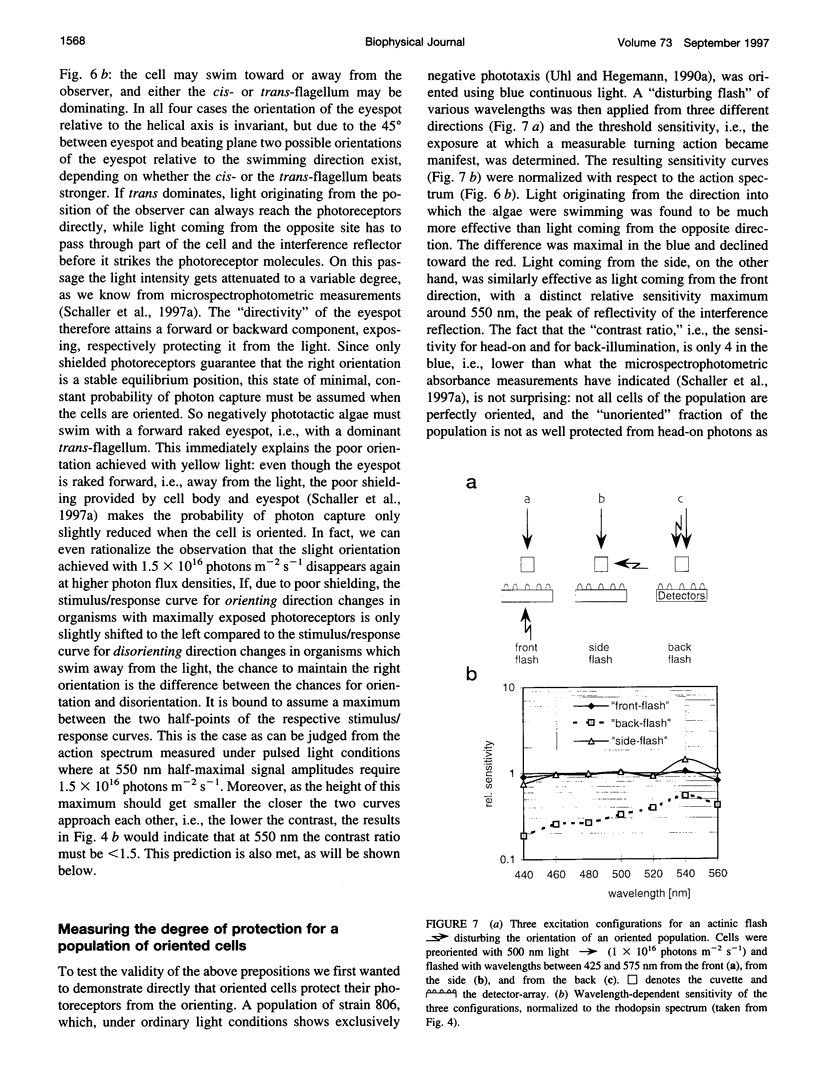
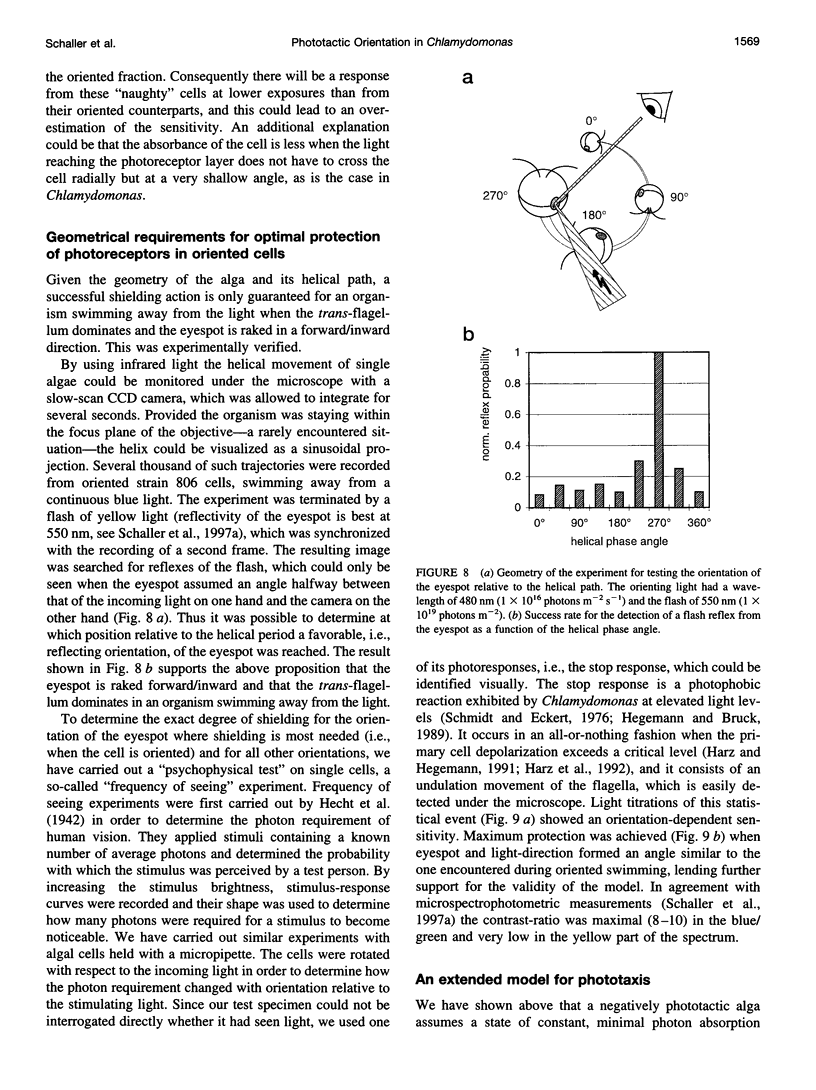
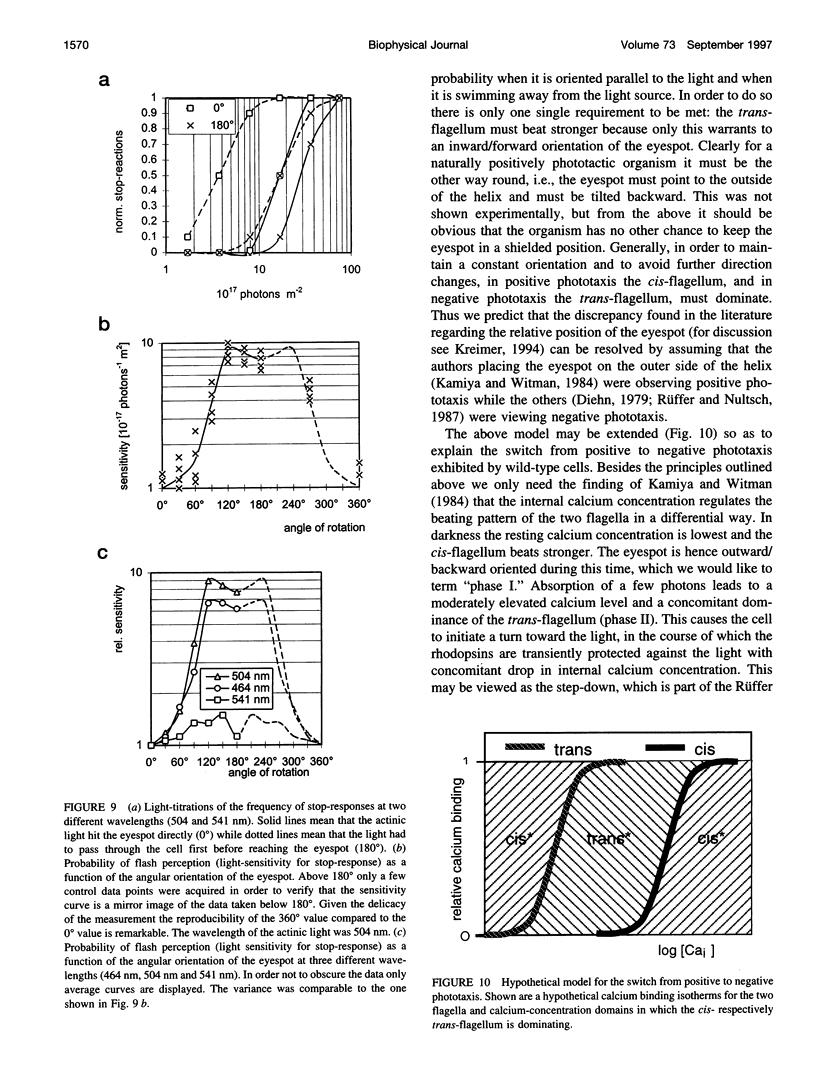
Abstract
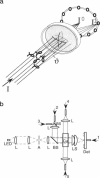
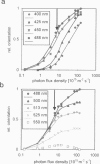
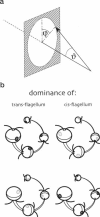
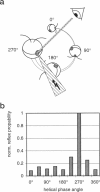
By using a real-time assay that allows measurement of the phototactic orientation of the unicellular alga Chlamydomonas with millisecond time resolution, it can be shown that single photons not only induce transient direction changes but that fluence rates as low as 1 photon cell(-1) s(-1) can already lead to a persistent orientation. Orientation is a binary variable, i.e., in a partially oriented population some organisms are fully oriented while the rest are still at random. Action spectra reveal that the response to a pulsed stimulus follows the Dartnall-nomogram for a rhodopsin while the response to a persistent stimulus falls off more rapidly toward the red end of the spectrum. Thus light of 540 nm, for which chlamy-rhodopsin is equally sensitive as for 440-nm light, induces no measurable persistent orientation while 440-nm light does. A model is presented which explains not only this behavior, but also how Chlamydomonas can track the light direction and switches between a positive and negative phototaxis. According to the model the ability to detect the direction of light, to make the right turn and to stay oriented, is a direct consequence of the helical path of the organism, the orientation of its eyespot relative to the helix-axis, and the special shielding properties of eyespot and cell body. The model places particular emphasis on the fact that prolonged swimming into the correct direction not only requires making a correct turn initially, but also avoiding further turns once the right direction has been reached.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckmann M., Hegemann P. In vitro identification of rhodopsin in the green alga Chlamydomonas. Biochemistry. 1991 Apr 16;30(15):3692–3697. doi: 10.1021/bi00229a014. [DOI] [PubMed] [Google Scholar]
- Deininger W., Kröger P., Hegemann U., Lottspeich F., Hegemann P. Chlamyrhodopsin represents a new type of sensory photoreceptor. EMBO J. 1995 Dec 1;14(23):5849–5858. doi: 10.1002/j.1460-2075.1995.tb00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinleib M. E. Phototactic response of Chlamydomonas to flashes of light. I. Response of cell populations. Photochem Photobiol. 1975 May;21(5):351–354. doi: 10.1111/j.1751-1097.1975.tb06683.x. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Saranak J., Patel N., Zarilli G., Okabe M., Kline T., Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellular eukaryote Chlamydomonas. Nature. 1984 Oct 25;311(5988):756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- Foster K. W., Smyth R. D. Light Antennas in phototactic algae. Microbiol Rev. 1980 Dec;44(4):572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann P., Hegemann U., Foster K. W. Reversible bleaching of Chlamydomonas reinhardtii rhodopsin in vivo. Photochem Photobiol. 1988 Jul;48(1):123–128. doi: 10.1111/j.1751-1097.1988.tb02796.x. [DOI] [PubMed] [Google Scholar]
- Kamiya R., Witman G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol. 1984 Jan;98(1):97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger P., Hegemann P. Photophobic responses and phototaxis in Chlamydomonas are triggered by a single rhodopsin photoreceptor. FEBS Lett. 1994 Mar 14;341(1):5–9. doi: 10.1016/0014-5793(94)80229-7. [DOI] [PubMed] [Google Scholar]
- Messler P., Harz H., Uhl R. Instrumentation for multiwavelengths excitation imaging. J Neurosci Methods. 1996 Nov;69(2):137–147. doi: 10.1016/S0165-0270(96)00032-5. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller K., Uhl R. A microspectrophotometric study of the shielding properties of eyespot and cell body in Chlamydomonas. Biophys J. 1997 Sep;73(3):1573–1578. doi: 10.1016/S0006-3495(97)78189-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Eckert R. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature. 1976 Aug 19;262(5570):713–715. doi: 10.1038/262713a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Kubota M., Watanabe M., Yoshihara K., Derguini F., Nakanishi K. Diversion of the sign of phototaxis in a Chlamydomonas reinhardtii mutant incorporated with retinal and its analogs. FEBS Lett. 1992 Dec 21;314(3):275–279. doi: 10.1016/0014-5793(92)81488-8. [DOI] [PubMed] [Google Scholar]
- Uhl R., Hegemann P. Probing visual transduction in a plant cell: Optical recording of rhodopsin-induced structural changes from Chlamydomonas reinhardtii. Biophys J. 1990 Nov;58(5):1295–1302. doi: 10.1016/S0006-3495(90)82469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman G. B. Chlamydomonas phototaxis. Trends Cell Biol. 1993 Nov;3(11):403–408. doi: 10.1016/0962-8924(93)90091-e. [DOI] [PubMed] [Google Scholar]
- Zacks D. N., Derguini F., Nakanishi K., Spudich J. L. Comparative study of phototactic and photophobic receptor chromophore properties in Chlamydomonas reinhardtii. Biophys J. 1993 Jul;65(1):508–518. doi: 10.1016/S0006-3495(93)81067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]