Abstract
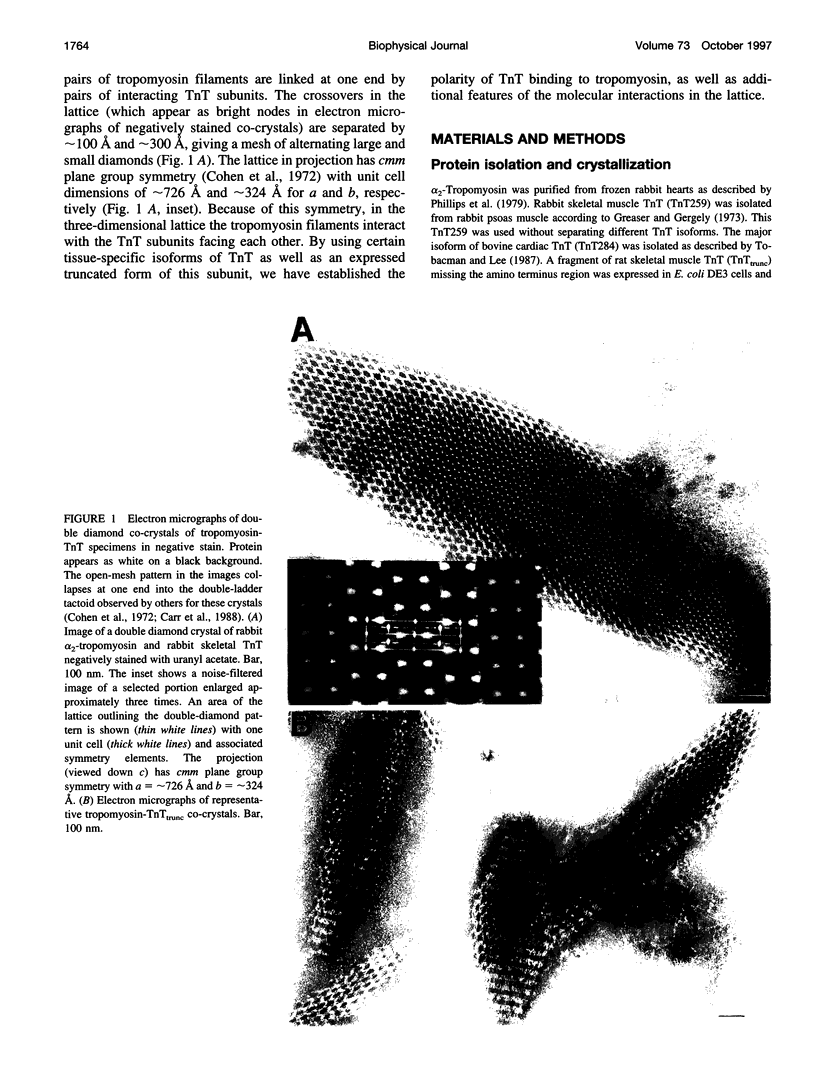
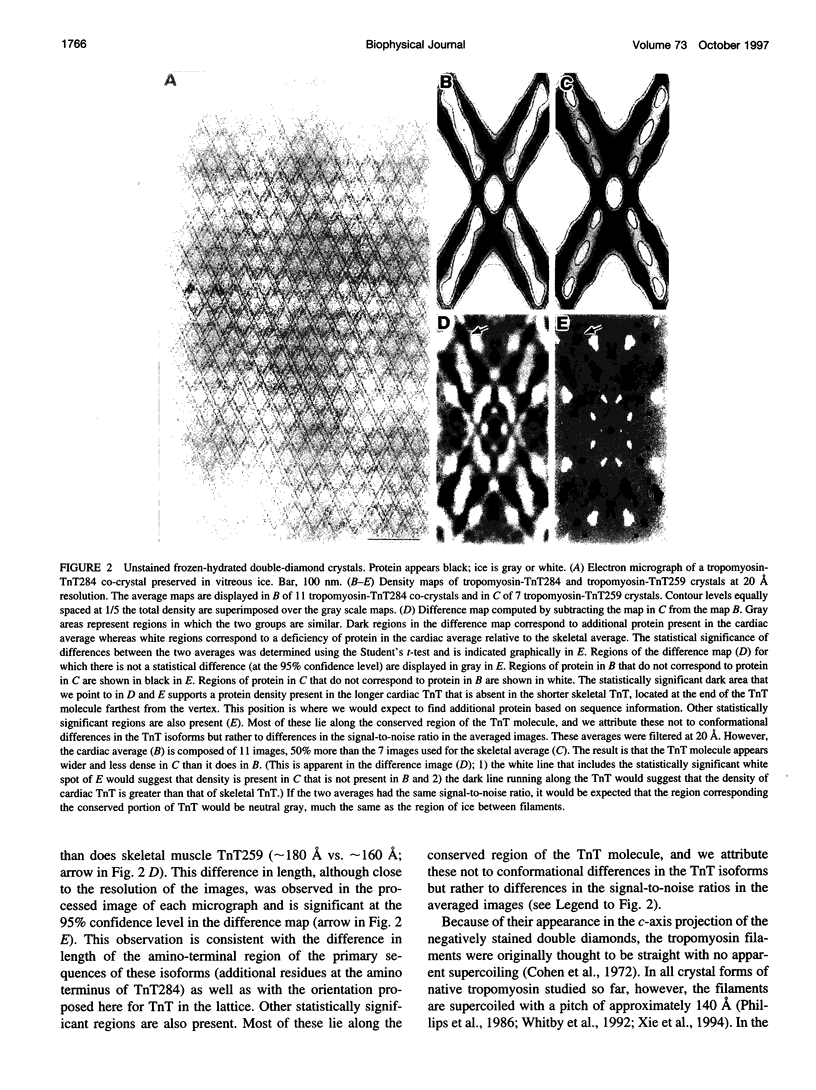
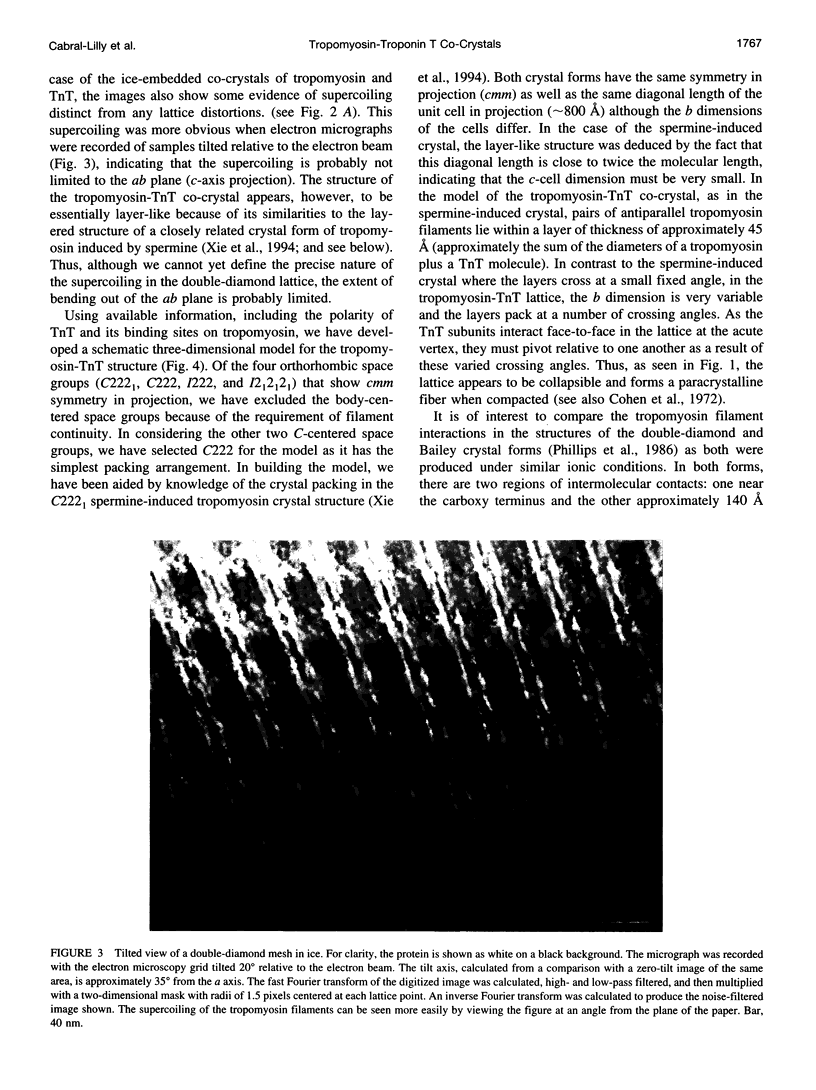
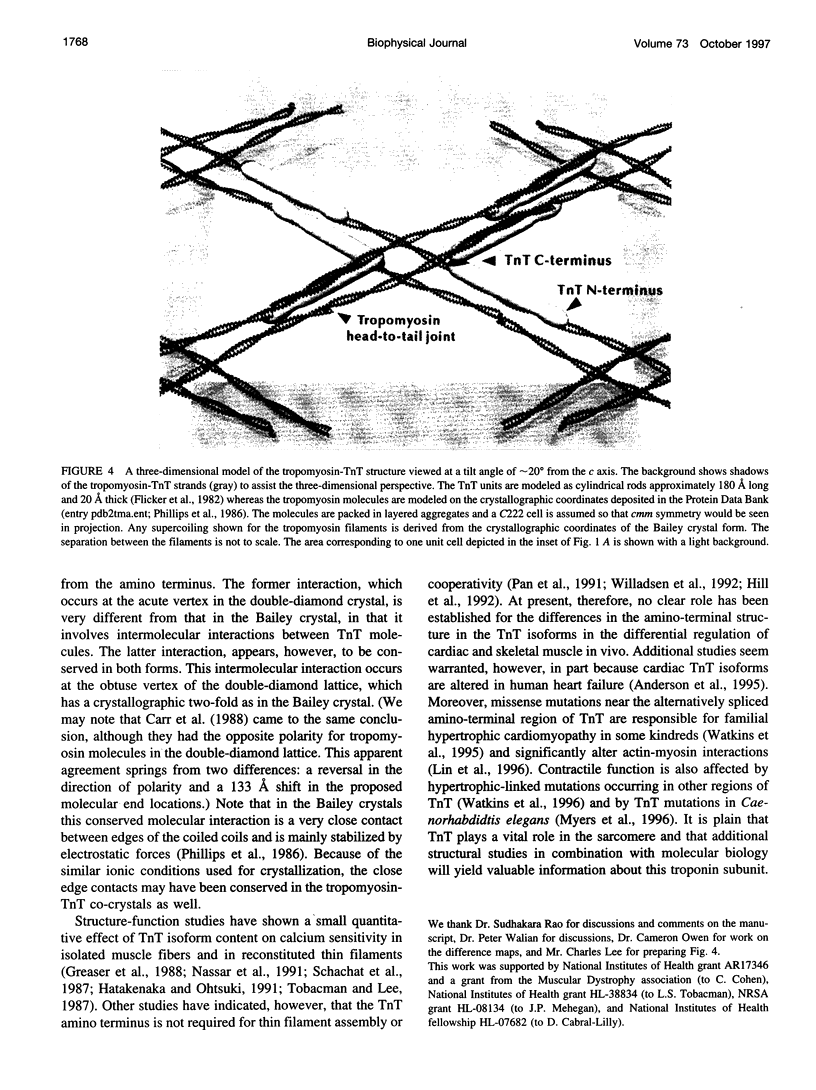
New features of the structure and interactions of troponin T and tropomyosin have been revealed by electron microscopy of so-called double-diamond co-crystals. These co-crystals were formed using rabbit alpha2 tropomyosin complexed with troponin T from either skeletal or cardiac muscle, which have different lengths in the amino-terminal region, as well as a bacterially expressed skeletal muscle troponin T fragment of 190 residues that lacks the amino-terminal region. Differences in the images of the co-crystals have allowed us to establish the polarities of both the troponin T subunit and tropomyosin in the projected lattice. Moreover, in agreement with their sequences, the amino-terminal region of a bovine cardiac muscle troponin T isoform appears to be longer than that from the rabbit skeletal muscle troponin T isoform and to span more of the amino terminus of tropomyosin at the head-to-tail filament joints. Images of crystals tilted relative to the electron beam also reveal the supercoiling of the tropomyosin filaments in this lattice. Based on these results, a three-dimensional model of the double-diamond lattice has been constructed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. A., Greig A., Mark T. M., Malouf N. N., Oakeley A. E., Ungerleider R. M., Allen P. D., Kay B. K. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res. 1995 Apr;76(4):681–686. doi: 10.1161/01.res.76.4.681. [DOI] [PubMed] [Google Scholar]
- Anderson P. A., Malouf N. N., Oakeley A. E., Pagani E. D., Allen P. D. Troponin T isoform expression in humans. A comparison among normal and failing adult heart, fetal heart, and adult and fetal skeletal muscle. Circ Res. 1991 Nov;69(5):1226–1233. doi: 10.1161/01.res.69.5.1226. [DOI] [PubMed] [Google Scholar]
- Bandman E. Contractile protein isoforms in muscle development. Dev Biol. 1992 Dec;154(2):273–283. doi: 10.1016/0012-1606(92)90067-q. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Briggs M. M., Schachat F. Origin of fetal troponin T: developmentally regulated splicing of a new exon in the fast troponin T gene. Dev Biol. 1993 Aug;158(2):503–509. doi: 10.1006/dbio.1993.1208. [DOI] [PubMed] [Google Scholar]
- Cabral-Lilly D., Phillips G. N., Jr, Sosinsky G. E., Melanson L., Chacko S., Cohen C. Structural studies of tropomyosin by cryoelectron microscopy and x-ray diffraction. Biophys J. 1991 Apr;59(4):805–814. doi: 10.1016/S0006-3495(91)82293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr H. J., O'Brien E. J., Morris E. P. Structure of tropomyosin-troponin T cocrystals. J Muscle Res Cell Motil. 1988 Oct;9(5):384–392. doi: 10.1007/BF01774065. [DOI] [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single cardiac troponin T gene generates embryonic and adult isoforms via developmentally regulated alternate splicing. J Biol Chem. 1985 Sep 15;260(20):11140–11148. [PubMed] [Google Scholar]
- Dahiya R., Butters C. A., Tobacman L. S. Equilibrium linkage analysis of cardiac thin filament assembly. Implications for the regulation of muscle contraction. J Biol Chem. 1994 Nov 25;269(47):29457–29461. [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Flicker P. F., Phillips G. N., Jr, Cohen C. Troponin and its interactions with tropomyosin. An electron microscope study. J Mol Biol. 1982 Dec 5;162(2):495–501. doi: 10.1016/0022-2836(82)90540-x. [DOI] [PubMed] [Google Scholar]
- Gahlmann R., Troutt A. B., Wade R. P., Gunning P., Kedes L. Alternative splicing generates variants in important functional domains of human slow skeletal troponin T. J Biol Chem. 1987 Nov 25;262(33):16122–16126. [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Purification and properties of the components from troponin. J Biol Chem. 1973 Mar 25;248(6):2125–2133. [PubMed] [Google Scholar]
- Greaser M. L., Moss R. L., Reiser P. J. Variations in contractile properties of rabbit single muscle fibres in relation to troponin T isoforms and myosin light chains. J Physiol. 1988 Dec;406:85–98. doi: 10.1113/jphysiol.1988.sp017370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig A., Hirschberg Y., Anderson P. A., Hainsworth C., Malouf N. N., Oakeley A. E., Kay B. K. Molecular basis of cardiac troponin T isoform heterogeneity in rabbit heart. Circ Res. 1994 Jan;74(1):41–47. doi: 10.1161/01.res.74.1.41. [DOI] [PubMed] [Google Scholar]
- Hatakenaka M., Ohtsuki I. Replacement of three troponin components with cardiac troponin components within single glycerinated skeletal muscle fibers. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1022–1027. doi: 10.1016/0006-291x(91)92039-m. [DOI] [PubMed] [Google Scholar]
- Hill L. E., Mehegan J. P., Butters C. A., Tobacman L. S. Analysis of troponin-tropomyosin binding to actin. Troponin does not promote interactions between tropomyosin molecules. J Biol Chem. 1992 Aug 15;267(23):16106–16113. [PubMed] [Google Scholar]
- Ishii Y., Lehrer S. S. Two-site attachment of troponin to pyrene-labeled tropomyosin. J Biol Chem. 1991 Apr 15;266(11):6894–6903. [PubMed] [Google Scholar]
- Jin J. P., Lin J. J. Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem. 1989 Aug 25;264(24):14471–14477. [PubMed] [Google Scholar]
- Katus H. A., Looser S., Hallermayer K., Remppis A., Scheffold T., Borgya A., Essig U., Geuss U. Development and in vitro characterization of a new immunoassay of cardiac troponin T. Clin Chem. 1992 Mar;38(3):386–393. [PubMed] [Google Scholar]
- Leszyk J., Dumaswala R., Potter J. D., Gusev N. B., Verin A. D., Tobacman L. S., Collins J. H. Bovine cardiac troponin T: amino acid sequences of the two isoforms. Biochemistry. 1987 Nov 3;26(22):7035–7042. doi: 10.1021/bi00396a027. [DOI] [PubMed] [Google Scholar]
- Lin D., Bobkova A., Homsher E., Tobacman L. S. Altered cardiac troponin T in vitro function in the presence of a mutation implicated in familial hypertrophic cardiomyopathy. J Clin Invest. 1996 Jun 15;97(12):2842–2848. doi: 10.1172/JCI118740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair J., Dienstl F., Puschendorf B. Cardiac troponin T in the diagnosis of myocardial injury. Crit Rev Clin Lab Sci. 1992;29(1):31–57. doi: 10.3109/10408369209105245. [DOI] [PubMed] [Google Scholar]
- Mak A. S., Golosinska K., Smillie L. B. Induction of nonpolymerizable tropomyosin binding to F-actin by troponin and its components. J Biol Chem. 1983 Dec 10;258(23):14330–14334. [PubMed] [Google Scholar]
- Mak A. S., Smillie L. B. Structural interpretation of the two-site binding of troponin on the muscle thin filament. J Mol Biol. 1981 Jul 5;149(3):541–550. doi: 10.1016/0022-2836(81)90486-1. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Cohen C. Letter: Troponin subunit interactions. J Mol Biol. 1973 Dec 15;81(3):409–413. doi: 10.1016/0022-2836(73)90150-2. [DOI] [PubMed] [Google Scholar]
- Mesnard L., Samson F., Espinasse I., Durand J., Neveux J. Y., Mercadier J. J. Molecular cloning and developmental expression of human cardiac troponin T. FEBS Lett. 1993 Aug 9;328(1-2):139–144. doi: 10.1016/0014-5793(93)80981-y. [DOI] [PubMed] [Google Scholar]
- Myers C. D., Goh P. Y., Allen T. S., Bucher E. A., Bogaert T. Developmental genetic analysis of troponin T mutations in striated and nonstriated muscle cells of Caenorhabditis elegans. J Cell Biol. 1996 Mar;132(6):1061–1077. doi: 10.1083/jcb.132.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar R., Malouf N. N., Kelly M. B., Oakeley A. E., Anderson P. A. Force-pCa relation and troponin T isoforms of rabbit myocardium. Circ Res. 1991 Dec;69(6):1470–1475. doi: 10.1161/01.res.69.6.1470. [DOI] [PubMed] [Google Scholar]
- Pan B. S., Gordon A. M., Potter J. D. Deletion of the first 45 NH2-terminal residues of rabbit skeletal troponin T strengthens binding of troponin to immobilized tropomyosin. J Biol Chem. 1991 Jul 5;266(19):12432–12438. [PubMed] [Google Scholar]
- Pearlstone J. R., Carpenter M. R., Smillie L. B. Amino acid sequence of rabbit cardiac troponin T. J Biol Chem. 1986 Dec 25;261(36):16795–16810. [PubMed] [Google Scholar]
- Pearlstone J. R., Johnson P., Carpenter M. R., Smillie L. B. Primary structure of rabbit skeletal muscle troponin-T. Sequence determination of the NH2-terminal fragment CB3 and the complete sequence of troponin-T. J Biol Chem. 1977 Feb 10;252(3):983–989. [PubMed] [Google Scholar]
- Phillips G. N., Jr, Fillers J. P., Cohen C. Tropomyosin crystal structure and muscle regulation. J Mol Biol. 1986 Nov 5;192(1):111–131. doi: 10.1016/0022-2836(86)90468-7. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Lattman E. E., Cummins P., Lee K. Y., Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979 Mar 29;278(5703):413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Diamond M. S., Brandt P. W. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987 Dec 5;198(3):551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Schaertl S., Lehrer S. S., Geeves M. A. Separation and characterization of the two functional regions of troponin involved in muscle thin filament regulation. Biochemistry. 1995 Dec 12;34(49):15890–15894. doi: 10.1021/bi00049a003. [DOI] [PubMed] [Google Scholar]
- Smillie L. B., Golosinska K., Reinach F. C. Sequences of complete cDNAs encoding four variants of chicken skeletal muscle troponin T. J Biol Chem. 1988 Dec 15;263(35):18816–18820. [PubMed] [Google Scholar]
- Tobacman L. S., Lee R. Isolation and functional comparison of bovine cardiac troponin T isoforms. J Biol Chem. 1987 Mar 25;262(9):4059–4064. [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J., Macnab R. M. Three-dimensional structure of the complex flagellar filament of Rhizobium lupini and its relation to the structure of the plain filament. J Mol Biol. 1987 Jun 5;195(3):603–620. doi: 10.1016/0022-2836(87)90185-9. [DOI] [PubMed] [Google Scholar]
- Watkins H., McKenna W. J., Thierfelder L., Suk H. J., Anan R., O'Donoghue A., Spirito P., Matsumori A., Moravec C. S., Seidman J. G. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995 Apr 20;332(16):1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- Watkins H., Seidman C. E., Seidman J. G., Feng H. S., Sweeney H. L. Expression and functional assessment of a truncated cardiac troponin T that causes hypertrophic cardiomyopathy. Evidence for a dominant negative action. J Clin Invest. 1996 Dec 1;98(11):2456–2461. doi: 10.1172/JCI119063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby F. G., Kent H., Stewart F., Stewart M., Xie X., Hatch V., Cohen C., Phillips G. N., Jr Structure of tropomyosin at 9 angstroms resolution. J Mol Biol. 1992 Sep 20;227(2):441–452. doi: 10.1016/0022-2836(92)90899-u. [DOI] [PubMed] [Google Scholar]
- White S. P., Cohen C., Phillips G. N., Jr Structure of co-crystals of tropomyosin and troponin. 1987 Feb 26-Mar 4Nature. 325(6107):826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Moir A. J., Waterfield M. D. The expression of multiple forms of troponin T in chicken-fast-skeletal muscle may result from differential splicing of a single gene. Eur J Biochem. 1984 Aug 15;143(1):47–56. doi: 10.1111/j.1432-1033.1984.tb08337.x. [DOI] [PubMed] [Google Scholar]
- Willadsen K. A., Butters C. A., Hill L. E., Tobacman L. S. Effects of the amino-terminal regions of tropomyosin and troponin T on thin filament assembly. J Biol Chem. 1992 Nov 25;267(33):23746–23752. [PubMed] [Google Scholar]
- Xie X., Rao S., Walian P., Hatch V., Phillips G. N., Jr, Cohen C. Coiled-coil packing in spermine-induced tropomyosin crystals. A comparative study of three forms. J Mol Biol. 1994 Mar 4;236(4):1212–1226. doi: 10.1016/0022-2836(94)90022-1. [DOI] [PubMed] [Google Scholar]